A Meta-Analysis of the Effects of Insects in Feed on Poultry Growth Performances
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
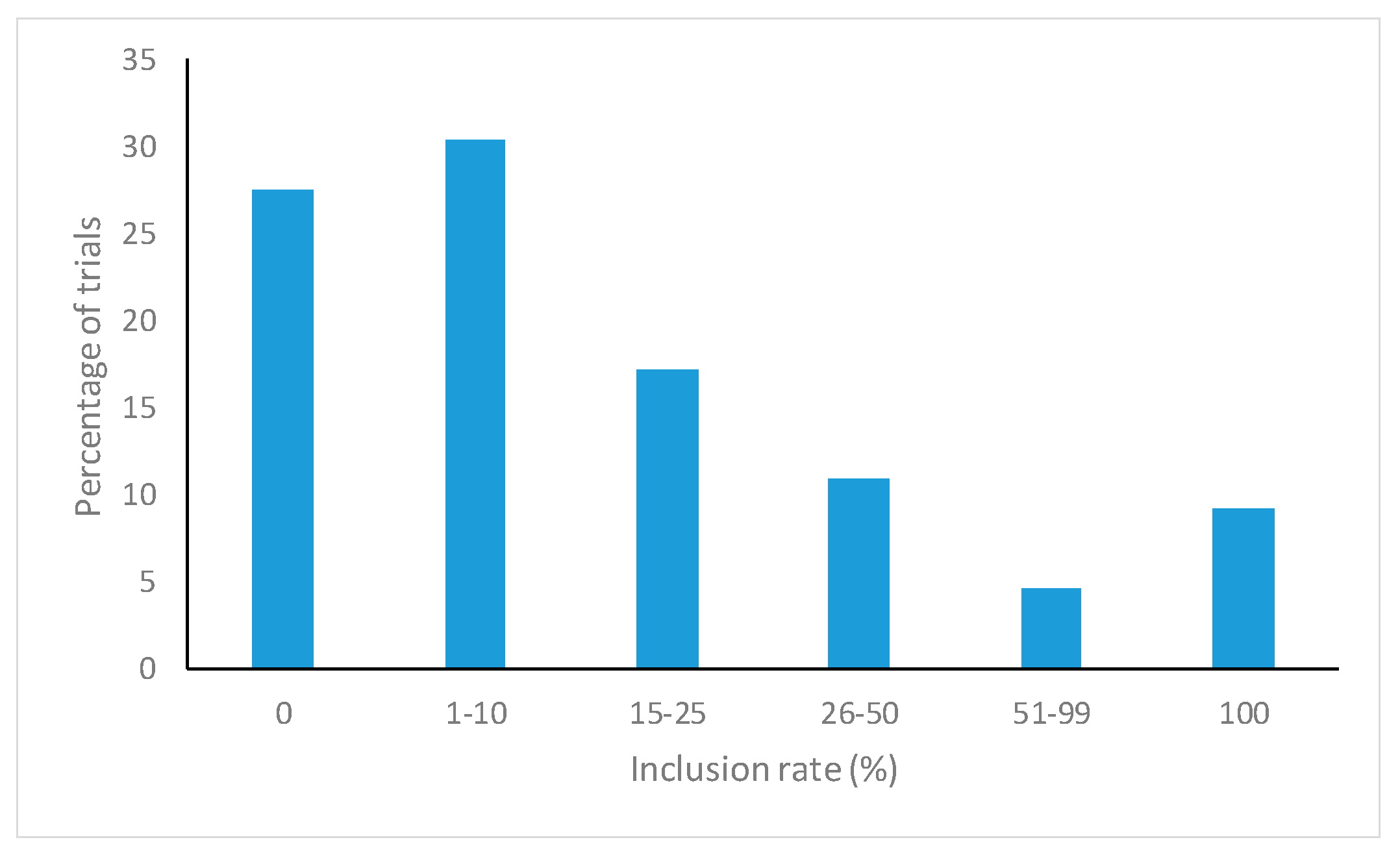
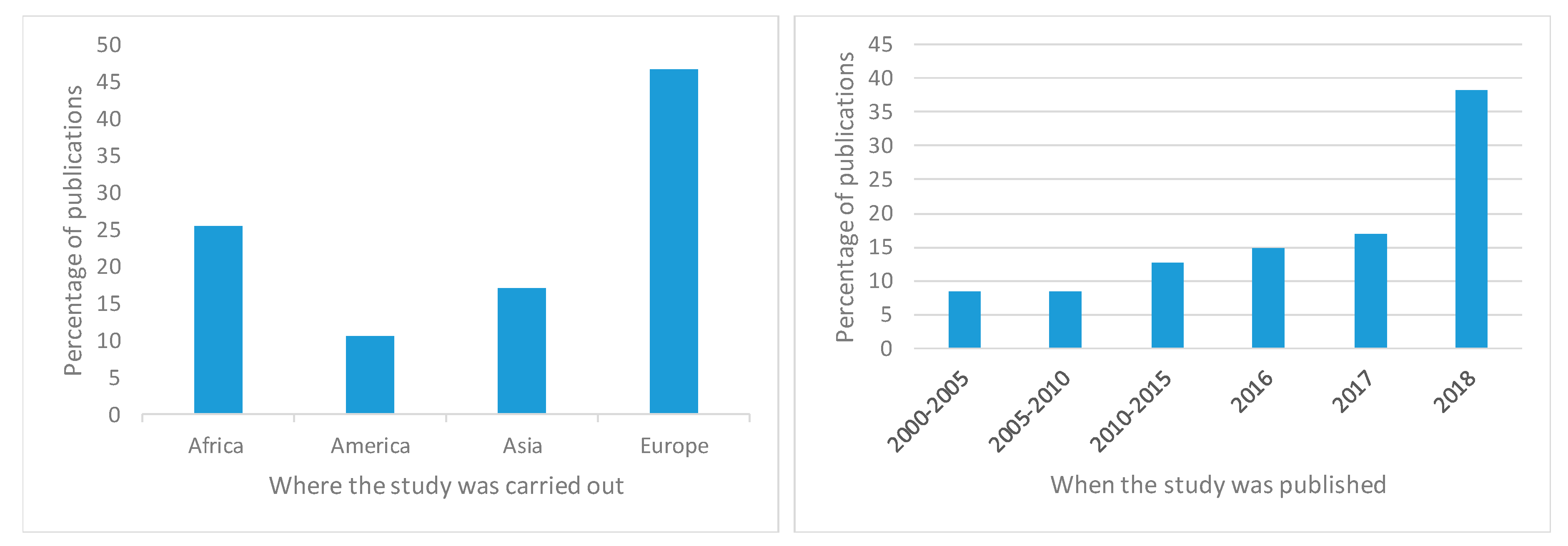
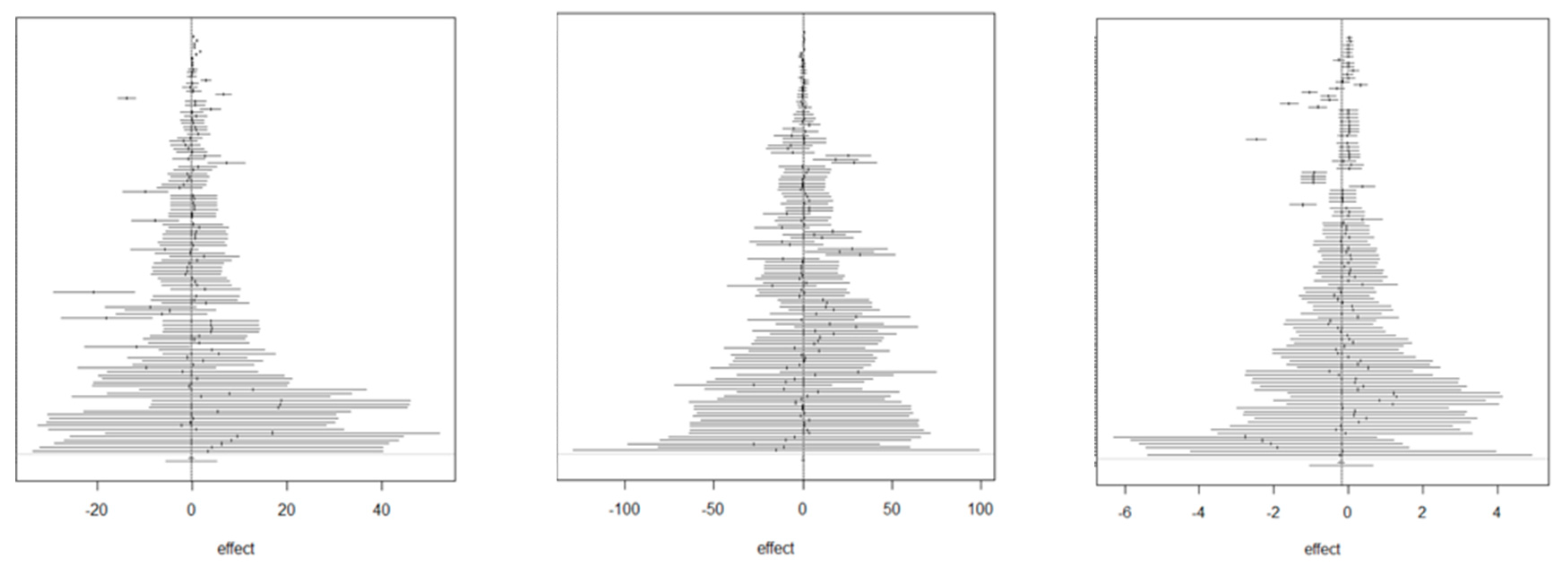
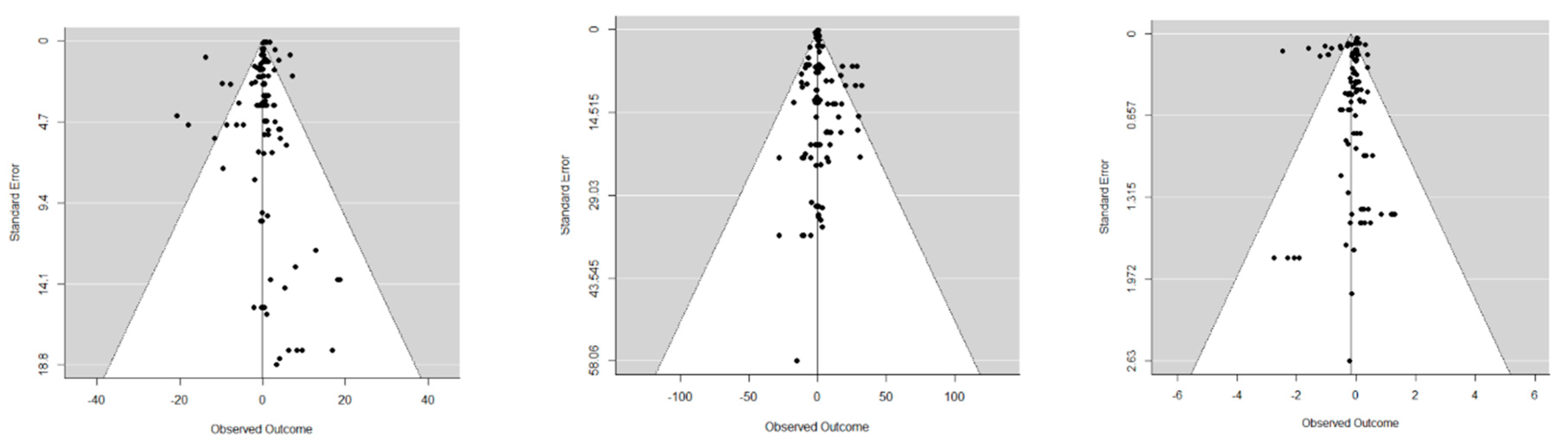
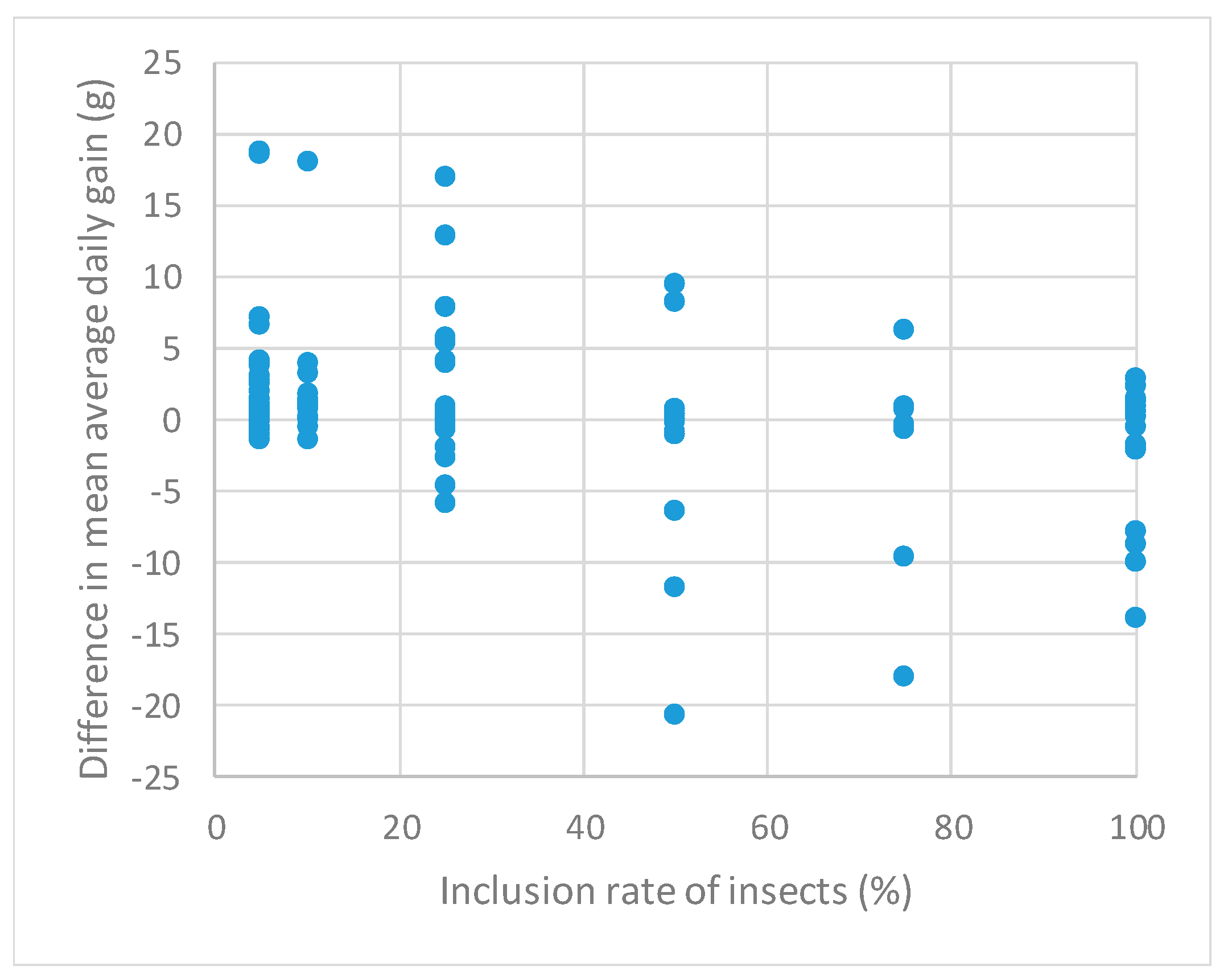
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Study Reference | Country | Inclusion Rate | Poultry Species | Insect Species | Average Daily Gain (g) | Feed Intake (g) | Feed Conversion Ratio | |||
| Mean | SD | Mean | SD | Mean | SD | |||||
| Velten et al., 2018 [49] | Germany | 0.0 | broiler | BSF | 64.54 | 3.40 | 87.27 | 4.72 | 1.35 | 0.04 |
| Velten et al., 2018 [49] | Germany | 50.0 | broiler | BSF | 43.86 | 2.71 | 75.37 | 6.17 | 1.72 | 0.17 |
| Schiavone et al., 2016 [47] | Italy | 0.0 | broiler | BSF | 48.62 | 3.84 | 55.10 | 6.32 | 1.48 | 0.03 |
| Schiavone et al., 2016 [47] | Italy | 50.0 | broiler | BSF | 49.08 | 3.84 | 61.20 | 6.32 | 1.51 | 0.03 |
| Schiavone et al., 2016 [47] | Italy | 100.0 | broiler | BSF | 50.02 | 3.84 | 65.40 | 6.32 | 1.52 | 0.03 |
| Onsongo et al., 2016 [45] | Kenya | 0.0 | broiler | BSF | 69.00 | 1.35 | 124.10 | 18.96 | 1.80 | 0.37 |
| Onsongo et al., 2016 [45] | Kenya | 5.0 | broiler | BSF | 71.70 | 1.02 | 126.20 | 14.26 | 1.80 | 0.29 |
| Onsongo et al., 2016 [45] | Kenya | 10.0 | broiler | BSF | 67.60 | 0.72 | 122.90 | 14.40 | 1.80 | 0.15 |
| Onsongo et al., 2016 [45] | Kenya | 15.0 | broiler | BSF | 68.30 | 1.03 | 119.10 | 6.64 | 1.70 | 0.22 |
| Gaffigan et al., 2017 [32] | USA | 0.0 | broiler | BSF | 66.59 | 18.77 | 99.88 | 22.70 | 1.50 | 0.25 |
| Gaffigan et al., 2017 [32] | USA | 100.0 | broiler | BSF | 75.30 | 10.96 | 108.05 | 4.54 | 1.44 | 0.20 |
| Dabbou et al., 2018 [28] | Italy | 0.0 | broiler | BSF | 65.56 | 2.54 | 91.76 | 9.01 | 1.60 | 0.06 |
| Dabbou et al., 2018 [28] | Italy | 5.0 | broiler | BSF | 65.41 | 2.54 | 90.90 | 9.01 | 1.59 | 0.06 |
| Dabbou et al., 2018 [28] | Italy | 10.0 | broiler | BSF | 65.84 | 2.54 | 92.44 | 9.01 | 1.60 | 0.06 |
| Dabbou et al., 2018 [28] | Italy | 15.0 | broiler | BSF | 59.76 | 2.54 | 89.83 | 9.01 | 1.72 | 0.06 |
| Cullere et al., 2016 [27] | Italy | 0.0 | other | BSF | 8.25 | 0.31 | 23.30 | 1.28 | 2.83 | 0.12 |
| Cullere et al., 2016 [27] | Italy | 10.0 | other | BSF | 8.40 | 0.31 | 24.40 | 1.28 | 2.90 | 0.12 |
| Cullere et al., 2016 [27] | Italy | 15.0 | other | BSF | 8.24 | 0.31 | 23.40 | 1.28 | 2.86 | 0.12 |
| Al-Qazzaz et al., 2016 [14] | Malaysia | 0.0 | layer | BSF | 27.63 | 2.14 | 78.90 | 4.33 | 2.86 | 0.16 |
| Al-Qazzaz et al., 2016 [14] | Malaysia | 5.0 | layer | BSF | 48.50 | 3.74 | 79.40 | 4.33 | 1.64 | 0.09 |
| Al-Qazzaz et al., 2016 [14] | Malaysia | 10.0 | layer | BSF | 28.08 | 2.15 | 79.79 | 4.33 | 2.84 | 0.15 |
| Mwaniki et al., 2018 [42] | Canada | 0.0 | layer | BSF | 5.21 | 2.44 | 92.20 | 2.10 | ||
| Mwaniki et al., 2018 [42] | Canada | 5.0 | layer | BSF | 5.84 | 2.44 | 92.00 | 2.10 | ||
| Mwaniki et al., 2018 [42] | Canada | 7.5 | layer | BSF | 5.89 | 2.44 | 95.70 | 2.10 | ||
| Cockcroft et al., 2018 [26] | South Africa | 0.0 | broiler | BSF | 47.37 | 11.00 | 79.39 | 17.25 | 1.69 | 0.46 |
| Cockcroft et al., 2018 [26] | South Africa | 15.0 | broiler | BSF | 60.20 | 5.19 | 85.94 | 4.56 | 1.41 | 0.15 |
| Cockcroft et al., 2018 [26] | South Africa | 15.0 | broiler | BSF | 52.70 | 9.22 | 88.37 | 10.49 | 1.67 | 0.46 |
| Cockcroft et al., 2018 [26] | South Africa | 15.0 | broiler | BSF | 55.27 | 7.13 | 86.07 | 14.26 | 1.55 | 0.15 |
| Borelli et al., 2017 [21] | Italy | 0.0 | layer | BSF | 125.80 | 6.79 | 2.47 | 0.14 | ||
| Borelli et al., 2017 [21] | Italy | 100.0 | layer | BSF | 108.31 | 10.77 | ||||
| Bovera et al., 2018 [22] | Germany | 0.0 | layer | BSF | 2.18 | 0.56 | 99.97 | 8.72 | 1.74 | 0.15 |
| Bovera et al., 2018 [22] | Germany | 15.0 | layer | BSF | 1.79 | 0.56 | 97.69 | 8.72 | 1.68 | 0.15 |
| Bovera et al., 2018 [22] | Germany | 25.0 | layer | BSF | 2.50 | 0.56 | 101.90 | 8.72 | 1.76 | 0.15 |
| Wallace et al., 2018 [50] | Ghana | 0.0 | other | BSF | 9.16 | 0.86 | 58.00 | 9.23 | 6.34 | 1.02 |
| Wallace et al., 2018 [50] | Ghana | 20.0 | other | BSF | 9.19 | 0.86 | 69.30 | 9.23 | 7.57 | 1.02 |
| Wallace et al., 2018 [50] | Ghana | 40.0 | other | BSF | 9.31 | 0.86 | 71.10 | 9.23 | 7.64 | 1.02 |
| Wallace et al., 2018 [50] | Ghana | 60.0 | other | BSF | 9.84 | 0.86 | 70.60 | 9.23 | 7.18 | 1.02 |
| Wallace et al., 2018 [50] | Ghana | 80.0 | other | BSF | 10.00 | 0.86 | 75.30 | 9.23 | 7.52 | 1.02 |
| Wallace et al., 2018 [50] | Ghana | 100.0 | other | BSF | 10.50 | 0.86 | 65.10 | 9.23 | 6.18 | 1.02 |
| Moula et al., 2017a [41] | Belgium | 0.0 | broiler | BSF | 20.48 | 11.21 | ||||
| Moula et al., 2017a [41] | Belgium | 2.0 | broiler | BSF | 21.36 | 11.21 | ||||
| Moula et al., 2017b [2] | Belgium | 0.0 | broiler | BSF | 20.59 | 0.96 | 1.51 | 0.14 | ||
| Moula et al., 2017b [2] | Belgium | 3.0 | broiler | BSF | 20.59 | 0.71 | 1.39 | 0.08 | ||
| Brah et al., 2018 [24] | Niger | 0.0 | broiler | GH | 44.21 | 3.45 | 83.00 | 25.46 | 1.77 | 1.36 |
| Brah et al., 2018 [24] | Niger | 25.0 | broiler | GH | 39.60 | 3.45 | 78.00 | 25.46 | 1.96 | 0.68 |
| Brah et al., 2018 [24] | Niger | 50.0 | broiler | GH | 37.81 | 3.45 | 73.00 | 25.46 | 1.92 | 0.68 |
| Brah et al., 2018 [24] | Niger | 75.0 | broiler | GH | 26.23 | 3.45 | 55.00 | 25.46 | 2.24 | 0.68 |
| Brah et al., 2018 [24] | Niger | 100.0 | broiler | GH | 35.50 | 3.43 | 72.00 | 25.46 | 2.06 | 0.68 |
| Brah et al., 2017 [25] | Niger | 0.0 | layer | GH | 46.00 | 15.87 | 83.00 | 15.87 | 1.80 | 1.00 |
| Brah et al., 2017 [25] | Niger | 25.0 | layer | GH | 39.00 | 15.87 | 78.00 | 15.87 | 2.00 | 1.00 |
| Brah et al., 2017 [25] | Niger | 50.0 | layer | GH | 37.00 | 15.87 | 73.00 | 15.87 | 1.97 | 1.00 |
| Brah et al., 2017 [25] | Niger | 75.0 | layer | GH | 25.00 | 15.87 | 55.00 | 15.87 | 2.20 | 1.00 |
| Brah et al., 2017 [25] | Niger | 100.0 | layer | GH | 35.00 | 15.87 | 72.00 | 15.87 | 2.06 | 1.00 |
| Das et al., 2014 [29] | India | 0.0 | other | GH | 3.05 | 0.03 | 13.31 | 0.08 | 4.37 | 0.07 |
| Das et al., 2014 [29] | India | 5.0 | other | GH | 3.36 | 0.03 | 13.69 | 0.08 | 4.07 | 0.07 |
| Das et al., 2014 [29] | India | 10.0 | other | GH | 4.04 | 0.03 | 13.40 | 0.08 | 3.33 | 0.07 |
| Das et al., 2014 [29] | India | 15.0 | other | GH | 3.59 | 0.03 | 13.79 | 0.08 | 3.84 | 0.07 |
| Das et al., 2014 [29] | India | 0.0 | other | GH | 3.23 | 0.04 | 14.72 | 0.07 | 4.56 | 0.08 |
| Das et al., 2014 [29] | India | 5.0 | other | GH | 3.74 | 0.04 | 15.14 | 0.07 | 4.05 | 0.08 |
| Das et al., 2014 [29] | India | 10.0 | other | GH | 5.01 | 0.04 | 14.86 | 0.07 | 2.97 | 0.08 |
| Das et al., 2014 [29] | India | 15.0 | other | GH | 4.11 | 0.04 | 15.34 | 0.07 | 3.74 | 0.08 |
| Das et al., 2014 [29] | India | 0.0 | other | GH | 0.12 | 0.09 | 33.60 | 4.38 | ||
| Das et al., 2014 [29] | India | 5.0 | other | GH | 0.17 | 0.09 | 26.40 | 4.38 | ||
| Das et al., 2014 [29] | India | 10.0 | other | GH | 0.18 | 0.09 | 25.10 | 4.38 | ||
| Das et al., 2014 [29] | India | 15.0 | other | GH | 0.16 | 0.09 | 27.60 | 4.38 | ||
| Khan et al., 2018 [38] | Brazil | 0.0 | broiler | HF | 10.78 | 0.95 | 58.91 | 13.75 | 2.08 | 1.36 |
| Khan et al., 2018 [38] | Brazil | 40.0 | broiler | HF | 11.61 | 0.73 | 89.63 | 17.58 | 1.93 | 1.59 |
| Khan et al., 2018 [38] | Brazil | 50.0 | broiler | HF | 11.42 | 0.54 | 88.65 | 6.52 | 1.88 | 0.68 |
| Khan et al., 2018 [38] | Brazil | 60.0 | broiler | HF | 11.53 | 0.53 | 88.44 | 10.95 | 1.74 | 1.01 |
| Awoniyi et al., 2003 [16] | Nigeria | 0.0 | broiler | HF | 45.93 | 2.26 | 100.14 | 6.67 | 2.21 | 0.04 |
| Awoniyi et al., 2003 [16] | Nigeria | 25.0 | broiler | HF | 43.33 | 0.93 | 95.75 | 29.52 | 2.21 | 0.04 |
| Awoniyi et al., 2003 [16] | Nigeria | 50.0 | broiler | HF | 34.29 | 5.17 | 88.24 | 6.45 | 2.60 | 0.27 |
| Awoniyi et al., 2003 [16] | Nigeria | 75.0 | broiler | HF | 36.36 | 7.02 | 92.55 | 6.74 | 2.60 | 0.47 |
| Awoniyi et al., 2003 [16] | Nigeria | 100.0 | broiler | HF | 36.06 | 0.93 | 90.93 | 0.58 | 2.53 | 0.08 |
| Okah et al., 2012 [44] | Nigeria | 0.0 | broiler | HF | 39.46 | 12.69 | 39.46 | 12.77 | 6.17 | 1.27 |
| Okah et al., 2012 [44] | Nigeria | 20.0 | broiler | HF | 56.33 | 12.69 | 56.33 | 12.77 | 3.40 | 1.27 |
| Okah et al., 2012 [44] | Nigeria | 30.0 | broiler | HF | 48.90 | 12.69 | 48.90 | 12.77 | 3.86 | 1.27 |
| Okah et al., 2012 [44] | Nigeria | 40.0 | broiler | HF | 47.70 | 12.69 | 47.70 | 12.77 | 4.08 | 1.27 |
| Okah et al., 2012 [44] | Nigeria | 50.0 | broiler | HF | 45.71 | 12.69 | 45.71 | 12.77 | 4.26 | 1.27 |
| Đorđević et al., 2008 [30] | Serbia | 0.0 | broiler | HF | 38.06 | 4.46 | 73.33 | 12.19 | 1.93 | 0.23 |
| Đorđević et al., 2008 [30] | Serbia | 50.0 | broiler | HF | 37.06 | 4.63 | 68.66 | 12.18 | 1.85 | 0.23 |
| Đorđević et al., 2008 [30] | Serbia | 100.0 | broiler | HF | 38.24 | 4.75 | 74.90 | 13.21 | 1.96 | 0.24 |
| Đorđević et al., 2008 [30] | Serbia | 100.0 | broiler | HF | 40.33 | 4.69 | 75.71 | 12.49 | 1.88 | 0.22 |
| Téguia et al., 2002 [48] | Cameroun | 0.0 | broiler | HF | 24.22 | 3.45 | 48.45 | 23.97 | 2.00 | 1.20 |
| Téguia et al., 2002 [48] | Cameroun | 5.0 | broiler | HF | 28.41 | 4.47 | 50.56 | 23.17 | 1.78 | 2.34 |
| Téguia et al., 2002 [48] | Cameroun | 10.0 | broiler | HF | 25.63 | 3.84 | 49.20 | 22.31 | 1.92 | 1.26 |
| Téguia et al., 2002 [48] | Cameroun | 15.0 | broiler | HF | 29.90 | 4.96 | 52.02 | 24.88 | 1.74 | 0.44 |
| Khan et al., 2016 [37] | Brazil | 0.0 | broiler | HF | 42.35 | 12.45 | 94.87 | 1.83 | 2.24 | 0.66 |
| Khan et al., 2016 [37] | Brazil | 10.0 | broiler | HF | 45.63 | 14.08 | 89.43 | 2.37 | 1.96 | 0.60 |
| Khan et al., 2016 [37] | Brazil | 20.0 | broiler | HF | 46.47 | 13.61 | 88.29 | 4.59 | 1.90 | 0.55 |
| Wang et al., 2005 [51] | China | 0.0 | broiler | OT | 29.25 | 1.77 | 47.33 | 5.30 | 6.18 | 0.57 |
| Wang et al., 2005 [51] | China | 5.0 | broiler | OT | 29.75 | 1.77 | 47.92 | 5.30 | 6.21 | 0.57 |
| Wang et al., 2005 [51] | China | 10.0 | broiler | OT | 29.33 | 1.77 | 46.50 | 5.30 | 6.31 | 0.57 |
| Wang et al., 2005 [51] | China | 15.0 | broiler | OT | 29.25 | 1.77 | 48.00 | 5.30 | 6.09 | 0.57 |
| Nobo et al., 2012 [43] | Botswana | 0.0 | other | OT | 13.80 | 1.47 | 46.21 | 0.61 | 3.83 | 0.36 |
| Nobo et al., 2012 [43] | Botswana | 4.0 | other | OT | 12.80 | 1.47 | 46.42 | 0.61 | 4.09 | 0.42 |
| Nobo et al., 2012 [43] | Botswana | 9.0 | other | OT | 13.30 | 1.47 | 46.35 | 0.61 | 3.94 | 0.39 |
| Nobo et al., 2012 [43] | Botswana | 34.0 | other | OT | 12.90 | 1.47 | 45.20 | 0.61 | 3.96 | 0.40 |
| Ijaiya et al., 2009 [35] | Nigeria | 0.0 | broiler | OT | 18.68 | 10.95 | 30.83 | 21.85 | 1.64 | 0.27 |
| Ijaiya et al., 2009 [35] | Nigeria | 25.0 | broiler | OT | 18.55 | 10.95 | 30.33 | 21.85 | 1.64 | 0.27 |
| Ijaiya et al., 2009 [35] | Nigeria | 50.0 | broiler | OT | 19.03 | 10.95 | 30.47 | 21.85 | 1.60 | 0.27 |
| Ijaiya et al., 2009 [35] | Nigeria | 75.0 | broiler | OT | 18.41 | 10.95 | 31.66 | 21.85 | 1.70 | 0.27 |
| Ijaiya et al., 2009 [35] | Nigeria | 100.0 | broiler | OT | 16.56 | 10.95 | 29.51 | 21.85 | 1.72 | 0.27 |
| Jozefiak et al., 2018 [36] | Poland | 0.0 | broiler | OT | 57.74 | 2.63 | 90.97 | 4.63 | 1.58 | 0.09 |
| Jozefiak et al., 2018 [36] | Poland | 0.1 | broiler | OT | 57.31 | 2.63 | 90.34 | 4.63 | 1.58 | 0.09 |
| Jozefiak et al., 2018 [36] | Poland | 0.1 | broiler | OT | 60.31 | 2.63 | 93.74 | 4.63 | 1.57 | 0.09 |
| Jozefiak et al., 2018 [36] | Poland | 0.1 | broiler | OT | 58.77 | 2.63 | 93.00 | 4.63 | 1.58 | 0.09 |
| Jozefiak et al., 2018 [36] | Poland | 0.1 | broiler | OT | 57.11 | 2.63 | 91.51 | 4.63 | 1.61 | 0.09 |
| Jozefiak et al., 2018 [36] | Poland | 0.0 | broiler | OT | 58.23 | 2.63 | 91.80 | 4.63 | 1.58 | 0.09 |
| Jozefiak et al., 2018 [36] | Poland | 0.2 | broiler | OT | 57.17 | 2.63 | 91.23 | 4.63 | 1.60 | 0.09 |
| Jozefiak et al., 2018 [36] | Poland | 0.2 | broiler | OT | 57.23 | 2.63 | 91.14 | 4.63 | 1.60 | 0.09 |
| Jozefiak et al., 2018 [36] | Poland | 0.2 | broiler | OT | 56.89 | 2.63 | 91.26 | 4.63 | 1.61 | 0.09 |
| Jozefiak et al., 2018 [36] | Poland | 0.2 | broiler | OT | 58.29 | 2.63 | 90.40 | 4.63 | 1.55 | 0.09 |
| Jozefiak et al., 2018 [36] | Poland | 0.0 | broiler | OT | 78.37 | 2.63 | 110.22 | 4.73 | 1.65 | 0.04 |
| Jozefiak et al., 2018 [36] | Poland | 0.1 | broiler | OT | 79.03 | 2.63 | 111.22 | 4.73 | 1.65 | 0.04 |
| Jozefiak et al., 2018 [36] | Poland | 0.1 | broiler | OT | 79.37 | 2.63 | 112.61 | 4.73 | 1.66 | 0.04 |
| Jozefiak et al., 2018 [36] | Poland | 0.2 | broiler | OT | 81.14 | 2.63 | 113.88 | 4.73 | 1.64 | 0.04 |
| Jozefiak et al., 2018 [36] | Poland | 0.0 | broiler | OT | 50.46 | 2.24 | 88.40 | 4.73 | 1.50 | 0.10 |
| Jozefiak et al., 2018 [36] | Poland | 0.2 | broiler | OT | 50.63 | 2.24 | 89.26 | 4.73 | 1.51 | 0.10 |
| Amao et al., 2010 [15] | Nigeria | 0.0 | layer | OT | -0.02 | 0.35 | 114.71 | 19.59 | ||
| Amao et al., 2010 [15] | Nigeria | 25.0 | layer | OT | 0.04 | 0.49 | 118.30 | 24.32 | ||
| Amao et al., 2010 [15] | Nigeria | 50.0 | layer | OT | -0.11 | 0.28 | 115.21 | 25.88 | ||
| Dutta et al., 2012 [31] | India | 0.0 | broiler | OT | 25.06 | 1.31 | 16.58 | 1.10 | 1.58 | 0.17 |
| Dutta et al., 2012 [31] | India | 25.0 | broiler | OT | 24.59 | 0.05 | 16.20 | 0.82 | 0.65 | 0.05 |
| Dutta et al., 2012 [31] | India | 50.0 | broiler | OT | 25.05 | 0.99 | 16.30 | 0.88 | 0.66 | 0.04 |
| Dutta et al., 2012 [31] | India | 75.0 | broiler | OT | 24.37 | 0.93 | 16.07 | 0.77 | 0.64 | 0.04 |
| Dutta et al., 2012 [31] | India | 100.0 | broiler | OT | 23.19 | 0.60 | 15.58 | 0.82 | 0.64 | 0.04 |
| Aigbodion et al., 2012 [13] | Nigeria | 0.0 | other | OT | 28.45 | 0.85 | ||||
| Aigbodion et al., 2012 [13] | Nigeria | 100.0 | other | OT | 14.64 | 0.45 | ||||
| Bovera et al., 2015 [23] | Italy | 0.0 | broiler | TM | 50.50 | 3.31 | 207.80 | 41.05 | 4.13 | 0.80 |
| Bovera et al., 2015 [23] | Italy | 100.0 | broiler | TM | 53.41 | 3.31 | 192.40 | 41.05 | 3.62 | 0.80 |
| Ballitoc et al., 2013 [17] | South Korea | 0.0 | broiler | TM | 38.46 | 9.81 | 23.14 | 14.29 | 2.10 | 0.40 |
| Ballitoc et al., 2013 [17] | South Korea | 0.5 | broiler | TM | 40.42 | 9.81 | 22.29 | 14.29 | 1.92 | 0.38 |
| Ballitoc et al., 2013 [17] | South Korea | 1.0 | broiler | TM | 57.15 | 9.81 | 24.57 | 14.29 | 1.90 | 0.26 |
| Ballitoc et al., 2013 [17] | South Korea | 2.0 | broiler | TM | 57.08 | 9.81 | 23.71 | 14.29 | 1.85 | 0.26 |
| Ballitoc et al., 2013 [17] | South Korea | 10.0 | broiler | TM | 56.54 | 9.81 | 21.29 | 14.29 | 1.72 | 0.26 |
| Hwangbo et al., 2009 [34] | South Korea | 0.0 | broiler | TM | 46.80 | 3.62 | 80.40 | 7.44 | 1.71 | 0.13 |
| Hwangbo et al., 2009 [34] | South Korea | 5.0 | broiler | TM | 50.71 | 3.62 | 79.77 | 7.44 | 1.57 | 0.13 |
| Hwangbo et al., 2009 [34] | South Korea | 10.0 | broiler | TM | 50.80 | 3.62 | 79.49 | 7.44 | 1.56 | 0.13 |
| Hwangbo et al., 2009 [34] | South Korea | 15.0 | broiler | TM | 51.00 | 3.62 | 79.17 | 7.44 | 1.55 | 0.13 |
| Hwangbo et al., 2009 [34] | South Korea | 20.0 | broiler | TM | 50.80 | 3.62 | 79.31 | 7.44 | 1.56 | 0.13 |
| Biasato et al., 2018 [20] | Italy | 0.0 | broiler | TM | 122.56 | 6.95 | 1.92 | 0.70 | ||
| Biasato et al., 2018 [20] | Italy | 5.0 | broiler | TM | 150.25 | 6.95 | 2.26 | 0.70 | ||
| Biasato et al., 2018 [20] | Italy | 10.0 | broiler | TM | 142.83 | 6.95 | 2.18 | 0.70 | ||
| Biasato et al., 2018 [20] | Italy | 15.0 | broiler | TM | 154.65 | 6.95 | 2.46 | 0.70 | ||
| Kieronczyk et al., 2018 [39] | Poland | 0.0 | broiler | TM | 55.04 | 3.29 | 80.89 | 1.99 | 1.47 | 0.06 |
| Kieronczyk et al., 2018 [39] | Poland | 100.0 | broiler | TM | 55.93 | 3.29 | 81.07 | 1.99 | 1.45 | 0.06 |
| Loponte et al., 2017 [40] | Italy | 0.0 | other | TM | 3.92 | 1.74 | 10.88 | 0.35 | 2.79 | 0.43 |
| Loponte et al., 2017 [40] | Italy | 25.0 | other | TM | 4.24 | 1.74 | 9.79 | 0.35 | 2.32 | 0.43 |
| Loponte et al., 2017 [40] | Italy | 50.0 | other | TM | 4.19 | 1.74 | 9.43 | 0.35 | 2.26 | 0.43 |
| Hussain et al., 2017 [33] | Pakistan | 0.0 | broiler | TM | 44.23 | 0.32 | 89.23 | 0.79 | 2.01 | 0.05 |
| Hussain et al., 2017 [33] | Pakistan | 1.0 | broiler | TM | 47.21 | 0.41 | 88.99 | 0.69 | 1.88 | 0.27 |
| Hussain et al., 2017 [33] | Pakistan | 2.0 | broiler | TM | 48.08 | 1.07 | 88.78 | 0.28 | 1.84 | 0.38 |
| Hussain et al., 2017 [33] | Pakistan | 3.0 | broiler | TM | 50.83 | 0.77 | 88.75 | 0.85 | 1.75 | 0.05 |
| Biasato et al., 2016 [18] | Italy | 0.0 | broiler | TM | 16.80 | 2.53 | 112.75 | 9.90 | 4.40 | 0.70 |
| Biasato et al., 2016 [18] | Italy | 100.0 | broiler | TM | 8.97 | 111.60 | 11.60 | 4.40 | 0.60 | |
| Ramos et al., 2002 [46] | Mexico | 0.0 | broiler | TM | 32.86 | 7.06 | 44.88 | 0.72 | 1.37 | 0.10 |
| Ramos et al., 2002 [46] | Mexico | 5.0 | broiler | TM | 32.79 | 7.05 | 45.79 | 0.73 | 1.39 | 0.10 |
| Ramos et al., 2002 [46] | Mexico | 10.0 | broiler | TM | 33.94 | 7.30 | 45.50 | 0.73 | 1.34 | 0.10 |
| Biasato et al., 2017 [19] | Italy | 0.0 | broiler | TM | 53.62 | 1.43 | 126.48 | 4.56 | 1.78 | 0.32 |
| Biasato et al., 2017 [19] | Italy | 5.0 | broiler | TM | 60.85 | 1.43 | 151.57 | 4.56 | 1.84 | 0.32 |
| Biasato et al., 2017 [19] | Italy | 10.0 | broiler | TM | 54.86 | 1.43 | 144.48 | 4.56 | 1.81 | 0.32 |
| Biasato et al., 2017 [19] | Italy | 15.0 | broiler | TM | 53.82 | 1.43 | 154.95 | 4.56 | 1.95 | 0.32 |
References
- FAO. Insects as Animal Feed. Available online: http://www.fao.org/3/i3253e/i3253e07.pdf (accessed on 20 April 2019).
- Moula, N.; Scippo, M.L.; Douny, C.; Degand, G.; Dawans, E.; Cabaraux, J.F.; Hornick, J.L.; Medigo, R.C.; Leroy, P.; Francis, F.; et al. Performances of local poultry breed fed black soldier fly larvae reared on horse manure. Anim. Nutr. 2017, 4, 73–78. [Google Scholar] [CrossRef] [PubMed]
- IPIFF. Insects as Food and Feed. Available online: http://ipiff.org/insects-eu-legislation_ (accessed on 20 April 2019).
- Tran, G.; Gnaedinger, C.; Mélin, C. Feedipedia, a Programme by INRA, CIRAD, AFZ and FAO. Available online: https://www.feedipedia.org/node/16401 (accessed on 9 December 2018).
- Ressing, M.; Blettner, M.; Klug, S.J. Systematic literature reviews and meta-analyses: Part 6 of a series on evaluation of scientific publications. Dtsch. Arztebl. Int. 2009, 106, 456–463. [Google Scholar] [PubMed]
- Walker, E.; Hernandez, A.V.; Kattan, M.W. Meta-analysis: Its strengths and limitations. Clev. Clin. J. Med. 2008, 75, 431–439. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Bax, L.; Ikeda, N.; Fukui, N.; Yaju, Y.; Tsuruta, H.; Moons, K.G.M. More than numbers: The power of graphs in meta-analysis. Am. J. Epidemiol. 2009, 169, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Baker, W.L.; White, C.M.; Cappelleri, J.C.; Kluger, J.; Coleman, C.I. Understanding heterogeneity in meta-analysis: The role of meta-regression. Int. J. Clin. Pract. 2009, 63, 1426–1434. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. A basic introduction to fixed effect and random effects models for meta-analysis. Res. Synth. Methods 2010, 1, 97–111. [Google Scholar] [CrossRef]
- López-López, J.A.; Marín-Martínez, F.; Sánchez-Meca, J.; Van den Noortgate, W.; Viechtbauer, W. Estimation of the predictive power of the model in mixed-effects meta-regression: A simulation study. Br. J. Math. Stat. Psychol. 2014, 67, 30–48. [Google Scholar] [CrossRef]
- Röver, C. Bayesian random-effects meta-analysis using the bayesmeta R package. J. Stat. Software 2018. Available online: https://arxiv.org/pdf/1711.08683.pdf (accessed on 9 November 2018).
- Aigbodion, F.I.; Egbon, I.N. A preliminary study on the entomophagous response of Gallus gallus domesticus (Galliformes: Phasianidae) to adult Periplaneta Americana (Blattaria: Blattidae). Int. J. Trop. Ins. Sci. 2012, 32, 123–125. [Google Scholar] [CrossRef]
- Al-Qazzaz, M.F.A.; Ismail, D.; Akit, H.; Idris, L.H. Effect of using insect larvae meal as a complete protein source on quality and productivity characteristics of laying hens. R. Bras. Zootec. 2016, 45, 518–523. [Google Scholar] [CrossRef]
- Amao, O.A.; Oladunjoye, I.O.; Togun, V.A.; Olubajo, K.; Oyaniyi, O. Effect of Westwood (Cirina forda) larva meal on the laying performance and egg characteristics of laying hen in a tropical environment. Int. J. Poultry Sci. 2010, 9, 450–454. [Google Scholar] [CrossRef]
- Awoniyi, T.A.M.; Aletor, V.A.; Aina, J.M. Performance of broiler—Chickens fed on maggot meal in place of fishmeal. Int. J. Poultry Sci. 2003, 2, 271–274. [Google Scholar]
- Ballitoc, D.A.; Sun, S. Ground yellow mealworms (Tenebrio molitor L.) feed supplementation improves growth performance and carcass yield characteristics in broilers. Open Sci. Reposit. Agric. 2013. Available online: http://www.open-science-repository.com/agriculture-24050425.html (accessed on 9 December 2018).
- Biasato, I.; De Marco, M.; Rotolo, L.; Renna, M.; Lussiana, C.; Dabbou, S.; Capucchio, M.T.; Biasibetti, E.; Gai, F.; Pozzo, L.; et al. Effects of dietary Tenebrio molitor meal inclusion in free-range chickens. J. Anim. Physiol. Anim. Nutr. 2016, 100, 1104–1112. [Google Scholar] [CrossRef]
- Biasato, I.; Gasco, L.; De Marco, M.; Renna, M.; Rotolo, L.; Dabbou, S.; Capucchio, M.T.; Biasibetti, E.; Tarantola, M.; Binachi, C.; et al. Effect of yellow mealworm larvae (Tenebrio molitor) inclusion in diets for female broiler chickens: Implications for animal health and gut histology. Anim. Feed Sci. Technol. 2017, 234, 253–263. [Google Scholar] [CrossRef]
- Biasato, I.; Gasco, L.; De Marco, M.; Renna, M.; Rotolo, L.; Dabbou, S.; Capucchio, M.T.; Biasibetti, E.; Tarantola, M.; Sterpone, L.; et al. Yellow mealworm larvae (Tenebrio molitor) inclusion in diets for male broiler chickens: Effects on growth performance, gut morphology, and histological findings. Poult. Sci. 2018, 97, 540–548. [Google Scholar] [CrossRef]
- Borelli, L.; Coretti, L.; Dipineto, L.; Bovera, F.; Menna, F.; Chiariotti, L.; Nizza, A.; Lembo, F.; Fioretti, A. Insect-based diet, a promising nutritional source, modulates gut microbiota composition and SCFAs production in laying hens. Sci. Rep. 2017, 24, 16269–16280. [Google Scholar] [CrossRef]
- Bovera, F.; Loponte, R.; Marono, S.; Piccolo, G.; Parisi, G.; Iaconisi, V.; Gasco, L.; Nizza, A. Use of Tenebrio molitor larvae meal as protein source in broiler diet: Effect on growth performance, nutrient digestibility, and carcass and meat traits. J. Anim. Sci. 2016, 94, 639–647. [Google Scholar] [CrossRef]
- Bovera, F.; Piccolo, G.; Gasco, L.; Marono, S.; Loponte, R.; Vassalotti, G.; Mastellone, V.; Lombardi, P.; Attia, Y.A.; Nizza, A. Yellow mealworm larvae (Tenebrio molitor, L.) as a possible alternative to soybean meal in broiler diets. Br. Poult. Sci. 2015, 56, 569–575. [Google Scholar] [CrossRef]
- Brah, N.; Houndonougbo, F.M.; Issa, S. Grasshopper meal (Ornithacris cavroisi) in broiler diets in Niger: Bioeconomic performance. Int. J. Poult. Sci. 2018, 17, 126–133. [Google Scholar] [CrossRef]
- Brah, N.; Salissou, I.; Houndonougbo, F. Effect of grasshopper meal on laying hens’ performance and egg quality characteristics. Indian J. Anim. Sci. 2017, 87, 1005–1010. [Google Scholar]
- Cockcroft, B.L. An Evaluation of Defatted Black Soldier Fly (Hermetia illucens) Larvae as a Protein Source for Broiler Chicken Diets. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, 2018. [Google Scholar]
- Cullere, M.; Tasoniero, G.; Giaccone, V.; Miotti-Scapin, R.; Claeys, E.; De Smet, S.; Dalle Zotte, A. Black soldier fly as dietary protein source for broiler quails: Apparent digestibility, excreta microbial load, feed choice, performance, carcass and meat traits. Animal 2016, 10, 1923–1930. [Google Scholar] [CrossRef]
- Dabbou, F.G.; Biasato, I.; Capucchio, M.T.; Biasibetti, E.; Dezzutto, D.; Meneguz, M.; Plachà, I.; Gasco, L.; Schiavone, A. Black soldier fly defatted meal as a dietary protein source for broiler chickens: Effects on growth performance, blood traits, gut morphology and histological features. J. Anim. Sci. Biotechnol. 2018, 9, 49. [Google Scholar] [CrossRef]
- Das, M.; Mandal, S.K. Axya hyla hyla (Orthoptera: Acridicae) as an alternative protein source for Japanese quail. Int. Sch. Res. Not. 2014, 3–4. [Google Scholar] [CrossRef]
- Dordević, M.; Radenković-Damnjanović, B.; Vučinić, M.; Baltić, M.; Teodorović, R.; Janković, L.; Vukašinović, M.; Rajković, M. Effects of substitution of fish meal with fresh and dehydrated larvae of the house fly (Musca domestica L) on productive performance and health of broilers. Acta Vet. 2008, 58, 357–368. [Google Scholar]
- Dutta, A.; Dutta, S.; Kumari, S. Growth of poultry chicks fed on formulated feed containing silk worm pupae meal as protein supplement and commercial diet. Online J. Anim. Feed Res. 2012, 2, 303–307. [Google Scholar]
- Gaffigan, M. Is Insect Protein a Sustainable Alternative to Soy and Fishmeal in Poultry Feed? Bachelor’s Thesis, University of Colorado, Boulder, CO, USA, 2017. [Google Scholar]
- Hussain, I.; Khan, S.; Sultan, A.; Chand, N.; Khan, R.; Alam, W.; Ahmad, N. Meal worm (Tenebrio molitor) as potential alternative source of protein supplementation in broiler. Int. J. Biosci. 2017, 10, 255–262. [Google Scholar]
- Hwangbo, J.E.; Hong, C. Utilization of house fly-maggots, a feed supplement in the production of broiler chickens. J. Environ. Biol. 2009, 30, 609–614. [Google Scholar]
- Ijaiya, A.T.; Eko, E.O. Effect of replacing dietary fishmeal with silkworm (Anaphe infracta) caterpillar meal on growth, digestibility and economics of production of starter broiler chickens. Pak. J. Nutr. 2009, 8, 845–849. [Google Scholar]
- Joszefiak, A.; Kieronczyk, B.; Rawski, M.; Mazurkiewicz, J.; Benzertiha, A.; Gobbi, P.; Nogales-Mérisa, S.; Swiatkiewicz, S.; Joszefiak, D. Full-fat insect meals as feed additive—The effect on broiler chicken growth performance and gastrointestinal tract microbiota. J. Anim. Feed Sci. 2018, 27, 131–139. [Google Scholar] [CrossRef]
- Khan, S.; Khan, R.U.; Sultan, A.; Khan, M.; Hayat, S.U.; Shahid, M.S. Evaluating the suitability of maggot meal as a partial substitute of soya bean on the productive traits, digestibility indices and organoleptic properties of broiler meat. J. Anim. Physiol. Anim. Nutr. 2016, 100, 649–656. [Google Scholar] [CrossRef]
- Khan, M.; Chand, N.; Khan, S.; Khan, R.U.; Sultan, A. Utilizing the house fly (Musca Domestica) larva as an alternative to soybean meal in broiler ration during the starter phase. Braz. J. Poult. Sci. 2018, 20, 9–14. [Google Scholar] [CrossRef]
- Kieronezyk, B.; Rawski, M.; J Jozefiak, A.; Mazurkiewicz, J.; Swiatkiewicz, S.; Siwek, M.; Bednarczyk, M.; Szumacher-Strabel, M.; Cieslak, A.; Benzertiha, A.; et al. Effects of replacing soybean oil with selected insect fats on broilers. Anim. Feed Sci. Technol. 2018, 240, 170–183. [Google Scholar] [CrossRef]
- Loponte, R.; Nizza, S.; Bovera, F.; De Riu, N.; Fliegerova, K.; Lombardi, P.; Vassalotti, G.; Mastellone, V.; Nizza, A.; Moniello, G. Growth performance, blood profiles and carcass traits of Barbary partridge (Alectoris barbara) fed two different insect larvae meals (Tenebrio molitor and Hermetia illucens). Res. Vet. Sci. 2017, 115, 183–188. [Google Scholar] [CrossRef]
- Moula, N.; Hornick, J.-L.; Cabaraux, J.-F.; Korsak, N.; Daube, G.; Dawans, E.; Antoine, N.; Taminiau, B.; Detilleux, J. Effects of dietary black soldier fly larvae on performance of broilers mediated or not through changes in microbiota. JIFF 2017, 4, 31–42. [Google Scholar] [CrossRef]
- Mwaniki, Z.; Neijat, M.; Kiarie, E. Egg production and quality responses of adding up to 7.5% defatted black soldier fly larvae meal in a corn–soybean meal diet fed to Shaver WhiteLeghorns from wk 19 to 27 of age. Poult. Sci. 2018, 97, 1–7. [Google Scholar] [CrossRef]
- Nobo, G.; Moreki, J.C.; Nsoso, S.J. Feed intake, body weight, average daily gain, feed conversion ratio and carcass characteristics of Helmeted Guinea fowl fed varying levels of Phane meal (Imbrasia belina) as replacement of fishmeal under intensive system. Int. J. Poult. Sci. 2012, 11, 378–384. [Google Scholar] [CrossRef]
- Okah, U.; Onwujiariri, E.B. Performance of finisher broiler chickens fed maggot meal as a replacement for fish meal. J. Agric. Technol. 2012, 8, 471–477. [Google Scholar]
- Onsongo, V.O.; Osuga, I.M.; Gachuiri, C.K.; Wachira, A.M.; Miano, D.M.; Tanga, C.M.; Ekesi, S.; Nakimbugwe, D.; Fiaboe, K.K.M. Insects for income generation through animal feed: Effect of dietary replacement of soybean and fish meal with black soldier fly meal on broiler growth and economic performance. J. Econ. Entomol. 2018, 111, 1966–1973. [Google Scholar] [CrossRef]
- Ramos-Elorduy, J.E.A.; Gonzalez, E.A. Use of Tenebrio molitor (Coleoptera: Tenebrionidae) to recycle organic wastes and as feed for broiler chickens. J. Econ. Entomol. 2002, 95, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, A.; Cullere, M.; De Marco, M.; Meneguez, M.; Biasato, I.; Bergagna, S.; Dezzutto, D.; Gai, F.; Dabbou, S.; Gasco, L.; et al. Partial or total replacement of soybean oil by black soldier fly larvae (Hermetia illucens L.) fat in broiler diets: Effect on growth performances, feed-choice, blood traits, carcass characteristics and meat quality. Ital. J. Anim. Sci. 2017, 16, 93–100. [Google Scholar] [CrossRef]
- Téguia, A.; Mpoame, M. The production performance of broiler birds as affected by the replacement of fish meal by maggot meal in the starter and finisher diets. Tropicultura 2002, 20, 187–192. [Google Scholar]
- Velten, S.; Neumann, C.; Bleyer, M.; Gruber-Dujardin, E.; Hanuszewska, M.; Przybylska-Gornowicz, B.; Liebert, F. Effects of 50 percent substitution of soybean meal by alternative proteins from Hermetia illucens or Spirulina platensis in meat-type chicken diets with graded amino acid supply. Open J. Anim. Sci. 2018, 8, 119–136. [Google Scholar] [CrossRef]
- Wallace, P.; Nyameasem, J.; Adu-Aboagye, G.; Affedzie-Obresi, S.; Nkegbe, E.; Karbo, N.; Murray, F.; Leschen, W.; Maquart, P. Impact of black soldier fly larval meal on growth performance, apparent digestibility, haematological and blood chemistry indices of guinea fowl starter kept under tropical conditions. Trop. Anim. Health Prod. 2007, 49, 1163–1169. [Google Scholar] [CrossRef]
- Wang, D.; Zhai, S.W.; Zhang, C.X.; Bai, Y.Y.; An, S.H.; Xu, Y.N. Evaluation on nutritional value of field crickets as a poultry feedstuff. Asian Aust. J. Anim. Sci. 2005, 18, 667–670. [Google Scholar] [CrossRef]
- Hossain, S.; Blair, R. Chitin utilization by broilers and its effects on body composition and blood metabolites. Br. Poult. Sci. 2007, 48, 33–38. [Google Scholar] [CrossRef]
- Tabata, E.; Kashimura, A.; Kikuchi, A.; Masuda, H.; Miyahara, R.; Hiruma, Y.; Wakita, S.; Ohno, M.; Sakaguchi, M.; Sugahara, Y.; et al. Chitin digestibility is dependent on feeding behaviors, which determine acidic chitinase mRNA levels in mammalian and poultry stomachs. Sci. Rep. 2018, 8, 1461–1472. [Google Scholar] [CrossRef]
- Moniello, G.; Ariano, A.; Panettieri, V.; Tulli, F.; Olivotto, I.; Messina, M.; Randazzo, B.; Severino, L.; Piccolo, G.; Musco, N.; et al. Intestinal morphometry, enzymatic and microbial activity in laying hens fed different levels of a Hermetia illucens larvae meal and toxic elements content of the insect meal and diets. Animals 2019, 9, 86–99. [Google Scholar] [CrossRef]
- Biasato, I.; Ferrocino, I.; Biasibetti, E.; Grego, E.; Dabbou, S.; Sereno, A.; Gai, F.; Gasco, L.; Schiavone, A. Modulation of intestinal microbiota, morphology and mucin composition by dietary insect meal inclusion in free-range chickens. BMV Vet. Res. 2018, 14, 383–398. [Google Scholar] [CrossRef]
- Laudadio, V.; Passantino, L.; Perillo, A.; Loprestu, G.; Passantino, A.; Khan, R.U.; Tufarelli, V. Productive performance and histological features of intestinal mucosa of broiler chickens fed different dietary protein levels. Poult. Sci. 2012, 91, 265–270. [Google Scholar] [CrossRef]
- Kers, J.G.; Velkers, F.C.; Fischer, E.A.J.; Hermes, G.D.A.; Stegeman, J.A.; Smidt, H. Host and environmental factors affecting the intestinal microbiota in chickens. Front. Mircrobiol. 2018, 9, 235. [Google Scholar] [CrossRef]
- Nir, I.; Melcion, J.P.; Picard, M. Effect of particle size of sorghum grains on feed intake and performance of young broilers. Poult. Sci. 1990, 69, 2177–2184. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Loannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analysis of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009. Available online: http://www.prisma-statement.org/documents/PRISMA%20EandE%202009.pdf (accessed on 29 January 2019). [CrossRef]
| Differences between Poultry Fed a Diet With vs. without Insects in Means of | Pooled Estimate | Heterogeneity (I2) |
|---|---|---|
| Average daily gain | −0.10 (−0.83 to 0.63) | 99.2 |
| Feed intake | 0.14 (−0.18 to 0.41) | 39.9 |
| Feed conversion ratio | −0.18 (−0.29 to −0.07) | 89.6 |
| Effects | DIFF_ADG (g) | DIFF_FI (g) | DIFF_FCR |
|---|---|---|---|
| Overall mean | −4.56 (−9.50 to 0.38) | 3.77 (−0.83 to 8.42) | 0.23 (−0.40 to 0.86) |
| Insects species | |||
| Black soldier fly larvae (reference) | 0 | 0 | 0 |
| Maggots | 3.13 (−1.05 to 7.31) | −6.56 * (−10.87 to −2.26) | −0.02 (−0.48 to 0.45) |
| Mealworms | 1.31 (−0.79 to 3.42) | −1.12 (−2.11 to −0.13) | 0.12 (−0.18 to 0.42) |
| Grasshoppers | −4.32 * (−6.83 to −1.81) | 3.83 * (1.43 to 6.24) | −0.21 (−0.67 to 0.24) |
| Other insects | −0.77 (−2.69 to 1.15) | −1.49 (−3.45 to 0.48) | −0.10 (−0.34 to 0.13) |
| Animal species | |||
| Broilers (reference) | 0 | 0 | 0 |
| Layers | 2.31 (0.04 to 4.57) | −1.25 (−5.66 to 3.15) | −0.05 (−0.88 to 0.17) |
| Other poultry | 1.42 (−0.31 to 3.15) | −4.41 (−6.60 to −2.21) | −0.12 (−0.47 to 0.22) |
| Inclusion rate | −0.05 * (−0.08 to −0.03) | −0.005 (−0.02 to 0.01) | −0.003 (−0.006 to −0.001) |
| Year of publication | 0.29 (0.04 to 0.54) | 0.044 (−0.19 to 0.28) | −0.007 (−0.039 to 0.024) |
| Continent | |||
| Europe (reference) | 0 | 0 | 0 |
| Africa | 0.58 (−1.15 to 2.31) | 1.44 (−0.91 to 3.80) | 0.14 (−0.17 to 0.45) |
| Asia and Oceania | 4.46 * (2.22 to 6.70) | −3.41 * (−5.70 to −1.13) | −0.50 * (−0.78 to −0.22) |
| America | −0.05 (−3.88 to 3.78) | −1.87 (−5.96 to 2.22) | −0.22 (−0.73 to 0.29) |
| Amount of heterogeneity accounted for (R2, %) | 52.82 | 76.93 | 47.37 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moula, N.; Detilleux, J. A Meta-Analysis of the Effects of Insects in Feed on Poultry Growth Performances. Animals 2019, 9, 201. https://doi.org/10.3390/ani9050201
Moula N, Detilleux J. A Meta-Analysis of the Effects of Insects in Feed on Poultry Growth Performances. Animals. 2019; 9(5):201. https://doi.org/10.3390/ani9050201
Chicago/Turabian StyleMoula, Nassim, and Johann Detilleux. 2019. "A Meta-Analysis of the Effects of Insects in Feed on Poultry Growth Performances" Animals 9, no. 5: 201. https://doi.org/10.3390/ani9050201
APA StyleMoula, N., & Detilleux, J. (2019). A Meta-Analysis of the Effects of Insects in Feed on Poultry Growth Performances. Animals, 9(5), 201. https://doi.org/10.3390/ani9050201




