Gut Microbiota and Mucin Composition in Female Broiler Chickens Fed Diets including Yellow Mealworm (Tenebrio molitor, L.)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds and Experimental Design
2.2. Intestinal Sampling
2.3. DNA Extraction and 16S rRNA Amplicon Target Sequencing
2.4. Histochemical Staining
2.5. Mucin Staining Intensity Evaluation
2.6. Bioinformatics and Statistical Analysis
3. Results
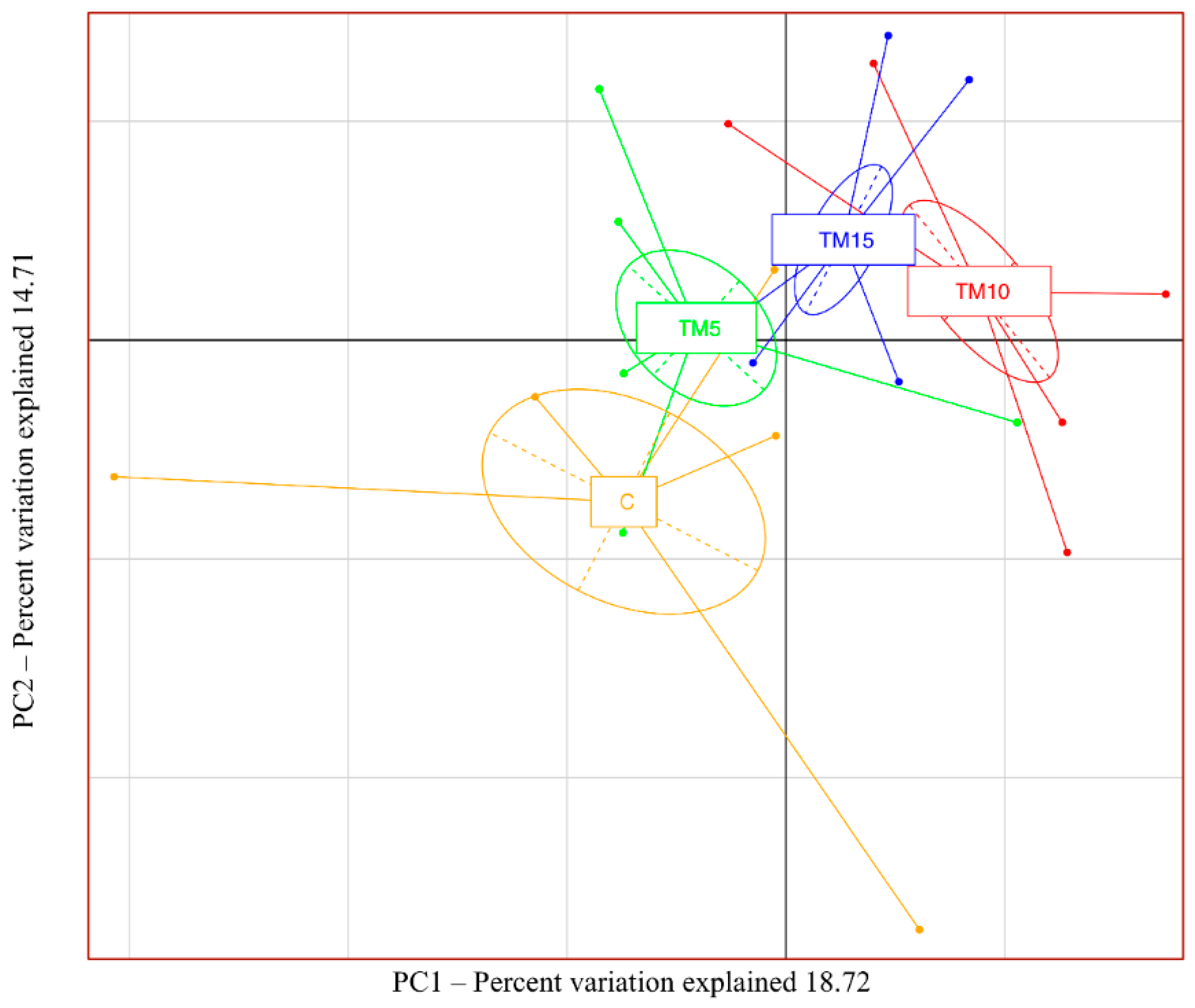
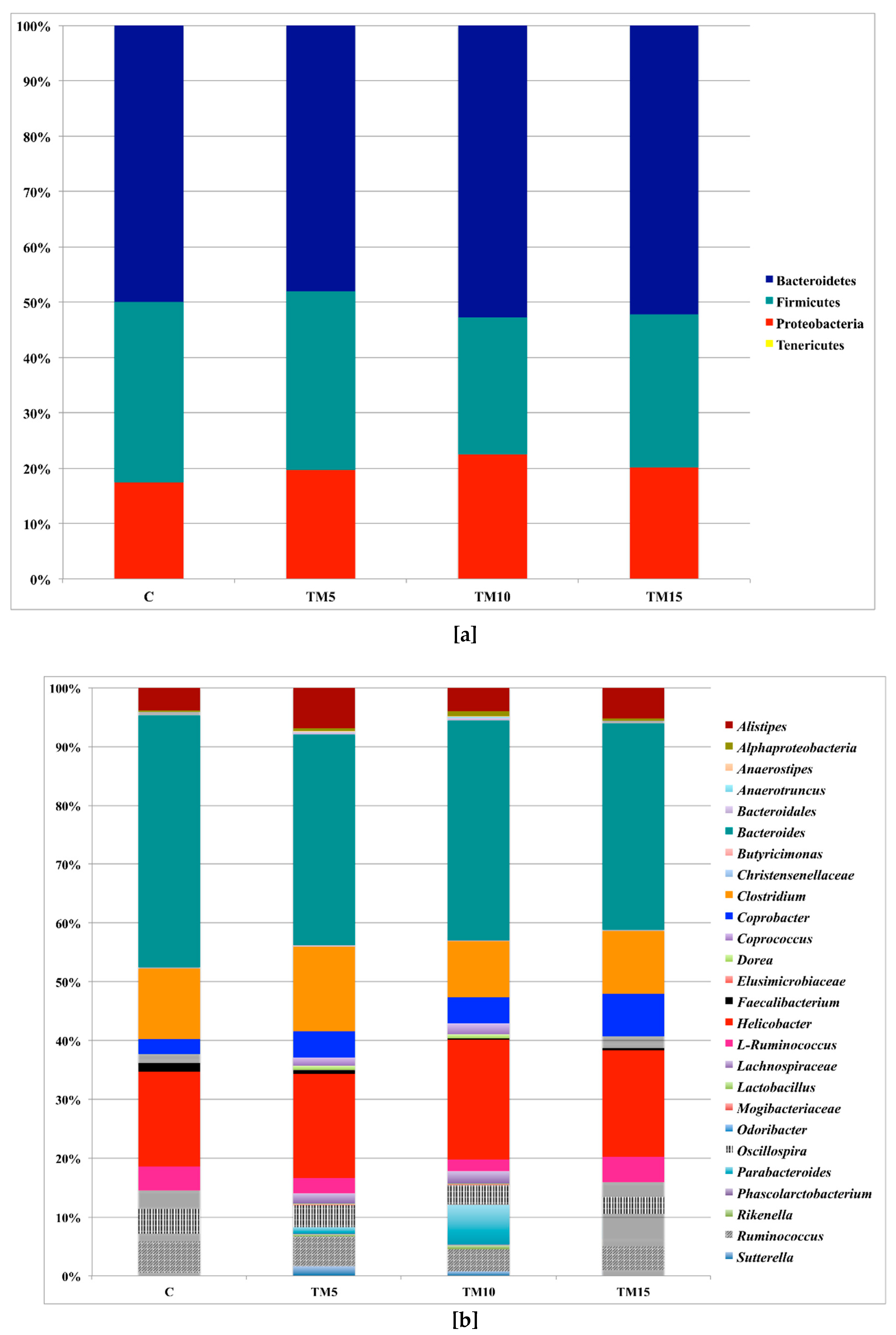
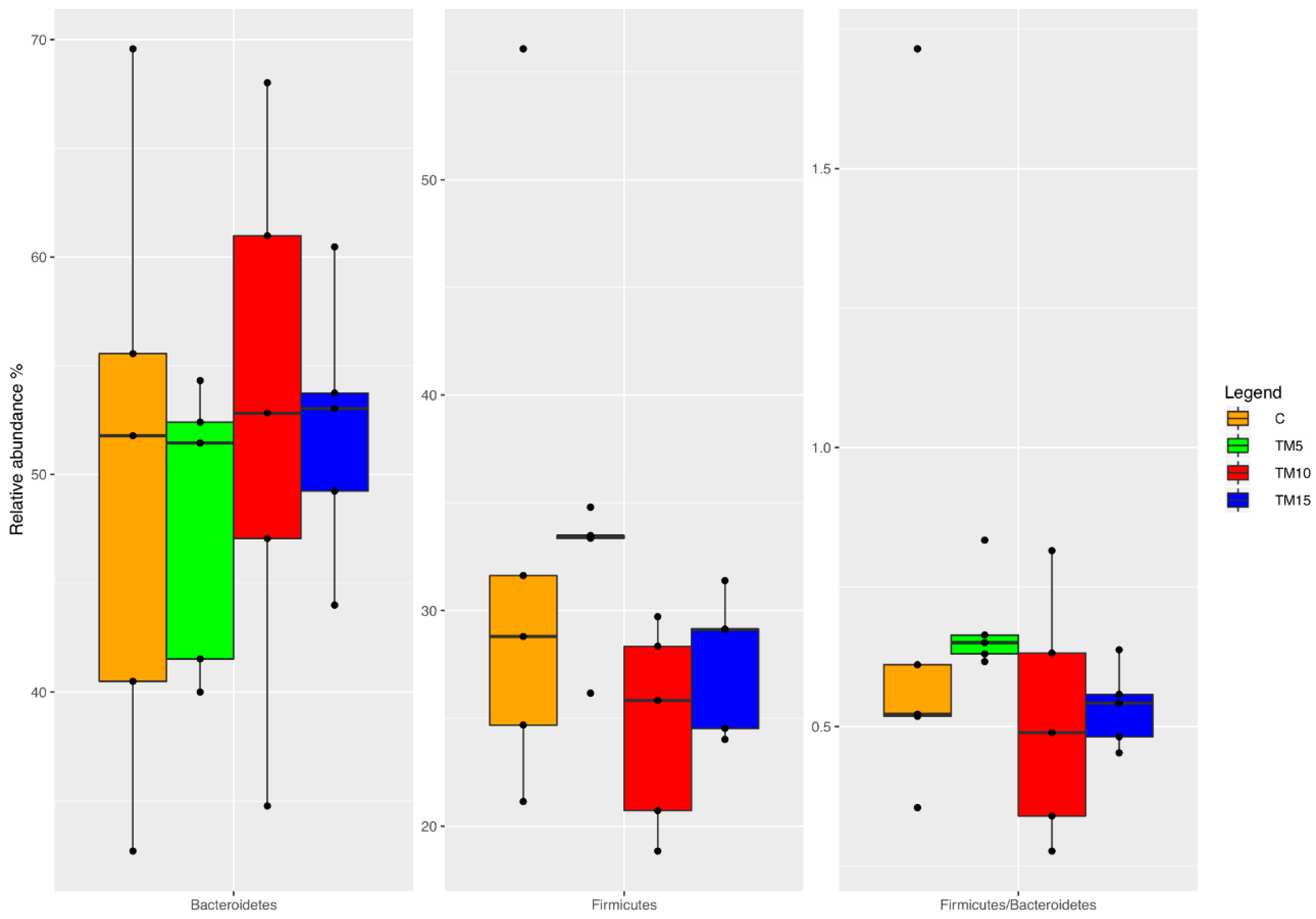
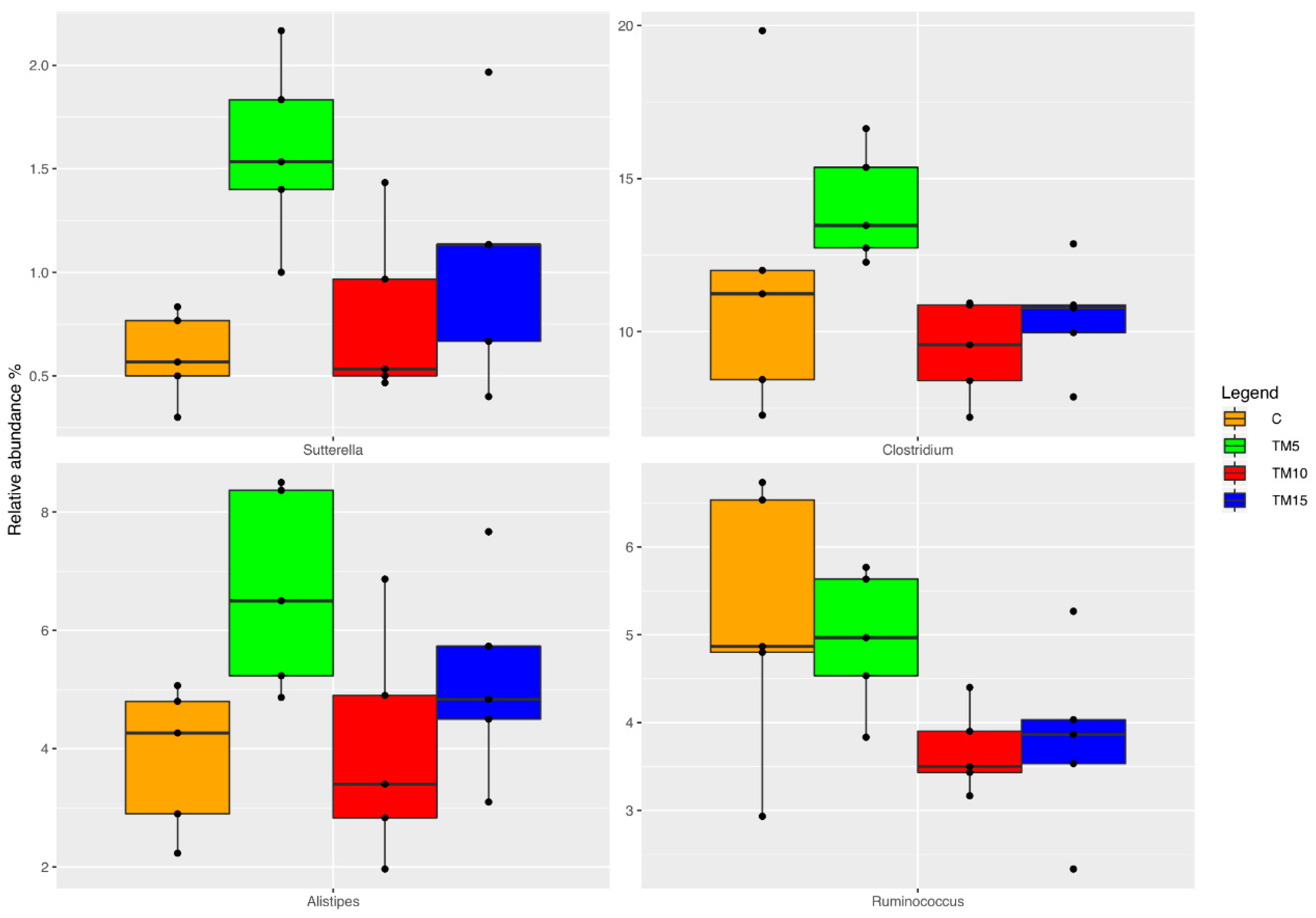
3.1. Cecal Microbiota Characterization
3.2. Intestinal Mucin Composition
4. Discussion
4.1. Cecal Microbiota Characterization
4.2. Intestinal Mucin Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Celi, P.; Cowieson, A.J.; Fru-Nji, F.; Steinert, R.E.; Kluenter, A.-M.; Verlha, V. Gastrointestinal functionality in animal nutrition and health: New opportunities for sustainable animal production. Anim. Feed Sci. Technol. 2017, 234, 88–100. [Google Scholar] [CrossRef]
- Conway, P.L. Function and regulation of the gastrointestinal microbiota of the pig. In Proceedings of the VI-th International Symposium on Digestive Physiology in Pigs, Bad Doberan, Germany, 4–6 October 1994; pp. 231–240. [Google Scholar]
- Forstner, J.F.; Oliver, M.G.; Sylvester, F.A. Production, structure and biologic relevance of gastrointestinal mucins. In Infections of the Gastrointestinal Tract; Guerrant, L.R., Ed.; Raven Press: New York, NY, USA, 1995; pp. 71–88. [Google Scholar]
- Forstner, G.; Forstner, J.F. Gastrointestinal mucus. In Physiology of the Gastrointestinal Tract; Johnson, L.R., Ed.; Raven Press: New York, NY, USA, 1994; pp. 1255–1284. [Google Scholar]
- Robertson, A.M.; Wright, D.P. Bacterial glycosulphatases and sulphomucin degradation. Can. J. Gastroenterol. 1997, 11, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Deplancke, B.; Gaskins, H.R. Microbial modulation of innate defense: Goblet cells and the intestinal mucus layer. Am. J. Clin. Nutr 2001, 73, 1131S–1141S. [Google Scholar] [CrossRef]
- Gasco, L.; Finke, M.; van Huis, A. Can diets containing insects promote animal health? J. Insects Food Feed 2018, 4, 1–4. [Google Scholar] [CrossRef]
- Biasato, I.; De Marco, M.; Rotolo, L.; Renna, M.; Dabbou, S.; Capucchio, M.T.; Biasibetti, E.; Tarantola, M.; Costa, P.; Gai, F.; et al. Effects of dietary Tenebrio molitor meal inclusion in free-range chickens. J. Anim. Physiol. Anim. Nutr. 2016, 100, 1104–1112. [Google Scholar] [CrossRef]
- Biasato, I.; Ferrocino, I.; Biasibetti, E.; Grego, E.; Dabbou, S.; Sereno, A.; Gai, F.; Gasco, L.; Schiavone, A.; Cocolin, L.; et al. Modulation of intestinal microbiota, morphology and mucin composition by dietary insect meal inclusion in free-range chickens. BMC Vet. Res. 2018, 14, 383. [Google Scholar] [CrossRef]
- Biasato, I.; Gasco, L.; De Marco, M.; Renna, M.; Rotolo, L.; Dabbou, S.; Capucchio, M.T.; Biasibetti, E.; Tarantola, M.; Bianchi, C.; et al. Effects of yellow mealworm larvae (Tenebrio molitor) inclusion in diets for female broiler chickens: Implications for animal health and gut histology. Anim. Feed Sci. Technol. 2017, 234, 253–263. [Google Scholar] [CrossRef]
- Biasato, I.; Gasco, L.; De Marco, M.; Renna, M.; Rotolo, L.; Dabbou, S.; Capucchio, M.T.; Biasibetti, E.; Tarantola, M.; Sterpone, L.; et al. Yellow mealworm larvae (Tenebrio molitor) inclusion in diets for male broiler chickens: Effects on growth performance, gut morphology and histological findings. Poult. Sci. 2018, 97, 540–548. [Google Scholar] [CrossRef]
- Dabbou, S.; Gai, F.; Biasato, I.; Capucchio, M.T.; Biasibetti, E.; Dezzutto, D.; Meneguz, M.; Plachà, I.; Gasco, L.; Schiavone, A. Black soldier fly defatted meal as a dietary protein source for broiler chickens: Effects on growth performance, blood traits gut morphology and histological features. J. Anim. Sci. Biotechnol. 2018, 9, 49. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, L.; Coretti, L.; Dipineto, L.; Bovera, F.; Menna, F.; Chiariotti, L.; Nizza, A.; Lembo, F.; Fioretti, A. Insect-based diet, a promising nutritional source, modulates gut microbiota composition and SCFAs production in laying hens. Sci. Rep. 2017, 7, 16269. [Google Scholar] [CrossRef]
- De Marco, M.; Martínez, S.; Hernandez, F.; Madrid, J.; Gai, F.; Rotolo, L.; Belforti, M.; Bergero, D.; Katz, H.; Dabbou, S.; et al. Nutritional value of two insect larval meals (Tenebrio molitor and Hermetia illucens) for broiler chickens: Apparent nutrient digestibility, apparent ileal amino acid digestibility and apparent metabolizable energy. Anim. Feed Sci. Technol. 2015, 209, 211–218. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Ferrocino, I.; Bellio, A.; Romano, A.; Macori, G.; Rantsiou, K.; Decastelli, L.; Cocolin, L. RNA-Based Amplicon Sequencing Reveals Microbiota Development during Ripening of Artisanal versus Industrial Lard d’Arnad. Appl. Environ. Microbiol. 2017, 83, e00983–e01017. [Google Scholar] [CrossRef] [PubMed]
- Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Tsirtsikos, P.; Fegeros, K.; Kominakis, A.; Balaskas, C.; Mountzouris, KC. Modulation of intestinal mucin composition and mucosal morphology by dietary phytogenic inclusion level in broilers. Animal 2012, 6, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Tsirtsikos, P.; Fegeros, K.; Kominakis, A.; Balaskas, C.; Mountzouris, KC. Dietary probiotic inclusion level modulates intestinal mucin composition and mucosal morphology in broilers. Poult. Sci. 2012, 91, 1860–1868. [Google Scholar] [CrossRef]
- Oakley, B.B.; Lillehoj, H.S.; Kogut, M.H.; Kim, W.K.; Maurer, J.J.; Pedroso, A.; Lee, M.D.; Collett, S.R.; Johnson, T.J.; Cox, N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014, 360, 100–112. [Google Scholar] [CrossRef]
- Wise, M.G.; Siragusa, G.R. Quantitative analysis of the intestinal bacterial community in one-to three-week-old commercially reared broiler chickens fed conventional or antibiotic-free vegetable-based diets. J. Appl. Microbiol. 2007, 102, 1138–1149. [Google Scholar] [CrossRef]
- Tillman, G.E.; Haas, G.J.; Wise, M.G.; Oakley, B.; Smith, M.A.; Siragusa, G.R. Chicken intestine microbiota following the administration of lupulone, a hop-based antimicrobial. FEMS Microbiol. Ecol. 2011, 77, 395–403. [Google Scholar] [CrossRef]
- Wei, S.; Morrison, M.; Yu, Z. Bacterial census of poultry intestinal microbiome. Poult. Sci. 2013, 92, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Vasaï, F.; Brugirard Ricaud, K.; Bernadet, M.D.; Cauquil, L.; Bouchez, O.; Combes, S.; Davail, S. Overfeeding and genetics affect the composition of intestinal microbiota in Anas platyrhynchos [Pekin] Cairina moschata [Muscovy] ducks. FEMS. Microbiol. Ecol. 2014, 87, 204–216. [Google Scholar] [CrossRef]
- Xu, Q.; Yuan, X.; Gu, T.; Li, Y.; Dai, W.; Shen, X.; Song, Y.; Zhang, Y.; Zhao, W.; Chang, G.; et al. Comparative characterization of bacterial communities in geese fed all-grass or high-grain diets. PLoS ONE 2017, 12, e0185590. [Google Scholar] [CrossRef]
- Scupham, A.J.; Patton, T.G.; Bent, E.; Bayles, D.O. Comparison of the cecal microbiota of domestic and wild turkeys. Microb. Ecol. 2008, 56, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, J.; Zhong, H.; Li, N.; Xu, H.; Zhu, Q.; Liu, Y. Effect of probiotics on the meat flavour and gut microbiota of chicken. Sci. Rep. 2017, 7, 6400. [Google Scholar] [CrossRef] [PubMed]
- Lan, P.T.; Sakamoto, M.; Sakata, S.; Benno, Y. Bacteroides barnesiae sp.nov. Bacteroides salanitronis sp. Nov. and Bacteroides gallinarum sp. Nov. isolated from chicken cecum. Inter. J. Syst. Evol. Microbiol. 2006, 56, 2853–2859. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Si, W.; Forster, R.J.; Huang, R.; Yu, H.; Yin, Y.; Yang, C.; Han, Y. 16S rRNA gene-based analysis of mucosa-associated bacterial community and phylogeny in the chicken gastrointestinal tracts: From crops to ceca. FEMS Microbiol. Ecol. 2007, 59, 147–157. [Google Scholar] [CrossRef]
- Danzeisen, J.L.; Kim, H.B.; Isaacson, R.E.; Tu, Z.J.; Johnson, T.J. Modulations of the chicken cecal microbiome and metagenome in response to anticoccidial and growth promoter treatment. PLoS ONE 2011, 6, e27949. [Google Scholar] [CrossRef]
- Stanley, D.; Denman, S.E.; Hughes, R.J.; Geier, M.S.; Crowley, T.M.; Chen, H.; Haring, V.R.; Moore, R.J. Intestinal microbiota associated with differential feed conversion efficiency in chickens. Appl. Microbiol. Biotechnol. 2012, 96, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.C.; Bessegatto, J.A.; Alfieri, A.A.; Weese, J.S.; Filho, J.A.; Oba, A. Different antibiotic growth promoters induce specific changes in the cecal microbiota membership of broiler chicken. PLoS ONE 2017, 12, e0171642. [Google Scholar] [CrossRef]
- Shkoporov, A.N.; Khokhlova, E.V.; Chaplin, A.V.; Kafarskaia, L.I.; Nikolin, A.A.; Polyakov, V.Y.; Shcherbakova, V.A.; Chernaia, Z.A.; Efimov, B.A. Coprobacter fastidiosus gen. nov., sp. nov., a novel member of the family Porphyromonadaceae isolated from infant faeces. Int. J. Syst. Evol. Microbiol. 2013, 63, 4181–4188. [Google Scholar] [CrossRef]
- Sergeant, M.J.; Constantinidou, C.; Cogan, T.A.; Bedford, M.R.; Penn, C.W.; Pallen, M.J. Extensive microbial and functional diversity within the chicken cecal microbiome. PLoS ONE 2014, 9, e91941. [Google Scholar] [CrossRef]
- Stanley, J.; Linton, D.; Burnens, A.P.; Dewhirst, F.E.; On, S.L.; Porter, A.; Owen, R.J.; Costas, M. Helicobacter pullorum sp. nov-genotype and phenotype of a new species isolated from poultry and from human patients with gastroenteritis. Microbiology 1994, 140, 3441–3449. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Zhang, W.; Wang, L.; Hou, R.; Zhang, M.; Fei, L.; Zhang, X.; Huang, H.; Bridgewater, L.C.; Jiang, Y.; et al. The bamboo-eating giant panda harbors a carnivore-like gut microbiota, with excessive seasonal variations. mBio 2015, 6, e00022–e00115. [Google Scholar] [CrossRef]
- Kuhn, I.; Katouli, M.; Lund, A.; Wallgren, P.; Mollby, R. Phenotype diversity and stability of intestinal coliform flora in piglets during the first three months of age. Microbial. Ecol. Health Dis. 1993, 6, 101–107. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Q.; Huang, Z.; Lv, L.; Liu, X.; Yin, C.; Yan, H.; Yuan, J. Effect of Bacillus subtilis CGMCC 1.1086 on the growth performance and intestinal microbiota of broilers. J. Appl. Microbiol. 2016, 120, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.M.; Shah, T.; Deshpande, S.; Jakhesara, S.J.; Koringa, P.G.; Rank, D.N.; Joshi, C.G. High through put 16S rRNA gene-based pyrosequencing analysis of the fecal microbiota of high FCR and low FCR broiler growers. Mol. Biol. Rep. 2012, 39, 10595–10602. [Google Scholar] [CrossRef] [PubMed]
- Pryde, S.E.; Duncan, S.H.; Hold, G.L.; Stewart, C.S.; Flint, H.J. The microbiology of butyrate formation in the human colon. FEMS Microbiol. Lett. 2002, 217, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, S.; Ding, J.; Dai, R.; He, C.; Xu, K.; Honaker, C.F.; Zhang, Y.; Siegel, P.; Meng, H. Gut microbiota co-microevolution with selection for host humoral immunity. Front. Microbiol. 2017, 8, 1243. [Google Scholar] [CrossRef]
- Onrust, L.; Ducatelle, R.; Van Driessche, K.; De Maesschalck, C.; Vermeulen, K.; Haesebrouck, F.; Eeckhaut, V.; Van Immerseel, F. Steering endogenous butyrate production in the intestinal tract of broilers as a tool to improve gut health. Front. Vet. Sci. 2015, 2, 75. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Finegold, S.M.; Song, Y.; Lawson, P.A. Reclassification of Clostridium coccoides, Ruminococcus hansenii, Ruminococcus hydrogenotrophicus, Ruminococcus luti, Ruminococcus productus and Ruminococcus schinkii as Blautia coccoides gen. nov., comb. nov., Blautia hansenii comb. nov., Blautia hydrogenotrophica comb. nov., Blautia luti comb. nov., Blautia producta comb. nov., Blautia schinkii comb. nov. and description of Blautia wexlerae sp. nov., isolated from human faeces. Int. J. Syst. Evol. Microbiol. 2008, 58, 1896–1902. [Google Scholar]
- Sunkara, L.T.; Jiang, W.; Zhang, G. Modulation of antimicrobial host defense peptide gene expression by free fatty acids. PLoS ONE 2012, 7, e49558. [Google Scholar] [CrossRef] [PubMed]
- Rehman, H.U.; Vahjen, W.; Awad, W.A.; Zentek, J. Indigenous bacteria and bacterial metabolic products in the gastrointestinal tract of broiler chickens. Arch. Anim. Nutr. 2007, 61, 319–335. [Google Scholar] [CrossRef]
- Rautio, M.; Eerola, E.; Väisänen-Tunkelrott, M.L.; Molitoris, D.; Lawson, P.; Collins, M.D.; Jousimies-Somer, H. Reclassification of Bacteroides putredinis [Weinberg et al., 1937] in a new genus Alistipes gen. nov., as Alistipes putredinis comb. nov., and description of Alistipes finegoldii sp. nov., from human sources. Syst. Appl. Microbiol. 2013, 26, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, M.J.; Stanley, D.; Chen, H.; Donald, J.A.; Nicholas, K.R.; Moore, R.J.; Crowley, T.M. Functional similarities between pigeon ‘milk’ and mammalian milk: Induction of immune gene expression and modification of the microbiota. PLoS ONE 2012, 7, e48363. [Google Scholar] [CrossRef] [PubMed]
- Forder, R.E.; Howarth, G.S.; Tivey, D.R.; Hughes, R.J. Bacterial modulation of small intestinal goblet cells and mucin composition during early posthatch development of poultry. Poult. Sci. 2007, 86, 2396–2403. [Google Scholar] [CrossRef]
- Dean-Nystrom, E.A.; Samuel, J.E. Age-related resistance to 987P fimbria-mediated colonization correlates with specific glycolipid receptors in intestinal mucus in swine. Infect. Immun. 1994, 62, 4789–4794. [Google Scholar]
- Uni, Z.; Smirnov, A.; Sklan, D. Pre-and posthatch development of goblet cells in the broiler small intestine: Effect of delayed access to feed. Poult. Sci. 2003, 82, 320–327. [Google Scholar] [CrossRef]
- Uni, Z.; Platin, R.; Sklan, D. Cell proliferation in chicken intestinal epithelium occurs both in the crypts and along the villus. J. Comp. Physiol. B 1998, 168, 241–247. [Google Scholar] [CrossRef]
| Factor | d.f. 6 | Chi-Square | P 7 |
|---|---|---|---|
| Crypts | |||
| Diet 1 | 3 | 3.736 | 0.291 |
| Mucin type 2 | 2 | 10.084 | 0.006 |
| Gut segment 3 | 3 | 216.132 | <0.001 |
| Fragment 4 | 2 | 112.127 | <0.001 |
| Villi | |||
| Diet | 3 | 12.569 | 0.006 |
| Mucin type | 2 | 0.762 | 0.683 |
| Gut segment 5 | 2 | 140.155 | <0.001 |
| Fragment | 2 | 6.561 | 0.038 |
| Gut Mucosal Element | Predictor | Predictor Factors | Mucin Staining Intensity 1,2 |
|---|---|---|---|
| Crypts | Diet | C | 1.23 ± 0.03 |
| TM5 | 1.26 ± 0.03 | ||
| TM10 | 1.31 ± 0.03 | ||
| TM15 | 1.26 ± 0.03 | ||
| Mucin type | Neutral | 1.33 ± 0.03 A | |
| Acidic sialylated | 1.23 ± 0.02 B | ||
| Acidic sulfated | 1.24 ± 0.02 B | ||
| Gut segment | Duodenum | 1.18 ± 0.03 C | |
| Jejunum | 1.40 ± 0.03 B | ||
| Ileum | 1.55 ± 0.03 A | ||
| Cecum | 1.00 ± 0.02 D | ||
| Fragment | Base | 1.49 ± 0.03 A | |
| Midsection | 1.18 ± 0.02 B | ||
| Tip | 1.15 ± 0.02 B | ||
| Villi | Diet | C | 1.82 ± 0.04 AB |
| TM5 | 1.92 ± 0.05 A | ||
| TM10 | 1.70 ± 0.04 C | ||
| TM15 | 1.77 ± 0.04 BC | ||
| Mucin type | Neutral | 1.83 ± 0.04 | |
| Acidic sialylated | 1.79 ± 0.04 | ||
| Acidic sulfated | 1.78 ± 0.04 | ||
| Gut segment | Duodenum | 1.50 ± 0.03 B | |
| Jejunum | 1.83 ± 0.04 A | ||
| Ileum | 2.13 ± 0.04 A | ||
| Fragment | Base | 1.87 ± 0.04 a | |
| Midsection | 1.79 ± 0.04 ab | ||
| Tip | 1.73 ± 0.04 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biasato, I.; Ferrocino, I.; Grego, E.; Dabbou, S.; Gai, F.; Gasco, L.; Cocolin, L.; Capucchio, M.T.; Schiavone, A. Gut Microbiota and Mucin Composition in Female Broiler Chickens Fed Diets including Yellow Mealworm (Tenebrio molitor, L.). Animals 2019, 9, 213. https://doi.org/10.3390/ani9050213
Biasato I, Ferrocino I, Grego E, Dabbou S, Gai F, Gasco L, Cocolin L, Capucchio MT, Schiavone A. Gut Microbiota and Mucin Composition in Female Broiler Chickens Fed Diets including Yellow Mealworm (Tenebrio molitor, L.). Animals. 2019; 9(5):213. https://doi.org/10.3390/ani9050213
Chicago/Turabian StyleBiasato, Ilaria, Ilario Ferrocino, Elena Grego, Sihem Dabbou, Francesco Gai, Laura Gasco, Luca Cocolin, Maria Teresa Capucchio, and Achille Schiavone. 2019. "Gut Microbiota and Mucin Composition in Female Broiler Chickens Fed Diets including Yellow Mealworm (Tenebrio molitor, L.)" Animals 9, no. 5: 213. https://doi.org/10.3390/ani9050213
APA StyleBiasato, I., Ferrocino, I., Grego, E., Dabbou, S., Gai, F., Gasco, L., Cocolin, L., Capucchio, M. T., & Schiavone, A. (2019). Gut Microbiota and Mucin Composition in Female Broiler Chickens Fed Diets including Yellow Mealworm (Tenebrio molitor, L.). Animals, 9(5), 213. https://doi.org/10.3390/ani9050213








