Digestibility and Retention Time of Coastal Bermudagrass (Cynodon dactylon) Hay by Horses
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Diet Digestibility
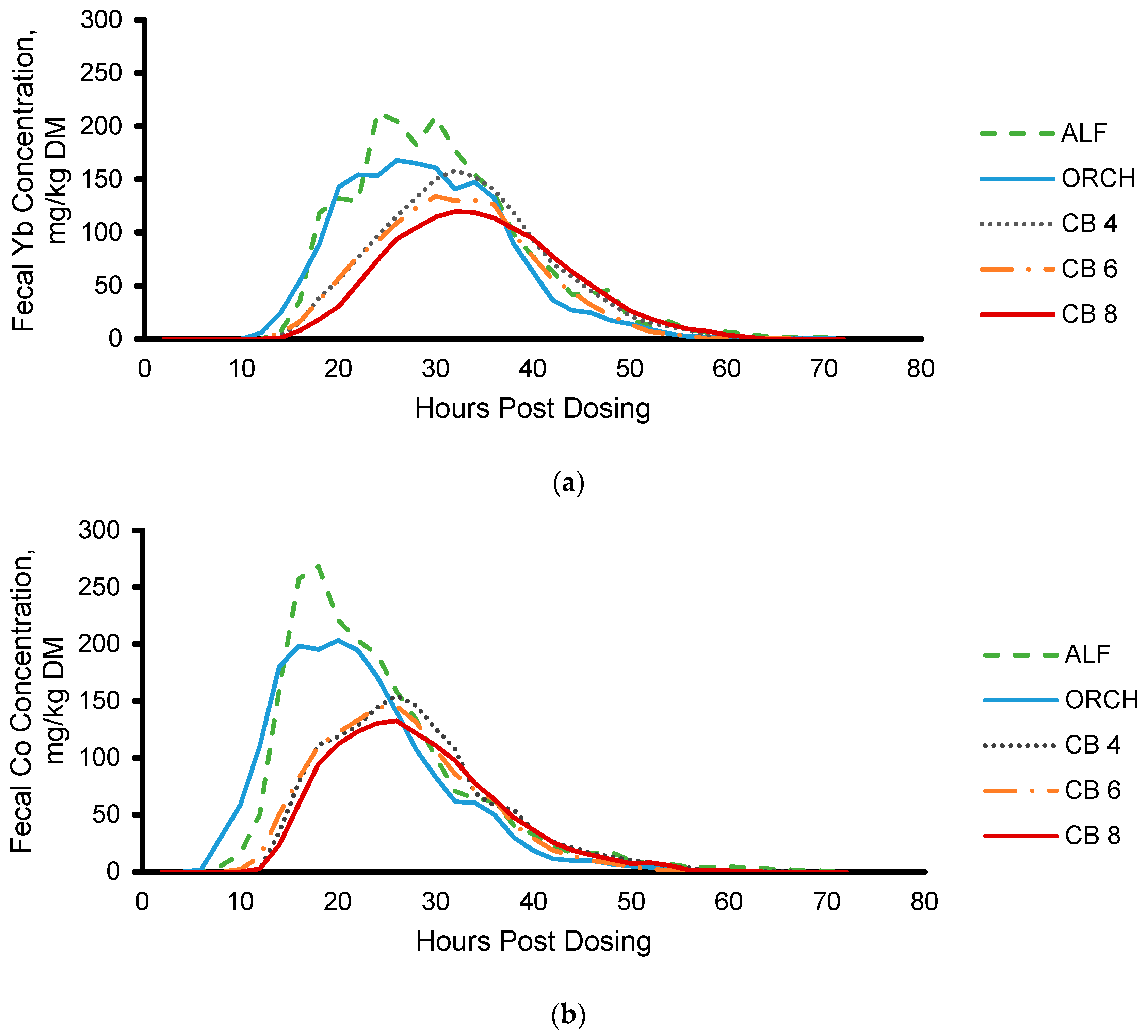
3.2. Fecal Marker Excretion
3.2.1. Marker Excretion and Recovery
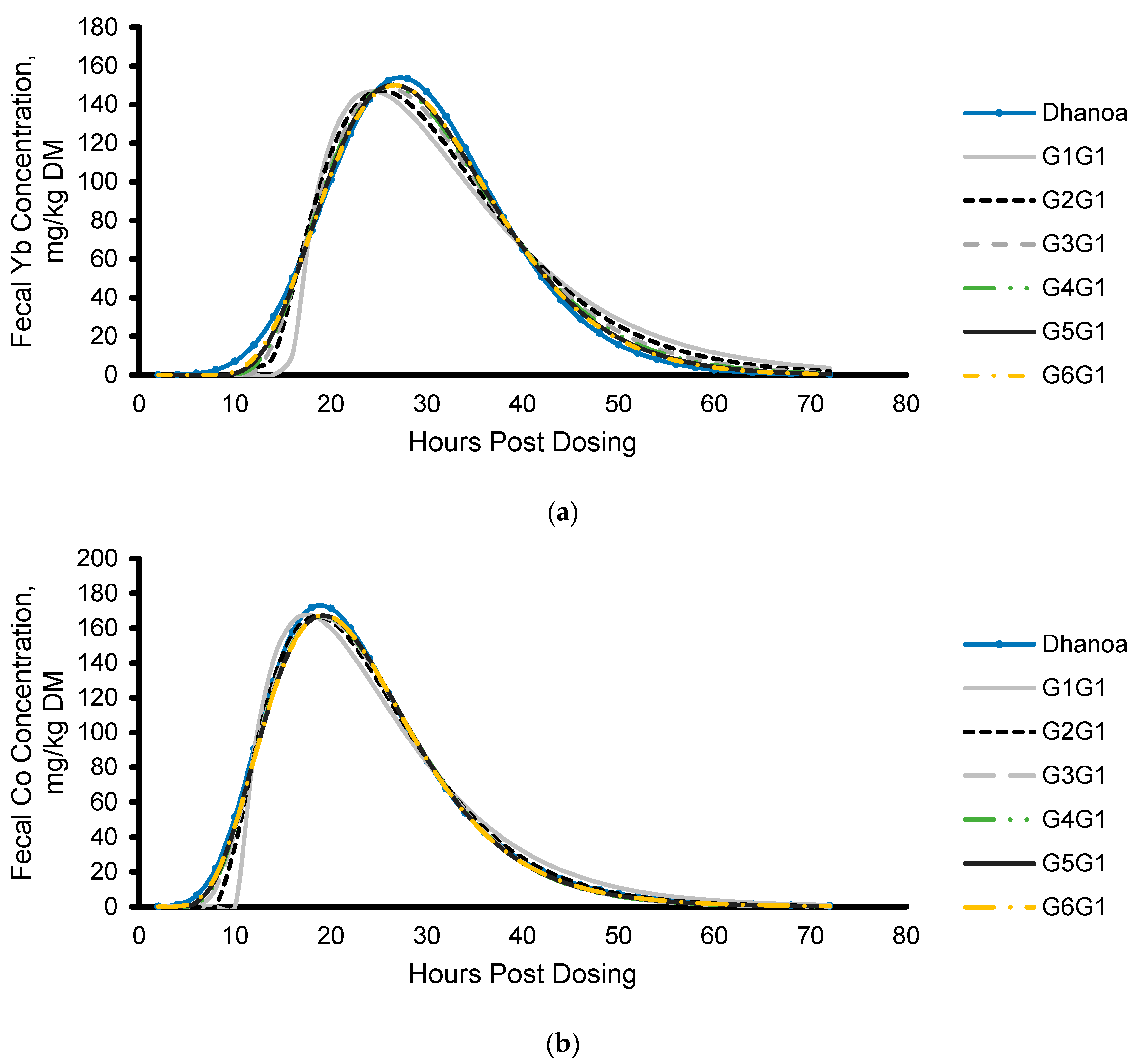
3.2.2. Modeling Fecal Marker Excretion
3.3. Digesta Mean Retention Time
3.3.1. Mean Retention Time Calculated from Model Parameters
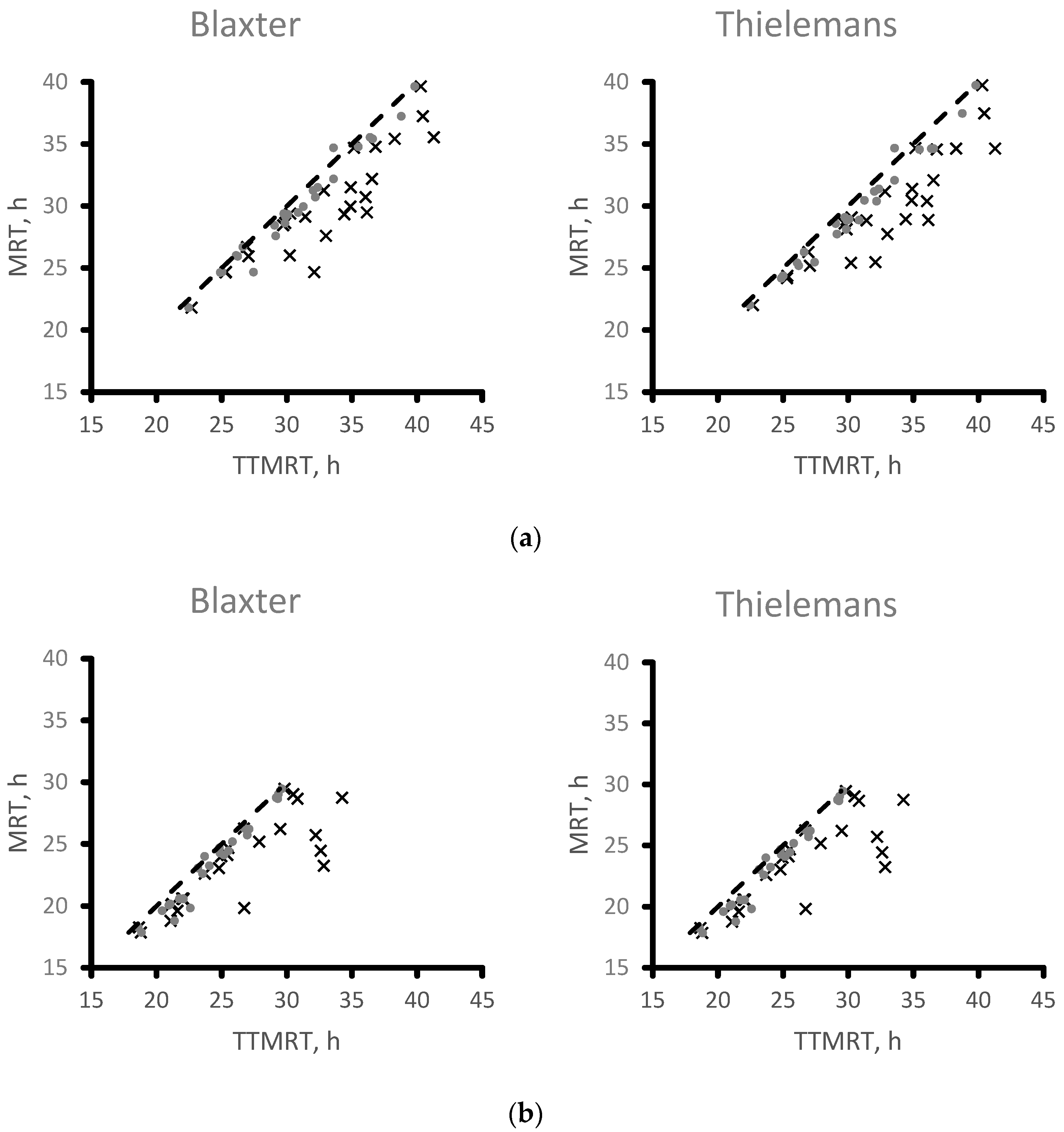
3.3.2. Arithmetically Calculated Mean Retention Time
3.3.3. Comparing Model-Derived and Arithmetically Calculated Mean Retention Time
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Little, D.; Blikslager, A.T. Factors associated with development of ileal impaction in horses with surgical colic: 78 cases (1986–2000). Equine Vet. J. 2002, 34, 464–468. [Google Scholar] [CrossRef]
- Blikslager, A.T. Colic prevention to avoid colic surgery: A surgeon’s perspective. J. Equine Vet. Sci. 2019, 76, 1–5. [Google Scholar] [CrossRef]
- Moore, K.J.; Boote, K.J.; Sanderson, M.A. Physiology and developmental morphology. In Warm-Season (C4) Grasses; Moser, L.E., Burson, B.L., Sollenberger, L.E., Eds.; ASA/CSSA/SSSA: Madison, WI, USA, 2014; pp. 179–216. [Google Scholar]
- Sollenberger, L.E.; Vanzant, E.S. Interrelationships among forage nutritive value and quantity and individual animal performance. Crop Sci. 2011, 51, 420–432. [Google Scholar] [CrossRef]
- Rohweder, D.A.; Barnes, R.F.; Jorgensen, N. Proposed hay grading standards based on laboratory analyses for evaluating quality. J. Anim. Sci. 1978, 47, 747–759. [Google Scholar] [CrossRef]
- Hansen, T.L.; Lawrence, L.M. Composition factors predicting forage digestibility by horses. J. Equine Vet. Sci. 2017, 58, 97–102. [Google Scholar] [CrossRef]
- Lowman, R.S.; Theodorou, M.K.; Hyslop, J.J.; Dhanoa, M.S.; Cuddeford, D. Evaulation of an in vitro batch culture technique for estimating the in vivo digestibility and digestible energy content of equine feeds using equine faeces as the source of microbial inoculum. Anim. Feed Sci. Technol. 1999, 80, 11–27. [Google Scholar] [CrossRef]
- Sunvold, G.D.; Hussein, H.S.; Fahey, G.C., Jr.; Merchen, N.R.; Reinhart, G.A. In vitro fermentation of cellulose, beet pulp, citrus pulp, and citrus pectin using fecal inoculum from cats, dogs, horses, humans, and pigs and ruminal fluid from cattle. J. Anim. Sci. 1995, 73, 3639–3648. [Google Scholar] [CrossRef]
- Pearson, R.A.; Archibald, R.F.; Muirhead, R.H. The effect of forage quality and level of feeding on digestibility and gastrointestinal transit time of oat straw and alfalfa given to ponies and donkeys. Br. J. Nutr. 2001, 85, 599–606. [Google Scholar] [CrossRef]
- Moore-Colyer, M.J.S.; Morrow, H.J.; Longland, A.C. Mathematical modelling of digesta passage rate, mean retention time and in vivo apparent digestibility of two different lengths of hay and big-bale grass silage in ponies. Br. J. Nutr. 2003, 90, 109–118. [Google Scholar] [CrossRef]
- Miyaji, M.; Ueda, K.; Hata, H.; Kondo, S. Effects of quality and physical form of hay on mean retention time of digesta and total tract digestibility in horses. Anim. Feed Sci. Technol. 2011, 165, 61–67. [Google Scholar] [CrossRef]
- Van Weyenberg, S.; Sales, J.; Janssens, G.P.J. Passage rate of digesta through the equine gastrointestinal tract: A review. Livest. Sci. 2006, 99, 3–12. [Google Scholar] [CrossRef]
- Hooda, S.; Metzler-Zebeli, B.U.; Vasanthan, T.; Zijlstra, R.T. Effects of viscosity and fermentability of dietary fibre on nutrient digestibility and digesta characteristics in ileal-cannulated grower pigs. Br. J. Nutr. 2011, 106, 664–674. [Google Scholar] [CrossRef]
- Dhanoa, M.S.; Siddons, R.C.; France, J.; Gale, D.L. A multicompartmental model to describe marker excretion patterns in ruminant faeces. Br. J. Nutr. 1985, 53, 663–671. [Google Scholar] [CrossRef]
- Pond, K.R.; Ellis, W.C.; Matis, J.H.; Ferreiro, H.M.; Sutton, J.D. Compartment models for estimating attributes of digesta flow in cattle. Br. J. Nutr. 1988, 60, 571–595. [Google Scholar] [CrossRef]
- Grovum, W.L.; Williams, V.J. Rate of passage of digesta in sheep. 4. Passage of marker through the alimentary tract and the biological relevance of rate-constants derived from the changes in concentration of marker in faeces. Br. J. Nutr. 1973, 30, 313–329. [Google Scholar] [CrossRef]
- Austbø, D.; Volden, H. Influence of passage model and caecal cannulation on estimated passage kinetics of roughage and concentrate in the gastrointestinal tract of horses. Livest. Sci. 2006, 100, 33–43. [Google Scholar] [CrossRef]
- Miyaji, M.; Ueda, K.; Hata, H.; Kondo, S. Effect of grass hay intake on fiber digestion and digesta retention time in the hindgut of horses. J. Anim. Sci. 2014, 92, 1574–1581. [Google Scholar] [CrossRef]
- Murray, J.A.M.D.; Sanderson, R.; Longland, A.C.; Moore-Colyer, M.J.S.; Hastie, P.M.; Dunnett, C. Assessment of mathematical models to describe the rate of passage of enzyme-treated or sugar beet pulp-substituted lucerne silage in equids. Anim. Feed Sci. Technol. 2009, 154, 228–240. [Google Scholar] [CrossRef]
- Rosenfeld, I.; Austbo, D.; Volden, H. Models for estimating digesta passage kinetics in the gastrointestinal tract of the horse. J. Anim. Sci. 2006, 84, 3321–3328. [Google Scholar] [CrossRef]
- Federation of Animal Science Societies (FASS). Guide for the Care and Use of Agricultural Animals in Research and Teaching, 3rd ed.; FASS: Champaign, IL, USA, 2010. [Google Scholar]
- Henneke, D.R.; Potter, G.D.; Kreider, J.L.; Yeates, B.F. Relationship between condition score, physical measurements and body fat percentage in mares. Equine Vet. J. 1983, 15, 371–372. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Horses: Sixth Revised Edition; The National Academies Press: Washington, DC, USA, 2007; p. 360. [Google Scholar]
- Fisher, R.A.; Yates, F. Statistical Tables for Biological, Agricultural and Medical Research; Oliver & Boyd Ltd.: London, UK, 1963; p. 146. [Google Scholar]
- Chizek, E.L. Comparison of Feed Intake Behavior between Warm- and Cool-Season Forages Offered to Horses. Master’s Thesis, University of Florida, Gainesville, FL, USA, 2016. [Google Scholar]
- Pagan, J. Nutrient digestibility in horses. In Advances in Equine Nutrition; Kentucky Equine Research, Inc.: Versailles, KY, USA, 1998; pp. 77–83. [Google Scholar]
- Udén, P.; Colucci, P.E.; Van Soest, P.J. Investigation of chromium, cerium and cobalt as markers in digesta rate of passage studies. J. Sci. Food Agric. 1980, 31, 625–632. [Google Scholar] [CrossRef]
- Ringler, J.E.; Lawrence, L.M. Development of a method to label forages used in passage rate studies in the horse. J. Equine Vet. Sci. 2009, 29, 389–390. [Google Scholar] [CrossRef]
- ANKOM Technology. Analytical Methods Fiber Analyzer A200. Available online: https://www.ankom.com/analytical-methods-support/fiber-analyzer-a200 (accessed on 20 January 2015).
- EPA. Method 3052: Microwave Assisted Acid Digestion of Siliceous and Organically Based Matrices; EPA: Washington, DC, USA, 1996.
- EPA. Method 200.7: Determination of Metals and Trace Elements in Water and Wastes by Inductively Coupled Plasma-Atomic Emmision Spectometry; EPA: Washington, DC, USA, 1994.
- Blaxter, K.L.; Graham, N.M.; Wainman, F.W. Some observations on the digestibility of food by sheep, and on related problems. Br. J. Nutr. 1956, 10, 69–91. [Google Scholar] [CrossRef]
- Thielemans, M.-F.; Francois, E.; Bodart, C.; Thewis, A. Gastrointestinal transit in the pig: Measurement using radioactive lanthanides and comparison with sheep. Ann. Biol. Anim. Biochim. Biophys. 1978, 18, 237–247. [Google Scholar] [CrossRef]
- Sturgeon, L.S.; Baker, L.A.; Pipkin, J.L.; Haliburton, J.C.; Chirase, N.K. The digestibility and mineral availability of Matua, Bermuda grass, and alfalfa hay in mature horses. J. Equine Vet. Sci. 2000, 20, 45–48. [Google Scholar] [CrossRef]
- Eckert, J.V.; Myer, R.O.; Warren, L.K.; Brendemuhl, J.H. Digestibility and nutrient retention of perennial peanut and bermudagrass hays for mature horses. J. Anim. Sci. 2010, 88, 2055–2061. [Google Scholar] [CrossRef]
- Earing, J.E.; Cassill, B.D.; Hayes, S.H.; Vanzant, E.S.; Lawrence, L.M. Comparison of in vitro digestibility estimates using the DaisyII incubator with in vivo digestibility estimates in horses. J. Anim. Sci. 2010, 88, 3954–3963. [Google Scholar] [CrossRef]
- Cymbaluk, N.; Christensen, D. Nutrient utilization of pelleted and unpelleted forages by ponies. Can. J. Anim. Sci. 1986, 66, 237–244. [Google Scholar] [CrossRef]
- Cymbaluk, N.F. Comparison of forage digestion by cattle and horses. Can. J. Anim. Sci. 1990, 70, 601–610. [Google Scholar] [CrossRef]
- Potts, L.; Hinkson, J.; Graham, B.; Löest, C.; Turner, J. Nitrogen retention and nutrient digestibility in geldings fed grass hay, alfalfa hay, or alfalfa cubes. J. Equine Vet. Sci. 2010, 30, 330–333. [Google Scholar] [CrossRef]
- Crozier, J.A.; Allen, V.G.; Jack, N.E.; Fontenot, J.P.; Cochran, M.A. Digestibility, apparent mineral absorption, and voluntary intake by horses fed alfalfa, tall fescue, and caucasian bluestem. J. Anim. Sci. 1997, 75, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.W.; Goering, H.K.; Gordon, C.H. Relationships of forage compositions with rates of cell wall digestion and indigestibility of cell walls. J. Dairy Sci. 1972, 55, 1140–1147. [Google Scholar] [CrossRef]
- Albrecht, K.A.; Wedin, W.F.; Buxton, D.R. Cell-wall composition and digestibility of alfalfa stems and leaves. Crop Sci. 1987, 27, 735–741. [Google Scholar] [CrossRef]
- Griffin, J.L.; Jung, G.A. Leaf and stem forage quality of big bluestem and switchgrass. Agron. J. 1983, 75, 723–726. [Google Scholar] [CrossRef]
- Bourquin, L.D.; Fahey, G.C., Jr. Ruminal digestion and glycosyl linkage patterns of cell wall components from leaf and stem fractions of alfalfa, orchardgrass, and wheat straw. J. Anim. Sci. 1994, 72, 1362–1374. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.R.; Akin, D.E.; McLeod, M.N.; Minson, D.J. Particle size reduction of the leaves of a tropical and a temperate grass by cattle. II. Relation of anatomical structure to the process of leaf breakdown through chewing and digestion. Grass Forage Sci. 1989, 44, 65–75. [Google Scholar] [CrossRef]
- Hastert, A.A.; Owensby, C.E.; Harbers, L.H. Rumen microbial degradation of Indiangrass and big bluestem leaf blades. J. Anim. Sci. 1983, 57, 1626–1636. [Google Scholar] [CrossRef]
- Akin, D.E.; Robinson, E.L.; Barton, F.E.; Himmelsbach, D.S. Changes with maturity in anatomy, histochemistry, chemistry, and tissue digestibility of bermudagrass plant parts. J. Agric. Food Chem. 1977, 25, 179–186. [Google Scholar] [CrossRef]
- Lieb, S.; Ott, E.A.; French, E.C. Digestible nutrients and voluntary intake of rhizomes peanut, alfalfa, bermudagrass and bahiagrass by equine. In Proceedings of the Thirteenth Equine Nutrition and Physiology Symposium, Gainesville, FL, USA, 21–23 January 1993; pp. 98–99. [Google Scholar]
- Lieb, S.; Mislevy, P. Comparative intake and nutrient digestibility of three grass forages: Florakirk and Tifton 85 bermudagrasses and Florona stargrass to Coastal bermudagrass fed to horses. In Proceedings of the Seventeenth Equine Nutrition and Physiology Symposium, Lexington, KY, USA, 31 May–2 June 2001; pp. 390–391. [Google Scholar]
- LaCasha, P.A.; Brady, H.A.; Allen, V.G.; Richardson, C.R.; Pond, K.R. Voluntary intake, digestibility, and subsequent selection of Matua bromegrass, coastal bermudagrass, and alfalfa hays by yearling horses. J. Anim. Sci. 1999, 77, 2766–2773. [Google Scholar] [CrossRef]
- Akin, D.E.; Hartley, R.D. UV Absorption microspectrophotometry and digestibility of cell types of bermudagrass internodes at different stages of maturity. J. Sci. Food Agric. 1992, 59, 437–447. [Google Scholar] [CrossRef]
- De Ruiter, J.M.; Burns, J.C.; Timothy, D.H. Hemicellulosic cell wall carbohydrate monomer composition in Panicum amarum, P. amarulum and P virgatum accessions. J. Sci. Food Agric. 1992, 60, 297–307. [Google Scholar] [CrossRef]
- Koller, B.L.; Hintz, H.F.; Robertson, J.B.; Van Soest, P.J. Comparative cell wall and dry matter digestion in the cecum of the pony and the rumen of the cow using in vitro and nylon bag techniques. J. Anim. Sci. 1978, 47, 209–215. [Google Scholar] [CrossRef]
- Coblentz, W.K.; Fritz, J.O.; Fick, W.H.; Cochran, R.C.; Shirley, J.E. In situ dry matter, nitrogen, and fiber degradation of alfalfa, red clover, and eastern gamagrass at four maturities. J. Dairy Sci. 1998, 81, 150–161. [Google Scholar] [CrossRef]
- Eastwood, M.A.; Kay, R.M. An hypothesis for the action of dietary fiber along the gastrointestinal tract. Am. J. Clin. Nutr. 1979, 32, 364–367. [Google Scholar] [CrossRef]
- Wen, J.; Phillips, S.F.; Sarr, M.G.; Kost, L.J.; Holst, J.J. PYY and GLP-1 contribute to feedback inhibition from the canine ileum and colon. Am. J. Physiol. 1995, 269, G945–G952. [Google Scholar] [CrossRef]
- Reimer, R.A.; McBurney, M.I. Dietary fiber modulates intestinal proglucagon messenger ribonucleic acid and postprandial secretion of glucagon-like peptide-1 and insulin in rats. Endocrinology 1996, 137, 3948–3956. [Google Scholar] [CrossRef]
- Cani, P.D.; Lecourt, E.; Dewulf, E.M.; Sohet, F.M.; Pachikian, B.D.; Naslain, D.; De Backer, F.; Neyrinck, A.M.; Delzenne, N.M. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am. J. Clin. Nutr. 2009, 90, 1236–1243. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Wolever, T.M.; Leeds, A.R.; Gassull, M.A.; Haisman, P.; Dilawari, J.; Goff, D.V.; Metz, G.L.; Alberti, K.G. Dietary fibres, fibre analogues, and glucose tolerance: Importance of viscosity. Br. Med. J. 1978, 1, 1392–1394. [Google Scholar] [CrossRef]
- Schwartz, S.E.; Levine, R.A.; Singh, A.; Scheidecker, J.R.; Track, N.S. Sustained pectin ingestion delays gastric emptying. Gastroenterology 1982, 83, 812–817. [Google Scholar]
- Jensen, R.B.; Austbo, D.; Bach Knudsen, K.E.; Tauson, A.H. The effect of dietary carbohydrate composition on apparent total tract digestibility, feed mean retention time, nitrogen and water balance in horses. Animal 2014, 8, 1788–1796. [Google Scholar] [CrossRef]
- Ellis, W.C.; Matis, J.H.; Hill, T.M.; Murphy, M.R. Methodology for Estimating Digestion and Passage Kinetics of Forages. In Forage Quality, Evaluation, and Utilization; Fahey, G.C., Ed.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, WI, USA, 1994; pp. 682–756. [Google Scholar]
- Argenzio, R.A.; Lowe, J.E.; Pickard, D.W.; Stevens, C.E. Digesta passage and water exchange in the equine large intestine. Am. J. Physiol. 1974, 226, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Miyaji, M.; Ueda, K.; Nakatsuji, H.; Tomioka, T.; Kobayashi, Y.; Hata, H.; Kondo, S. Mean retention time of digesta in the different segments of the equine hindgut. Anim. Sci. J. 2008, 79, 89–96. [Google Scholar] [CrossRef]
- Hansen, T.L. Modeling Digestibility and Rate of Passage in Horses. Master’s Thesis, University of Kentucky, Lexington, KY, USA, 2014. [Google Scholar]
| Nutrient a | Alfalfa | Orchardgrass | Coastal 4 Weeks | Coastal 6 Weeks | Coastal 8 Weeks | Vit/Min Suppl 1 b | Vit/Min Suppl 2 c |
|---|---|---|---|---|---|---|---|
| DM, % | 88.4 | 90.9 | 90.0 | 91.8 | 91.6 | 89.4 | 90.5 |
| DE d, Mcal/kg | 2.50 | 2.09 | 1.95 | 1.90 | 1.85 | 2.76 | 3.31 |
| CP, % | 23.2 | 11.5 | 18.5 | 12.7 | 12.6 | 15.3 | 37.2 |
| NDF, % | 37.7 | 57.2 | 67.5 | 70.9 | 73.3 | 43.3 | 16.9 |
| ADF, % | 29.5 | 42.0 | 32.7 | 34.7 | 35.1 | 25.9 | 8.4 |
| ADL, % | 8.3 | 2.8 | 4.6 | 5.0 | 6.0 | n.m. | n.m. |
| Starch, % | 1.3 | 0.2 | 1.6 | 1.6 | 2.5 | n.m. | n.m. |
| ESC, % | 5.9 | 9.6 | 4.3 | 4.4 | 4.4 | n.m. | n.m. |
| WSC, % | 6.3 | 12.5 | 3.6 | 4.3 | 4.8 | n.m. | n.m. |
| Ca, % | 1.58 | 0.32 | 0.57 | 0.39 | 0.38 | 1.31 | 3.12 |
| P, % | 0.24 | 0.23 | 0.30 | 0.27 | 0.18 | 1.78 | 1.19 |
| Na, % | 0.067 | 0.44 | 0.067 | 0.023 | 0.12 | 0.23 | 0.40 |
| K, % | 2.33 | 2.12 | 1.58 | 1.74 | 0.82 | 1.03 | 1.60 |
| Cl, % | 0.93 | 1.56 | 0.45 | 0.33 | 0.17 | 0.60 | 0.75 |
| uNDFom, % e | 20.9 | 11.6 | 21.4 | 25.8 | 38.6 | n.m. | n.m. |
| Item | ALF | ORCH | CB 4 | CB 6 | CB 8 |
|---|---|---|---|---|---|
| Ingredient, % DMI | |||||
| Alfalfa | 93.7 | ||||
| Orchardgrass | 92.8 | ||||
| Coastal Bermuda, 4 weeks | 91.8 | ||||
| Coastal Bermuda, 6 weeks | 91.8 | ||||
| Coastal Bermuda, 8 weeks | 91.4 | ||||
| Vit/Min Suppl 1 a | 5.9 | ||||
| Vit/Min Suppl 2 b | 7.2 | 7.2 | 7.2 | 7.1 | |
| Sodium Chloride | 0.4 | 0.3 | 0.4 | 0.2 | |
| Potassium Chloride | 0.7 | 0.6 | 1.3 | ||
| Daily Intake | |||||
| DM, % BW | 1.71 | 1.73 | 1.74 | 1.74 | 1.75 |
| DE c, Mcal/kg BW | 0.043 | 0.038 | 0.035 | 0.35 | 0.034 |
| CP, g/kg BW | 3.87 | 2.31 | 3.43 | 2.50 | 2.48 |
| NDF, g/kg BW | 6.47 | 9.36 | 11.01 | 11.56 | 11.94 |
| ADF, g/kg BW | 4.98 | 6.83 | 5.34 | 5.65 | 5.72 |
| Ca, mg/kg BW | 265.9 | 90.23 | 130.2 | 101.4 | 99.82 |
| P, mg/kg BW | 56.2 | 51.69 | 62.89 | 58.09 | 43.69 |
| K, mg/kg BW | 383.1 | 359.2 | 332.8 | 348.4 | 261.2 |
| Na, mg/kg BW | 39.3 | 75.03 | 37.92 | 30.88 | 35.6 |
| Cl, mg/kg BW | 197.8 | 359.2 | 174.5 | 145.9 | 159.4 |
| Variable | ALF | ORCH | CB 4 | CB 6 | CB 8 | SEM | Diet 2 p-Value | Contrast 3 p-Value |
|---|---|---|---|---|---|---|---|---|
| Defecation Frequency, times/d | 10.0 c | 11.5 b,c | 14.1 a,b | 15.3 a | 14.0 a,b | 0.5 | <0.001 | <0.001 |
| Fecal Excretion, kg DM/d | 3.55 d | 4.41 c | 5.04 b | 5.84 a | 5.87 a | 0.20 | <0.001 | <0.001 |
| Fecal DM, % | 19.7 | 20.9 | 20.0 | 20.5 | 22.5 | 0.42 | 0.074 | 0.255 |
| Urination Frequency, times/d | 10.6 | 10.6 | 8.7 | 8.3 | 10.7 | 0.58 | 0.324 | 0.161 |
| Digestibility, % | ||||||||
| DM | 62.1 a | 51.2 b | 47.2 b | 36.0 c | 36.8 c | 2.1 | <0.001 | <0.001 |
| OM | 63.1 a | 52.3 b | 46.8 c | 37.3 d | 37.6 d | 2.1 | <0.001 | <0.001 |
| NDF | 43.1 a | 42.4 a | 46.2 a | 31.1 b | 31.8 b | 1.7 | <0.001 | <0.001 |
| ADF | 40.2 a | 39.8 a | 39.8 a | 23.9 b | 24.3 b | 1.9 | <0.001 | <0.001 |
| Variable | ALF | ORCH | CB 4 | CB 6 | CB 8 | SEM | Diet 2 p-Value | Contrast 3 p-Value |
|---|---|---|---|---|---|---|---|---|
| Particulate, % | 80.6 | 86.3 | 85.5 | 82.5 | 85.0 | 1.44 | 0.549 | 0.948 |
| Liquid, % | 85.5 | 95.1 | 79.4 | 80.3 | 82.6 | 2.17 | 0.075 | 0.025 |
| Variable | Dhanoa a | G1G1 b | G2G1 b | G3G1 b | G4G1 b | G5G1 b | G6G1 b |
|---|---|---|---|---|---|---|---|
| Particulate | |||||||
| RMSE | 11.70 | 20.48 | 16.30 | 14.83 | 14.02 | 12.43 | 12.91 |
| AIC | 128.8 | 163.1 | 148.1 | 142.3 | 138.7 | 134.3 | 137.0 |
| Models with Nonzero Rate Parameters, % | 4 | 0 | 20 | 72 | 76 | 84 | 92 |
| A | 2.68 × 1011 ± 2.65 × 1011 | ||||||
| C, mg Yb/kg digesta | −476 ± 26.6 | 636 ± 39.6 | 761 ± 49.6 | 856 ± 57.6 | 901 ± 56.3 | 916 ± 63.0 | |
| k1, h−1 | 0.265 ± 0.017 | 0.133 ± 0.007 | |||||
| k2, h−1 | 0.355 ± 0.011 | 0.142 ± 0.008 | 0.187 ± 0.006 | 0.223 ± 0.008 | 0.253 ± 0.008 | 0.271 ± 0.009 | 0.277± 0.012 |
| λ1, h−1 | 0.192 ± 0.007 | 0.235 ± 0.011 | 0.279 ± 0.014 | 0.321 ± 0.018 | 0.370 ± 0.024 | ||
| N | 48 ± 12.9 | ||||||
| TT, h | 25.8 ± 1.15 | 17.5 ± 0.71 | 15.6 ± 0.64 | 13.4 ± 0.69 | 11.9 ± 0.66 | 10.6 ± 0.62 | 9.50 ± 0.61 |
| CMRT1, h | 4.20 ± 0.29 | 8.01 ± 0.40 | 10.7 ± 0.34 | 13.3 ± 0.47 | 15.0 ± 0.56 | 16.4 ± 0.63 | 17.4 ± 0.77 |
| CMRT2, h | 2.89 ± 0.096 | 7.48 ± 0.33 | 5.46 ± 0.15 | 4.61 ± 0.17 | 4.07 ± 0.17 | 3.82 ± 0.18 | 3.87 ± 0.26 |
| TTMRT, h | 32.9 ± 1.00 | 33.0 ± 0.99 | 31.7 ± 0.93 | 31.3 ± 0.92 | 31.0 ± 0.90 | 30.8 ± 0.90 | 30.8 ± 0.88 |
| Liquid | |||||||
| RMSE | 13.88 | 17.51 | 15.57 | 14.95 | 14.04 | 12.43 | 14.01 |
| AIC | 141.1 | 154.6 | 147.2 | 145.0 | 142.6 | 143.0 | 143.5 |
| Models with Nonzero Rate Parameters, % | 16 | 0 | 52 | 76 | 80 | 84 | 84 |
| A | 8.00 × 108 ± 4.33 × 108 | ||||||
| C, mg Co/kg digesta | −470 ± 26.1 | 527 ± 21.4 | 582 ± 33.7 | 630 ± 42.6 | 642 ± 47.6 | 639 ± 66.3 | |
| k1, h−1 | 0.201 ± 0.020 | 0.202 ± 0.031 | |||||
| k2, h−1 | 0.443 ± 0.045 | 0.144 ± 0.006 | 0.166 ± 0.008 | 0.184 ± 0.011 | 0.202 ± 0.015 | 0.206 ± 0.017 | 0.213 ± 0.020 |
| λ1, h−1 | 0.333 ± 0.055 | 0.430 ± 0.072 | 0.517 ± 0.086 | 0.593 ± 0.097 | 0.655 ± 0.105 | ||
| N | 8.34 × 104 ± 83191 | ||||||
| TT, h | 17.3 ± 1.27 | 11.8 ± 0.48 | 10.1 ± 0.41 | 8.49 ± 0.38 | 7.47 ± 0.39 | 6.32 ± 0.43 | 5.41 ± 0.46 |
| CMRT1, h | 6.18 ± 0.53 | 6.20 ± 0.40 | 8.12 ± 0.64 | 9.81 ± 0.85 | 10.9 ± 0.97 | 12.0 ± 1.10 | 12.8 ± 1.17 |
| CMRT2, h | 2.57 ± 0.15 | 7.23 ± 0.29 | 6.39 ± 0.34 | 6.09 ± 0.44 | 5.85 ± 0.51 | 5.86 ± 0.52 | 5.87 ± 0.55 |
| TTMRT, h | 26.0 ± 0.92 | 25.2 ± 0.79 | 24.6 ± 0.71 | 24.4 ± 0.68 | 24.3 ± 0.65 | 24.1 ± 0.63 | 24.1 ± 0.63 |
| Item | ALF | ORCH | CB 4 | CB 6 | CB 8 | SEM | Diet 2 p-Value | Contrast 3 p-Value |
|---|---|---|---|---|---|---|---|---|
| Particulate | ||||||||
| Dhanoa A | ||||||||
| A | 2.4 × 109 | 3.0 × 109 | 1.3 × 1012 | 3.1 × 109 | 3.5 × 109 | 4.3 × 108 | ||
| k1, h−1 | 0.210 | 0.287 | 0.248 | 0.294 | 0.259 | 0.0536 | 0.223 | 0.239 |
| k2, h−1 | 0.357 | 0.380 | 0.336 | 0.372 | 0.328 | 0.0450 | 0.338 | 0.312 |
| N | 107 | 34 | 29 | 36 | 33 | 83191 | 0.179 | 0.054 |
| TT, h | 22.0 y | 23.5 x,y | 27.9 x,y | 26.9 x,y | 28.7 x | 1.27 | 0.035 | 0.004 |
| CMRT1, h | 5.59 x | 3.77 x,y | 3.82 x,y | 3.63 y | 4.17 x,y | 0.532 | 0.049 | 0.051 |
| CMRT2, h | 2.92 | 2.68 | 3.03 | 2.70 | 3.12 | 0.148 | 0.303 | 0.479 |
| TTMRT, h | 30.5 y | 30.0 y | 34.7 x,y | 33.2 x,y | 35.9 x | 0.921 | 0.020 | 0.003 |
| G5G1 B | ||||||||
| C, mg Yb/kg digesta | 1134 | 1007 | 906 | 780 | 681 | 56.3 | ||
| λ1, h−1 | 0.396 | 0.342 | 0.277 | 0.319 | 0.272 | 0.018 | 0.069 | 0.014 |
| k2, h−1 | 0.249 | 0.279 | 0.280 | 0.274 | 0.272 | 0.009 | 0.751 | 0.489 |
| TT, h | 11.4 | 9.02 | 10.3 | 11.1 | 11.4 | 0.616 | 0.362 | 0.948 |
| CMRT1, h | 13.6 b,y | 15.4 a,b | 18.2 a,b,x | 16.1 a,b | 18.5 a | 0.634 | 0.008 | 0.002 |
| CMRT2, h | 4.47 | 3.64 | 3.59 | 3.71 | 3.71 | 0.182 | 0.508 | 0.218 |
| TTMRT, h | 29.5 a,b | 28.1 b | 32.1 a,b | 30.9 a,b | 33.6 a | 0.900 | 0.022 | 0.007 |
| Liquid | ||||||||
| Dhanoa A | ||||||||
| A | 1919 | 3.0 × 109 | 1.5 × 109 | 1.3 × 109 | 9.8 × 107 | 2.7 × 1011 | ||
| k1, h−1 | 0.106 x | 0.234 | 0.145 | 0.111 y | 0.120 | 0.0165 | 0.042 | 0.023 |
| k2, h−1 | 0.708 x | 0.440 | 0.373 | 0.376 | 0.320 y | 0.0108 | 0.042 | 0.016 |
| N | 4.2 × 105 | 50 | 49 | 33 | 31.2 | 13 | 0.661 | 0.075 |
| TT, h | 11.3 b | 13.6 a,b | 19.9 a | 20.7 a | 20.7 a | 1.14 | 0.005 | <0.001 |
| CMRT1, h | 9.52 a | 6.14 b | 5.57 b | 4.0 b | 5.58 b | 0.291 | <0.001 | <0.001 |
| CMRT2, h | 1.75 b | 2.48 a,b | 2.75 a,b | 2.70 a,b | 3.17 a | 0.096 | 0.009 | 0.005 |
| TTMRT, h | 22.6 c,z | 22.3 b,c,y,z | 28.2 a,b,x,y | 27.5 a,b,c,x | 29.5 a | 1.00 | 0.002 | <0.001 |
| G4G1 B | ||||||||
| C, mg Yb/kg digesta | 465 | 801 | 614 | 704 | 565 | 43 | ||
| λ1, h−1 | 1.095 a,x | 0.491 a,b,y | 0.359 b | 0.329 b | 0.310 b | 0.0856 | 0.002 | 0.001 |
| k2, h−1 | 0.107 b,y | 0.216 a,b,x | 0.203 a,b | 0.247 a | 0.236 a,b,x | 0.0152 | 0.010 | 0.012 |
| TT, h | 8.42 x | 5.45 y | 8.15 | 7.34 | 8.00 | 0.391 | 0.042 | 0.194 |
| CMRT1, h | 4.71 b | 10.3 a | 12.6 a | 13.4 a | 13.6 a | 0.973 | <0.001 | <0.001 |
| CMRT2, h | 9.44 a | 5.32 b | 5.61 b | 4.20 b | 4.69 b | 0.513 | 0.001 | 0.002 |
| TTMRT, h | 22.6 b,c | 21.0 c | 26.4 a | 25.0 a,b | 26.3 a | 0.650 | <0.001 | <0.001 |
| Equation | ALF | ORCH | CB 4 | CB 6 | CB 8 | SEM | Diet 2 p-Value | Contrast 3 p-Value |
|---|---|---|---|---|---|---|---|---|
| Blaxter et al. [32] | ||||||||
| Particulate | 29.2 a,b | 26.9 b | 31.1 a,b | 30.0 a,b | 32.7 a | 0.88 | 0.010 | 0.006 |
| Liquid | 21.3 b,c,y | 20.1 c | 25.7 a | 24.3 a,b,x | 25.9 a | 0.70 | <0.001 | <0.001 |
| Thielemans et al. [33] | ||||||||
| Particulate | 28.9 a,b | 27.0 b | 30.4 a,b | 29.6 a,b | 32.6 a | 0.88 | 0.010 | 0.008 |
| Liquid | 21.2 b,c,y | 20.6 c | 25.4 a | 24.1 a,b,x | 25.9 a | 0.67 | <0.001 | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansen, T.L.; Chizek, E.L.; Zugay, O.K.; Miller, J.M.; Bobel, J.M.; Chouinard, J.W.; Adkin, A.M.; Skurupey, L.A.; Warren, L.K. Digestibility and Retention Time of Coastal Bermudagrass (Cynodon dactylon) Hay by Horses. Animals 2019, 9, 1148. https://doi.org/10.3390/ani9121148
Hansen TL, Chizek EL, Zugay OK, Miller JM, Bobel JM, Chouinard JW, Adkin AM, Skurupey LA, Warren LK. Digestibility and Retention Time of Coastal Bermudagrass (Cynodon dactylon) Hay by Horses. Animals. 2019; 9(12):1148. https://doi.org/10.3390/ani9121148
Chicago/Turabian StyleHansen, Tayler L., Elisabeth L. Chizek, Olivia K. Zugay, Jessica M. Miller, Jill M. Bobel, Jessie W. Chouinard, Angie M. Adkin, Leigh Ann Skurupey, and Lori K. Warren. 2019. "Digestibility and Retention Time of Coastal Bermudagrass (Cynodon dactylon) Hay by Horses" Animals 9, no. 12: 1148. https://doi.org/10.3390/ani9121148
APA StyleHansen, T. L., Chizek, E. L., Zugay, O. K., Miller, J. M., Bobel, J. M., Chouinard, J. W., Adkin, A. M., Skurupey, L. A., & Warren, L. K. (2019). Digestibility and Retention Time of Coastal Bermudagrass (Cynodon dactylon) Hay by Horses. Animals, 9(12), 1148. https://doi.org/10.3390/ani9121148




