Biogas Production by Anaerobic Co-Digestion of Dairy Wastewater with the Crude Glycerol from Slaughterhouse Sludge Cake Transesterification
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Crude Glycerol Collection and Preparation
2.2. Design of Digesters for Co-Digestion of Crude Glycerol and Dairy Cattle Wastewater
2.3. Time Course Experiments of Co-Digestion using Crude Glycerol and Dairy Cattle Wastewater as Feedstock
2.4. Sample Analysis
2.4.1. Water Quality of Wastewater Samples
2.4.2. Gas Contents of Gas Samples
2.4.3. Volatile Fatty Acids of Liquid Samples
2.5. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Feedstock for Anaerobic Co-Digestion
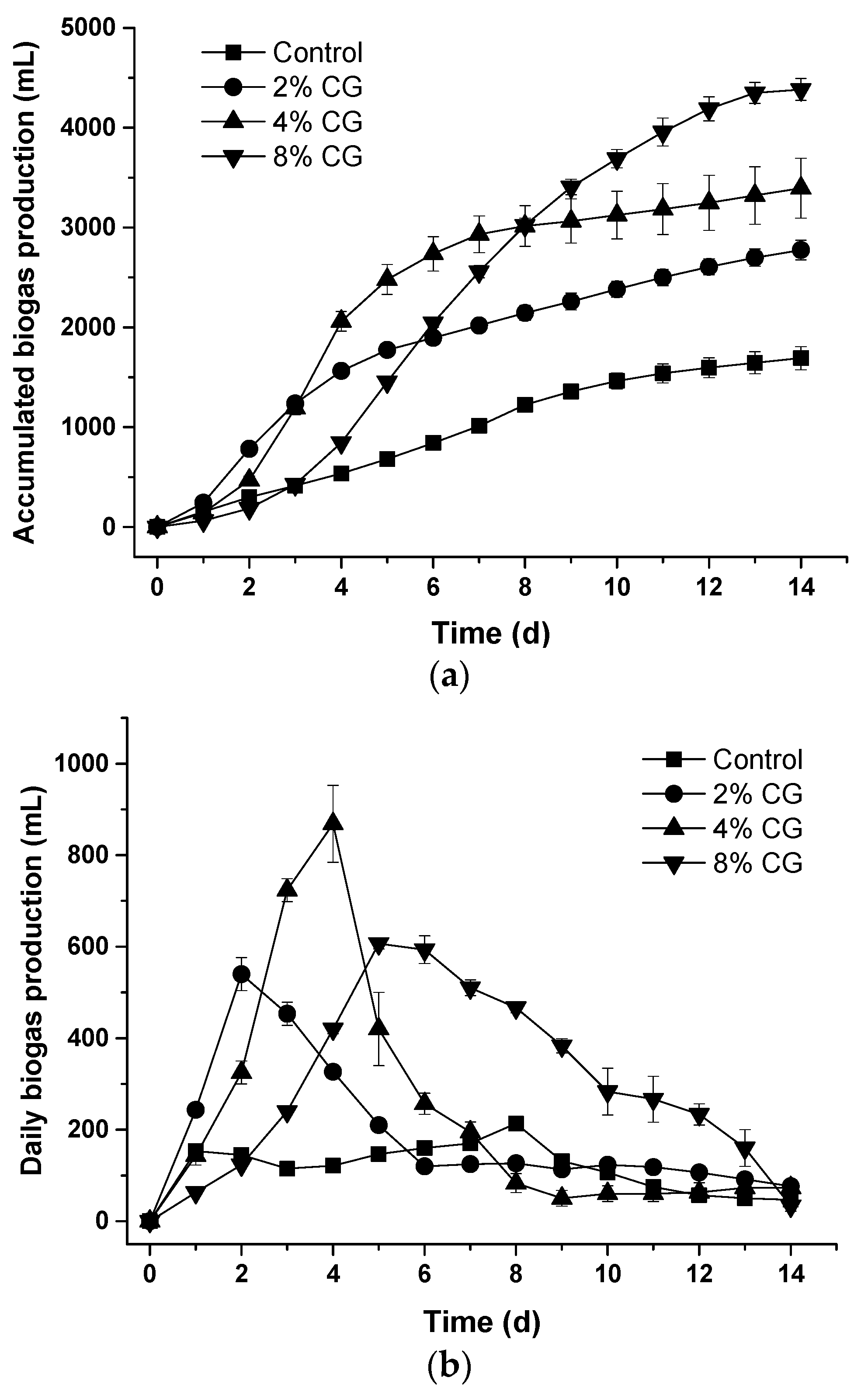
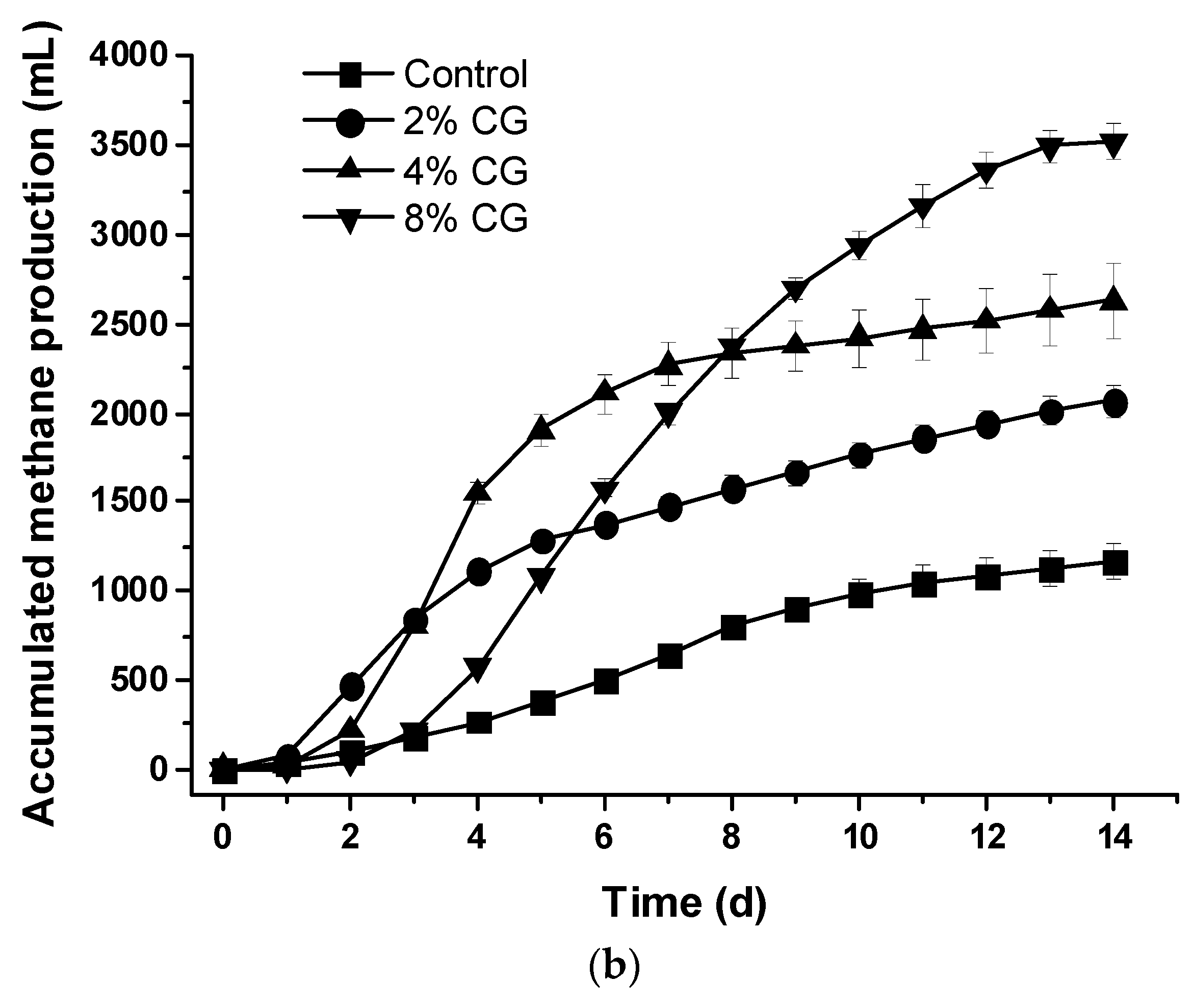
3.2. Biogas Production During the Co-Digestion of Dairy Cattle Wastewater and Crude Glycerol
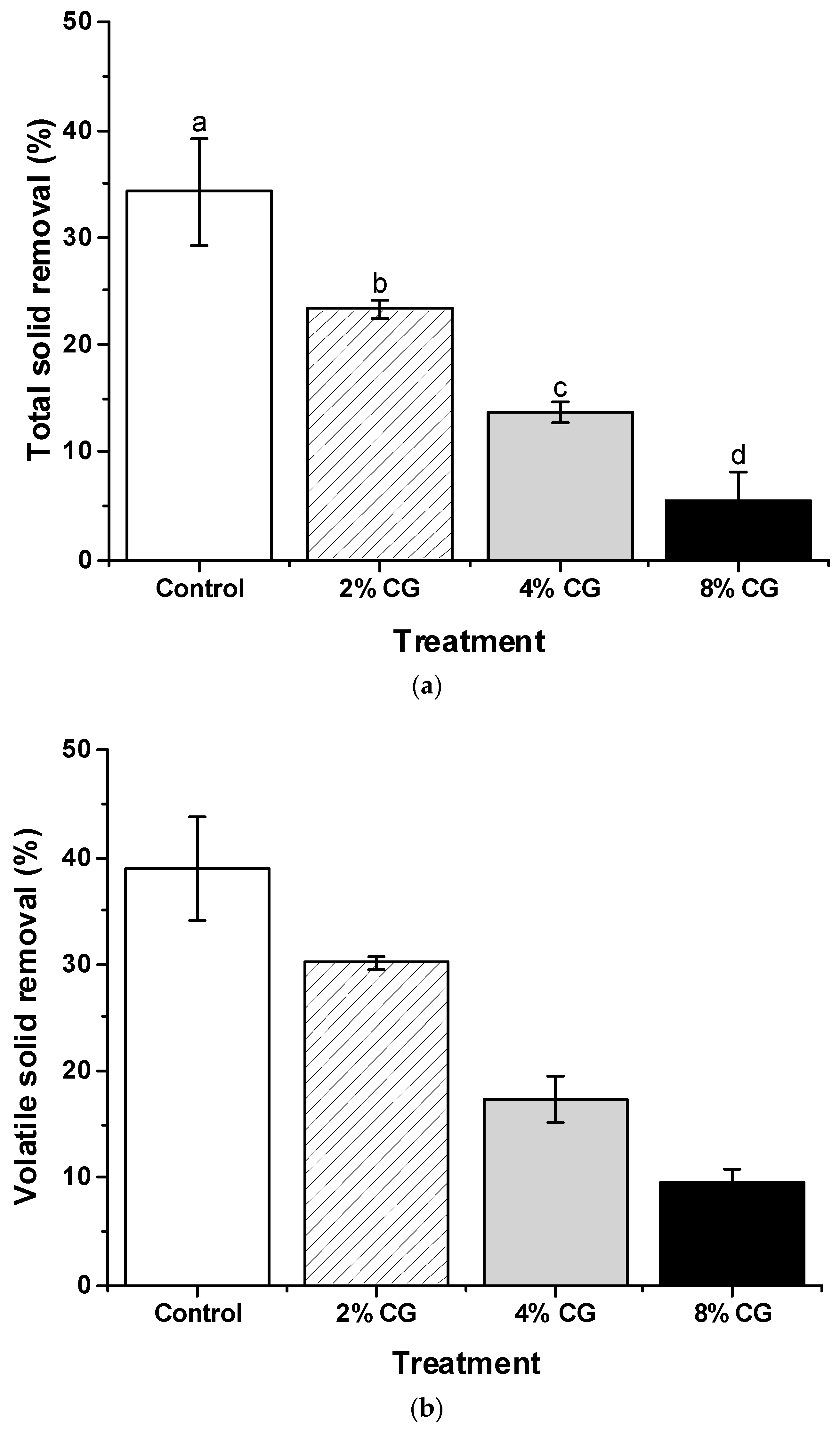
3.3. Removal Efficiency of TS and VS During the Co-Digestion Process
3.4. Comparison of the Specific Biogas Production Among the CG2, CG4, and CG8 Sets During the Co-Digestion Process
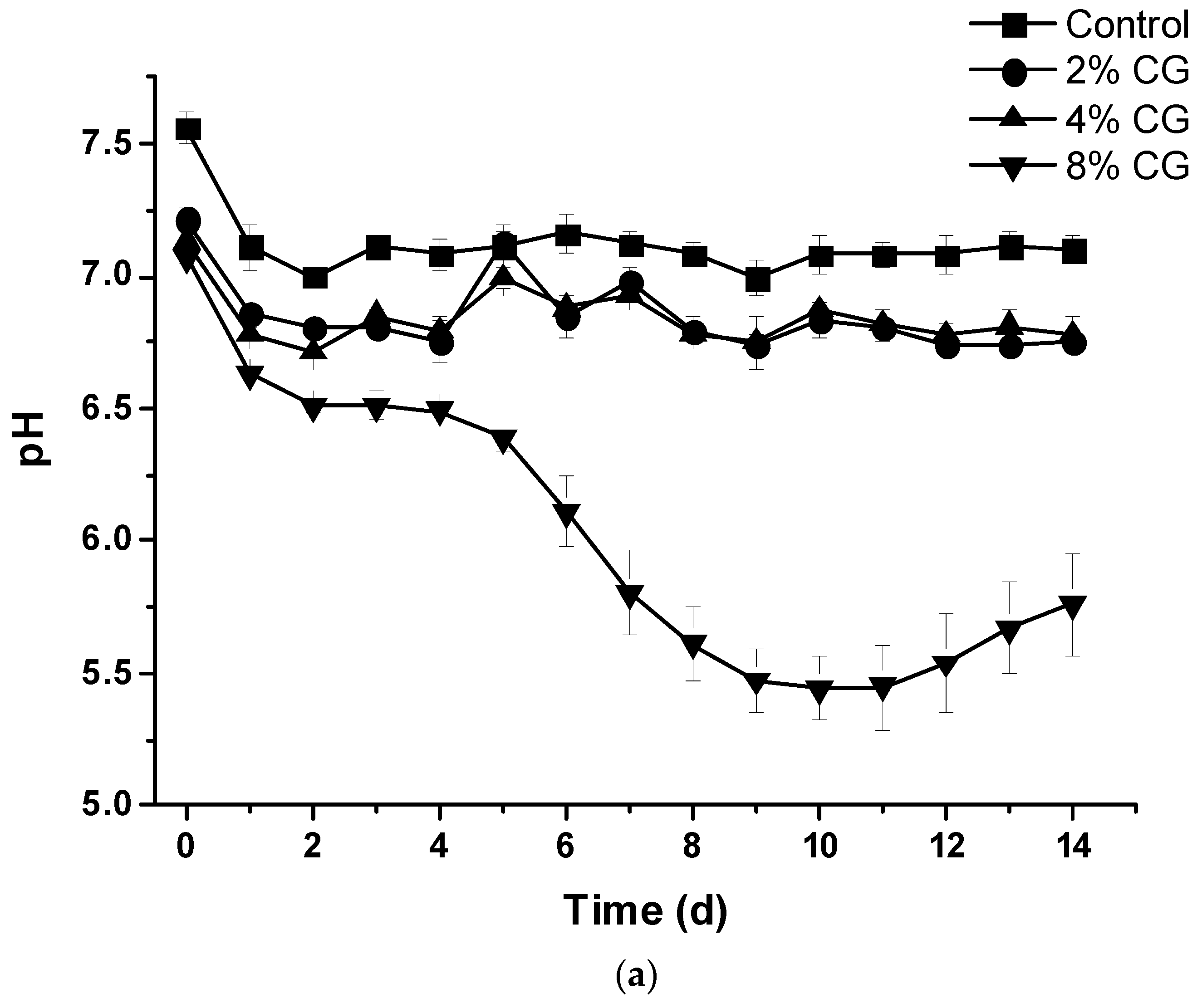
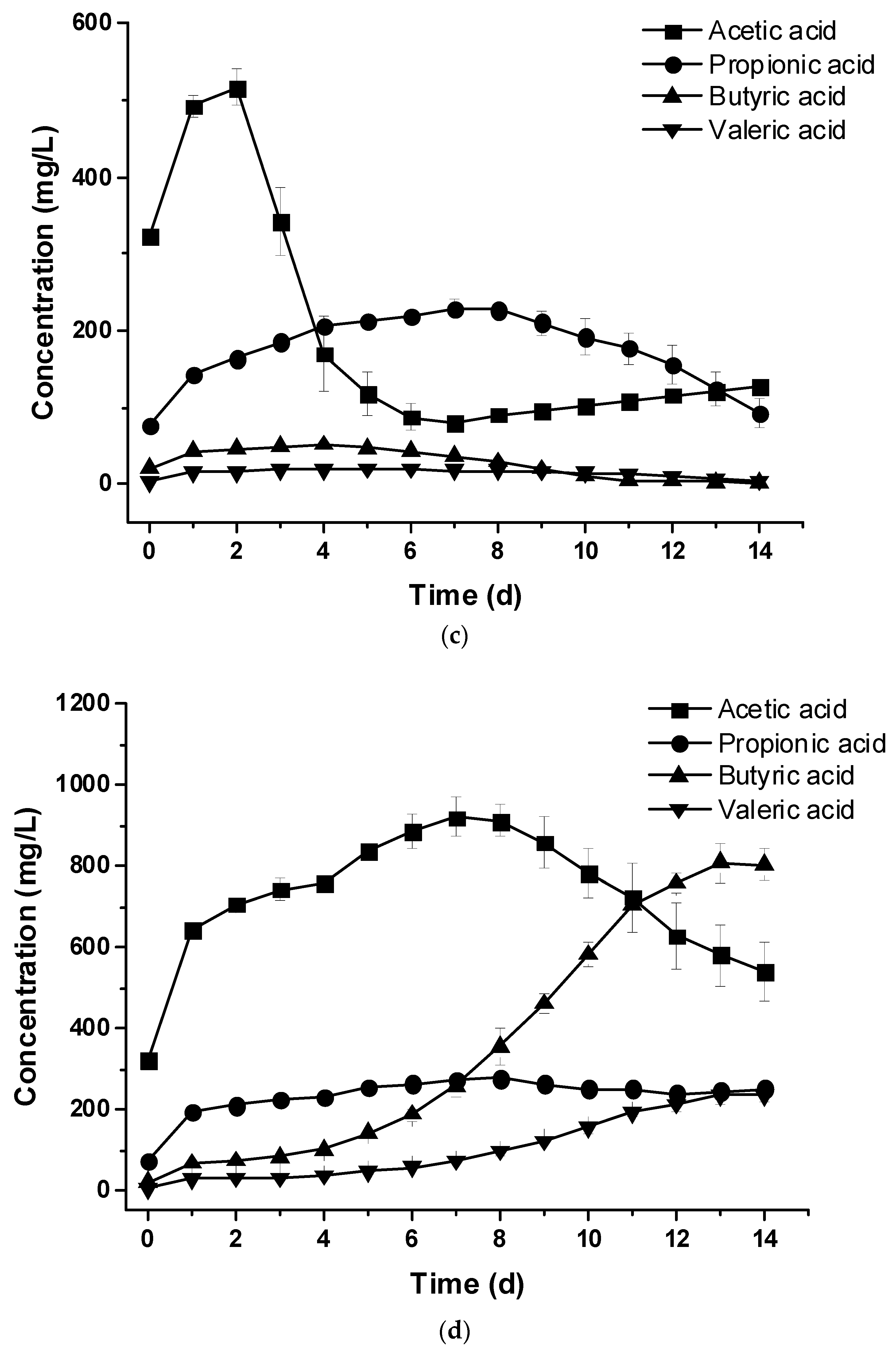
3.5. Changes of Water Quality Indexes During Anaerobic Co-Digestion of Crude Glycerol and Cattle Wastewater
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Park, S.; Zhu, J. Solid-state anaerobic digestion for methane production from organic waste. Renew. Sustain. Energy Rev. 2011, 15, 821–826. [Google Scholar] [CrossRef]
- Kim, J.; Oh, B.; Chun, Y.; Kim, S. Effects of temperature and hydraulic retention time on anaerobic digestion of food waste. J. Biosci. Bioeng. 2006, 102, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Trzcinski, A.; Stuckey, D. Treatment of municipal solid waste leachate using a submerged anaerobic membrane bioreactor at mesophilic and psychrophilic temperatures: Analysis of recalcitrants in the permeate using GC-MS. Water Res. 2010, 44, 671–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández, J.; Pérez, M.; Romero, L.I. Effect of substrate concentration on dry mesophilic anaerobic digestion of organic fraction of municipal solid waste (OFMSW). Bioresour. Technol. 2008, 99, 6075–6080. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.; Hobbs, P.; Holliman, P.; Jones, D. Optimisation of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef]
- Zhu, B.; Gikas, P.; Zhang, R.; Lord, J.; Jenkins, B.; Li, X. Characteristics and biogas production potential of municipal solid wastes pretreated with a rotary drum reactor. Bioresour. Technol. 2009, 100, 1122–1129. [Google Scholar] [CrossRef]
- Khalid, A.; Arshad, M.; Anjum, M.; Mahmood, T.; Dawson, L. The anaerobic digestion of solid organic waste. Waste Manag. 2011, 31, 1737–1744. [Google Scholar] [CrossRef]
- Kim, J.; Park, C.; Kim, T.-H.; Lee, M.; Kim, S.; Kim, S.-W.; Lee, J. Effects of various pretreatments for enhanced anaerobic digestion with waste activated sludge. J. Biosci. Bioeng. 2003, 95, 271–275. [Google Scholar] [CrossRef]
- Ren, N.; Liu, M.; Wang, A.; Ding, J.; Li, H. Organic acids conversion in methanogenic-phase reactor of the two-phase anaerobic process. Huan Jing Ke Xue 2003, 24, 89–93. [Google Scholar]
- Wang, Y.; Zhang, Y.; Wang, J.; Meng, L. Effects of volatile fatty acid concentrations on methane yield and methanogenic bacteria. Biomass Bioenergy 2009, 33, 848–853. [Google Scholar] [CrossRef]
- Siegert, I.; Banks, C. The effect of volatile fatty acid additions on the anaerobic digestion of cellulose and glucose in batch reactors. Process. Biochem. 2005, 40, 3412–3418. [Google Scholar] [CrossRef]
- Ziels, R.M.; Carlsson, A.; Beck, D.A.C.; Ejlertsson, J.; Yekta, S.S.; Bjorn, A.; Stensel, H.D.; Svensson, B.H. Microbial community adaptation influences long-chain fatty acid conversion during anaerobic codigestion of fats, oils, and grease with municipal sludge. Water Res. 2016, 103, 372–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Zhao, Q.B.; Laurens, L.L.M.; Jarvis, E.E.; Nagle, N.J.; Chen, S.; Frear, C.S. Mechanism, kinetics and microbiology of inhibition caused by long-chain fatty acids in anaerobic digestion of algal biomass. Biotechnol. Biofuels 2015, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- Dasa, K.T.; Westman, S.Y.; Millati, R.; Cahyanto, M.N.; Taherzadeh, M.J.; Niklasson, C. Inhibitory effect of long-chain fatty acids on biogas production and the protective effect of membrane bioreactor. BioMed Res. Int. 2016, 2016, 7263974. [Google Scholar] [PubMed]
- Wang, X.; Lu, X.; Li, F.; Yang, G. Effects of temperature and carbon-nitrogen (C/N) ratio on the performance of anaerobic co-digestion of dairy manure, chicken manure and rice straw: Focusing on ammonia inhibition. PLoS ONE 2014, 9, e97265. [Google Scholar] [CrossRef] [PubMed]
- Ağdağ, O.; Sponza, D. Co-digestion of mixed industrial sludge with municipal solid wastes in anaerobic simulated landfilling bioreactors. J. Hazard. Mater. 2007, 140, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Dasari, M.A.; Kiatsimkul, P.P.; Sutterlin, W.R.; Suppes, G.J. Low-pressure hydrogenolysis of glycerol to propylene glycol. Appl. Catal. A Gen. 2005, 281, 225–231. [Google Scholar] [CrossRef]
- Kolesárová, N.; Hutňan, M.; Bodík, I.; Špalková, V. Utilization of Biodiesel By-Products for Biogas Production. J. Biomed. Biotechnol. 2011, 2011, 126798. [Google Scholar] [CrossRef]
- Pachauri, N.; He, B. Value-added utilization of crude glycerol from biodiesel production: A survey of current research activities. In Proceedings of the ASABE Annual International Meeting, Portland, OR, USA, 9–12 July 2006; Available online: https://www.webpages.uidaho.edu/~bhe/pdfs/asabe066223.pdf (accessed on 27 August 2019).
- Ayoub, M.; Abdullah, A. Critical review on the current scenario and significance of crude glycerol resulting from biodiesel industry towards more sustainable renewable energy industry. Renew. Sustain. Energy Rev. 2012, 16, 2671–2686. [Google Scholar] [CrossRef]
- Fountoulakis, M.S.; Petousi, I.; Manios, T. Co-digestion of sewage sludge with glycerol to boost biogas production. Waste Manag. 2010, 30, 1849–1853. [Google Scholar] [CrossRef]
- Robra, S.; Serpa da Cruz, R.; de Oliveira, A.M.; Almeida Neto, J.A.; Santos, J.V. Generation of biogas using crude glycerin from biodiesel production as a supplement to cattle slurry. Biomass Bioenergy 2010, 34, 1330–1335. [Google Scholar] [CrossRef]
- Castrillón, L.; Fernández-Nava, Y.; Ormaechea, P.; Marañón, E. Optimization of biogas production from cattle manure by pre-treatment with ultrasound and co-digestion with crude glycerin. Bioresour. Technol. 2011, 102, 7845–7849. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 1995; pp. 2-53–2-58, 5-3–5-6, 5-12–5-16. [Google Scholar]
- Wee, C.Y.; Su, J.J. Biofuel produced from solid-state anaerobic digestion of dairy cattle manure in coordination with black soldier fly larvae decomposition. Energies 2019, 12. [Google Scholar] [CrossRef]
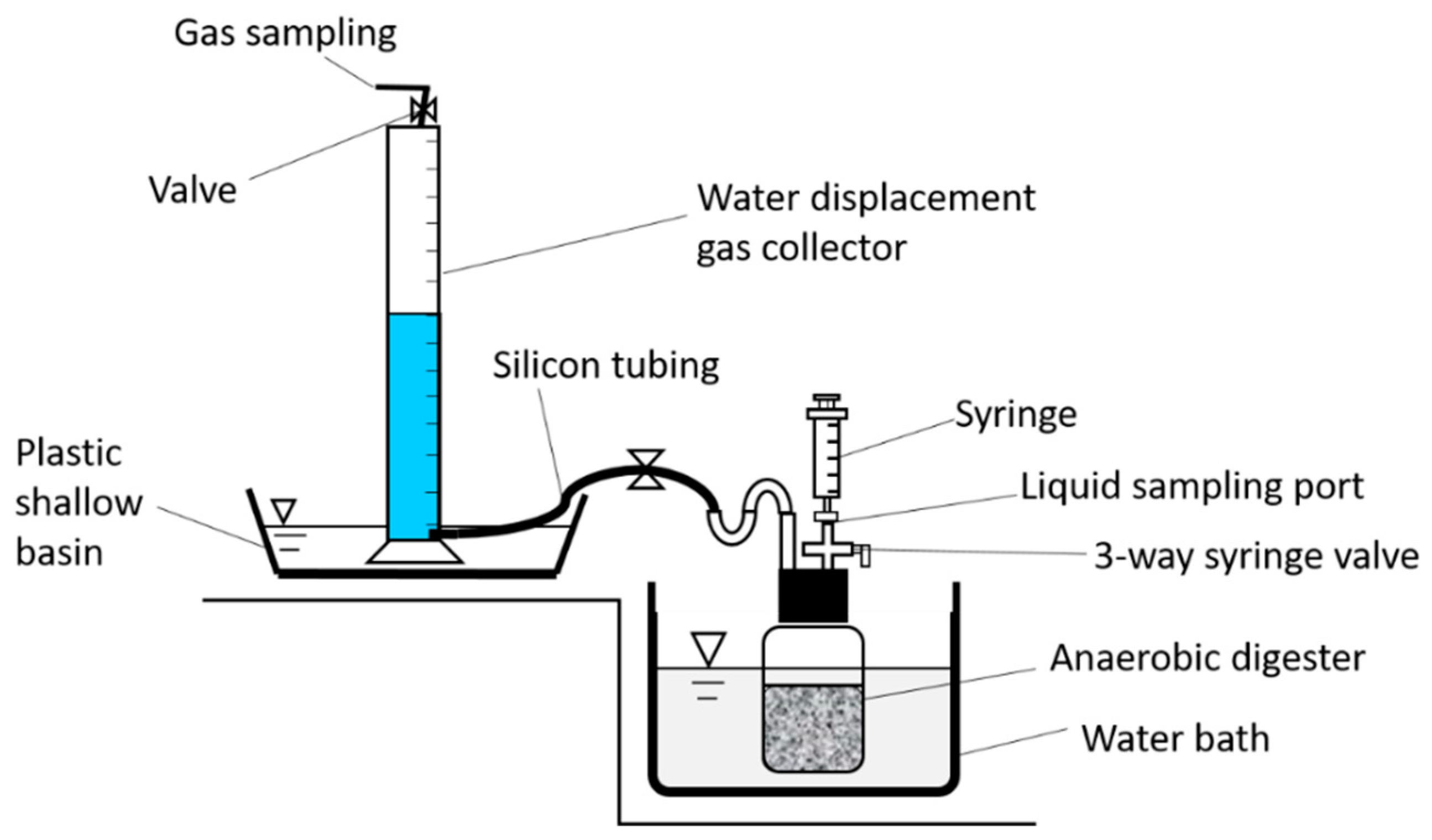







| Feedstock (mL) | Control Set | Experimental Sets | ||
|---|---|---|---|---|
| CG2 | CG4 | CG8 | ||
| Cattle Sludge (as inoculum) | 300 | 300 | 300 | 300 |
| Dairy cattle wastewater | 700 | 680 | 660 | 620 |
| Crude glycerol | 0 | 20 | 40 | 80 |
| Total (mL) | 1000 | 1000 | 1000 | 1000 |
| Parameters | Sludge | Dairy Wastewater | Crude Glycerol | Control | CG2 | CG4 | CG8 |
|---|---|---|---|---|---|---|---|
| pH | 7.16 | 7.86 | 6.83 | 7.56 ± 0.06 | 7.21 ± 0.05 | 7.13 ± 0.04 | 7.07 ± 0.03 |
| Moisture (%) | 96.91 | 99.45 | 75.56 | 98.48 ± 0.08 | 98.06 ± 0.05 | 97.63 ± 0.01 | 96.85 ± 0.07 |
| TS (%) | 3.09 | 0.55 | 24.44 | 1.52 ± 0.08 | 1.94 ± 0.05 | 2.37 ± 0.01 | 3.15 ± 0.07 |
| VS * (%) | 80.71 | 77.84 | 54.42 | 77.85 ± 0.27 | 71.46 ± 0.46 | 67.93 ± 0.71 | 63.24 ± 1.14 |
| Experimental Sets | Day 0 | Day 14 | Removal (%) |
|---|---|---|---|
| COD (mg/L) | |||
| Control | 2598.7 ± 212.5 d | 1614.4 ± 93.3 d | 37.9 ± 8.9 b |
| 2% CG | 7598.9 ± 362.7 c | 3057.8 ± 304.2 c | 59.8 ± 5.3 a |
| 4% CG | 13134.2 ± 370.1 b | 6304.2 ± 598.3 b | 52.0 ± 3.2 a |
| 8% CG | 22689.0 ± 399.7 a | 14316.0 ± 189.1 a | 36.9 ± 1.9 b |
| p-value | <0.05 | <0.05 | NS |
| BOD (mg/L) | |||
| Control | 1219.1 ± 53.5 d | 108.0 ± 19.4 d | 91.2 ± 1.3 a |
| 2% CG | 3417.5 ± 190.5 c | 309.5 ± 34.7 c | 90.9 ± 0.8 a |
| 4% CG | 5954.0 ± 129.5 b | 559.4 ± 31.9 b | 90.6 ± 0.7 a |
| 8% CG | 10065.0 ± 420.8 a | 3830.3 ± 176.7 a | 61.9 ± 1.5 b |
| p-value | <0.05 | <0.05 | NS |
| SS (mg/L) | |||
| Control | 270.0 ± 36.1 c | 66.7 ± 23.1 b | 75.6 ± 7.2 a |
| 2% CG | 426.7 ± 23.1 b | 100.0 ± 13.2 b | 76.4 ± 4.5 a |
| 4% CG | 493.3 ± 64.3 b | 156.7 ± 27.5 b | 68.4 ± 1.6 a |
| 8% CG | 693.3 ± 23.1 a | 610.0 ± 10.0 a | 12.0 ± 1.8 b |
| p-value | NS | NS | NS |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, Y.-C.; Su, J.-J. Biogas Production by Anaerobic Co-Digestion of Dairy Wastewater with the Crude Glycerol from Slaughterhouse Sludge Cake Transesterification. Animals 2019, 9, 618. https://doi.org/10.3390/ani9090618
Chou Y-C, Su J-J. Biogas Production by Anaerobic Co-Digestion of Dairy Wastewater with the Crude Glycerol from Slaughterhouse Sludge Cake Transesterification. Animals. 2019; 9(9):618. https://doi.org/10.3390/ani9090618
Chicago/Turabian StyleChou, Yu-Chun, and Jung-Jeng Su. 2019. "Biogas Production by Anaerobic Co-Digestion of Dairy Wastewater with the Crude Glycerol from Slaughterhouse Sludge Cake Transesterification" Animals 9, no. 9: 618. https://doi.org/10.3390/ani9090618
APA StyleChou, Y.-C., & Su, J.-J. (2019). Biogas Production by Anaerobic Co-Digestion of Dairy Wastewater with the Crude Glycerol from Slaughterhouse Sludge Cake Transesterification. Animals, 9(9), 618. https://doi.org/10.3390/ani9090618





