Maturation of the Goat Rumen Microbiota Involves Three Stages of Microbial Colonization
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Handling and Sample Collection
2.2. DNA Extraction, PCR Amplification, and Illumina MiSeq Sequencing
2.3. Data Analysis
2.4. Ethical Statement
3. Results
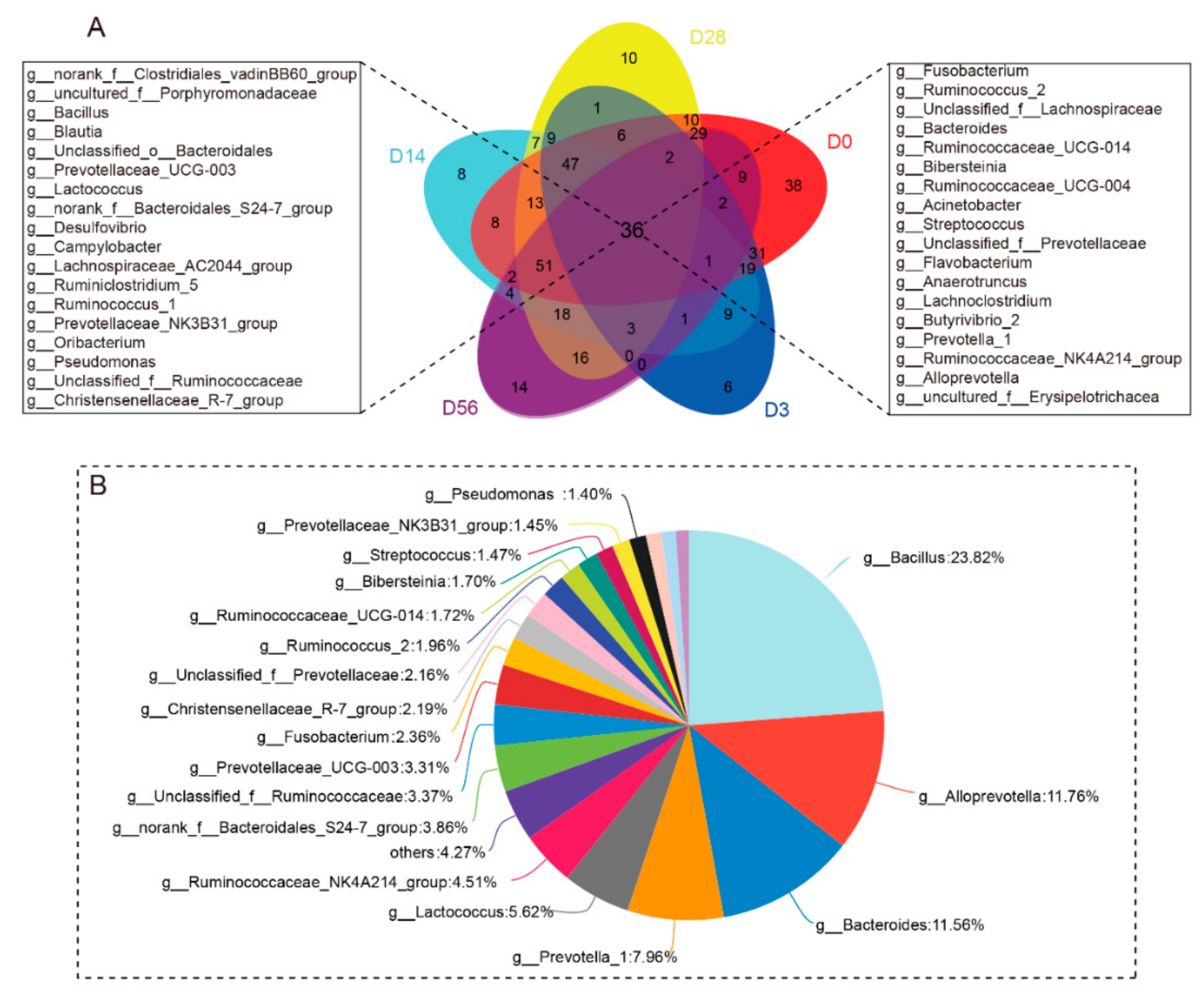
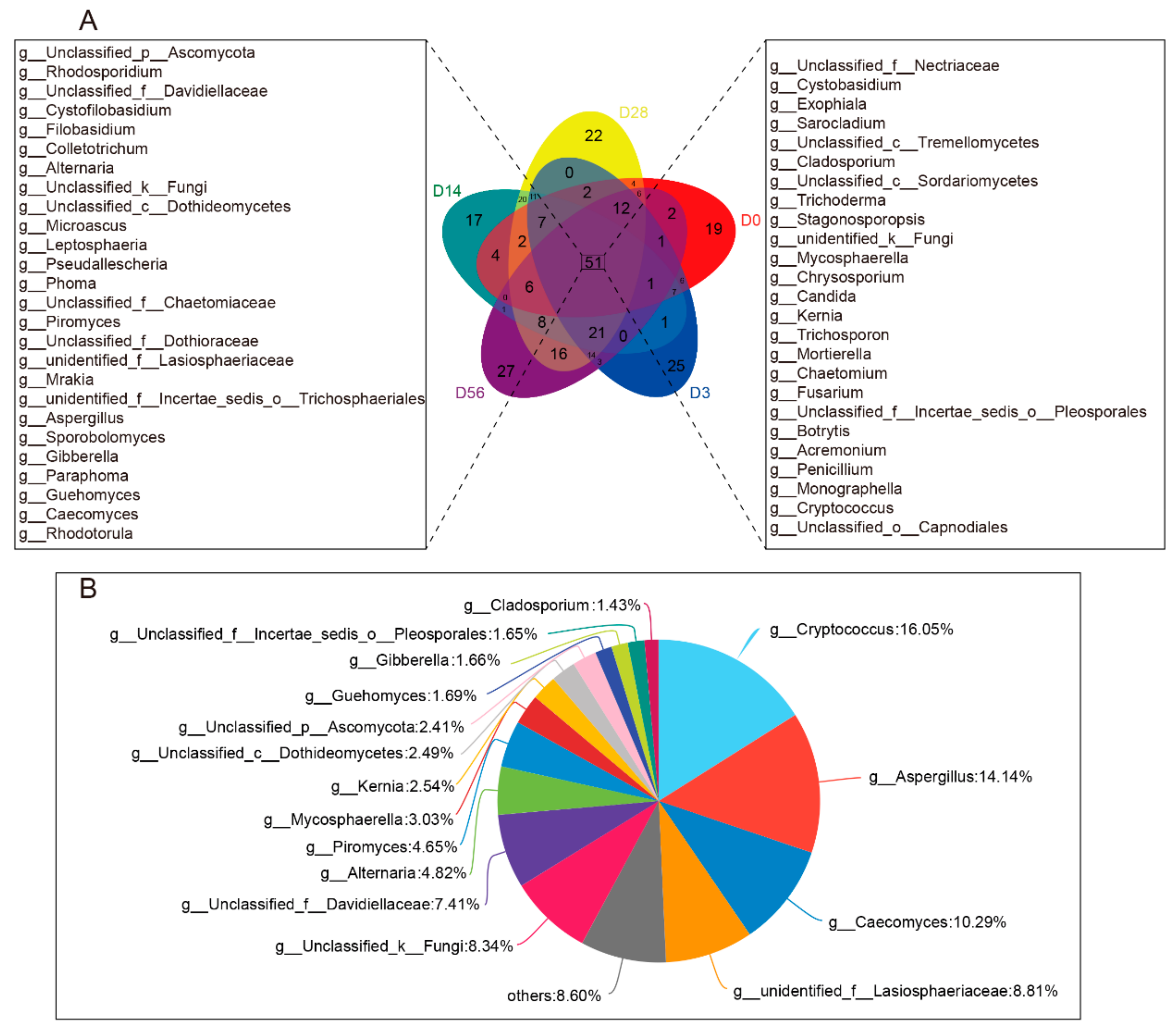
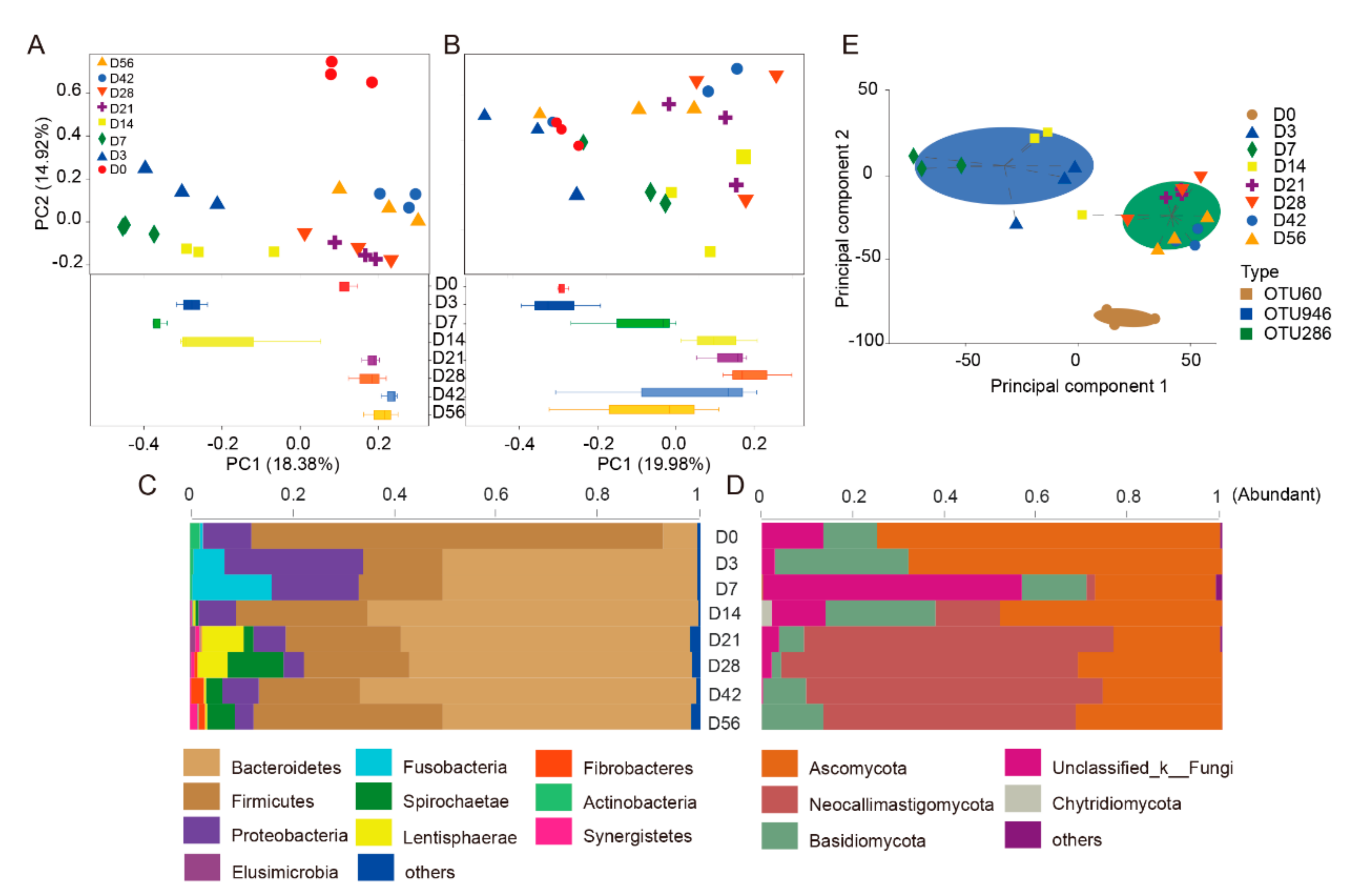
3.1. Diversity and Commonality Analysis
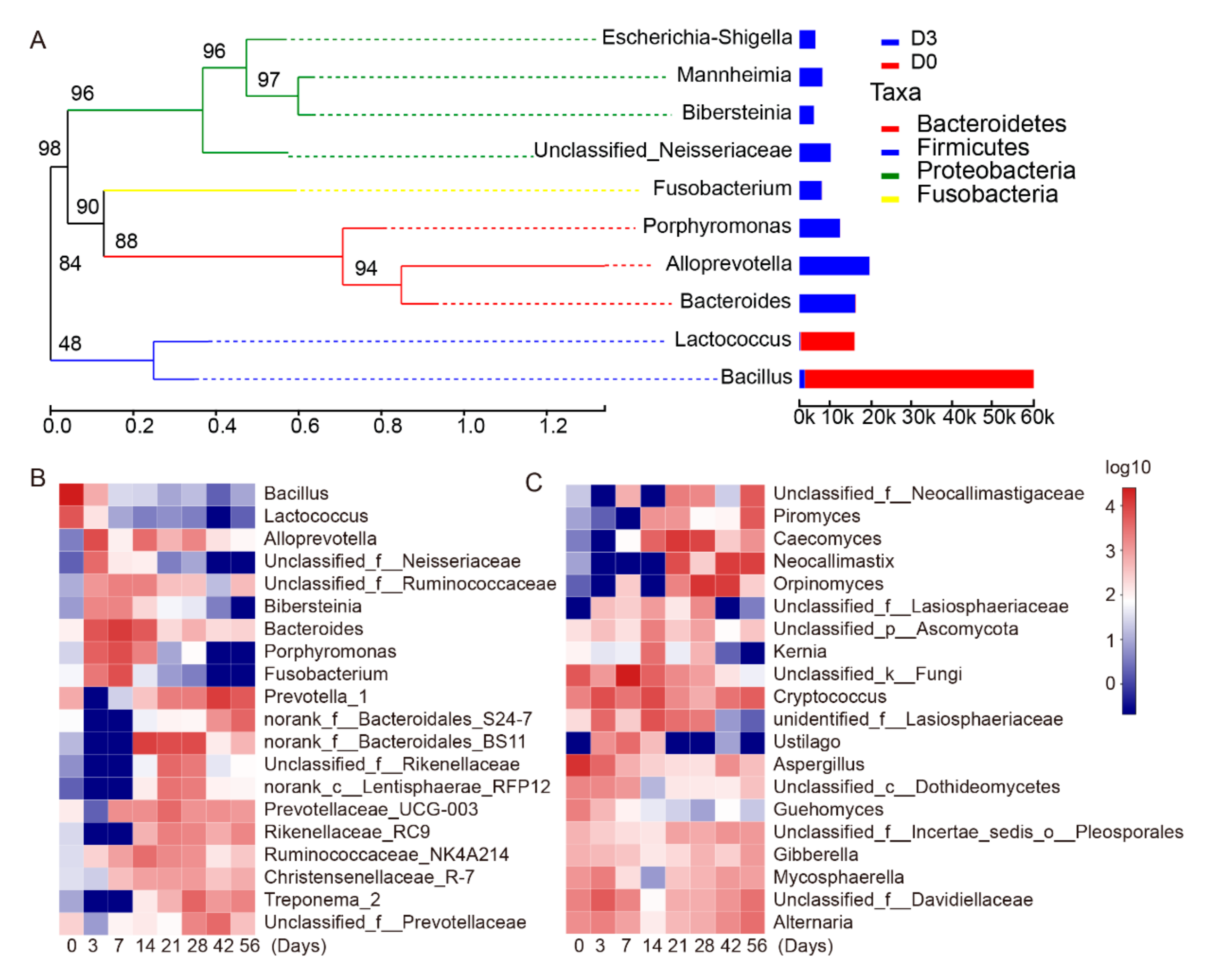
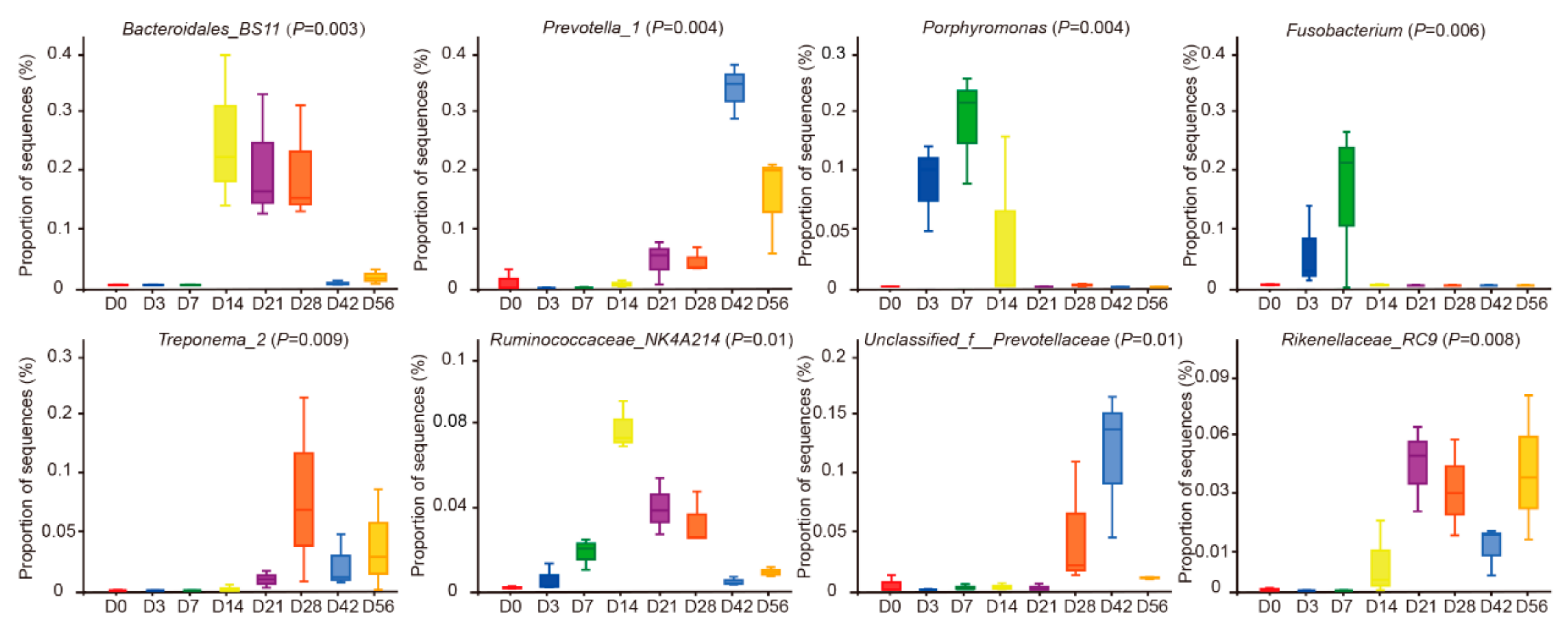
3.2. Temporal Succession of Ruminal Bacterial Community at Pre-Weaning
3.3. Temporal Succession of Ruminal Fungal Community at Pre-Weaning
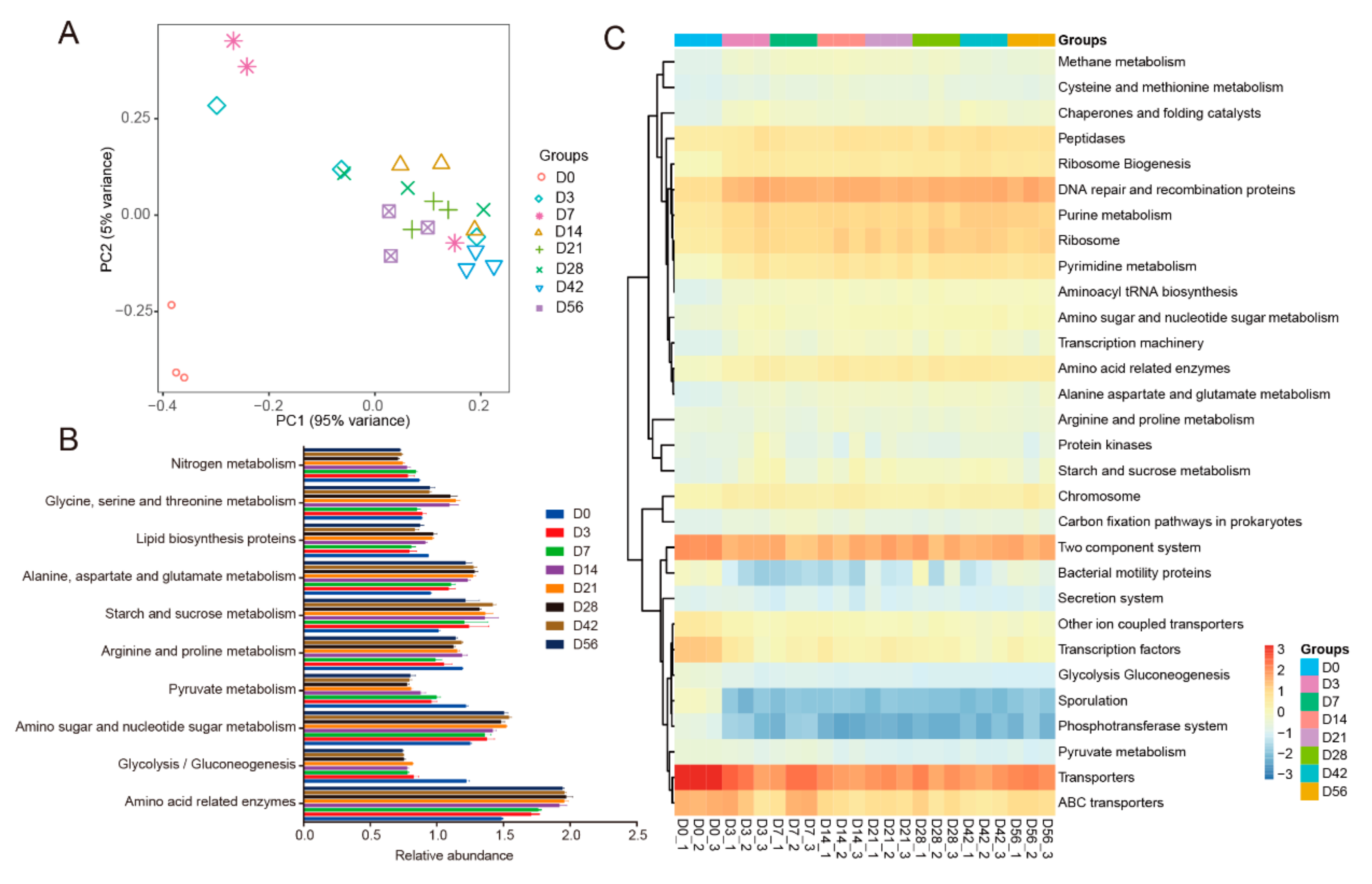
3.4. Predicted Molecular Functions of the Bacterial Microbiota
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huws, S.A.; Creevey, C.J.; Oyama, L.B.; Mizrahi, I.; Denman, S.E.; Popova, M.; Munoz-Tamayo, R.; Forano, E.; Waters, S.M.; Hess, M.; et al. Addressing global ruminant agricultural challenges through understanding the rumen microbiome: Past, present, and future. Front. Microbiol. 2018, 9, 2161. [Google Scholar] [CrossRef]
- Jami, E.; Israel, A.; Kotser, A.; Mizrahi, I. Exploring the bovine rumen bacterial community from birth to adulthood. ISME J. 2013, 7, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Sasson, G.; Benshabat, S.K.; Seroussi, E.; Doronfaigenboim, A.; Shterzer, N.; Yaacoby, S.; Miller, M.E.B.; White, B.A.; Halperin, E.; Mizrahi, I. Heritable bovine rumen bacteria are phylogenetically related and correlated with the cow’s capacity to harvest energy from its feed. MBio 2017, 8, e00703-17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, D.; Wang, L.; Hao, J.; Wang, J.; Zhou, X.; Wang, W.; Qiu, Q.; Huang, X.; Zhou, J.; et al. Convergent evolution of rumen microbiomes in high-altitude mammals. Curr. Biol. 2016, 26, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Yeoman, C.J.; Ishaq, S.L.; Bichi, E.; Olivo, S.K.; Lowe, J.; Aldridge, B.M. Biogeographical differences in the influence of maternal microbial sources on the early successional development of the bovine neonatal gastrointestinal tract. Sci. Rep. 2018, 8, 3197. [Google Scholar] [CrossRef]
- Owens, F.N.; Basalan, M. Ruminal Fermentation. In Rumenology; Springer: Cham, Switzerland, 2016; pp. 63–102. [Google Scholar]
- Taschuk, R.; Griebel, P.J. Commensal microbiome effects on mucosal immune system development in the ruminant gastrointestinal tract. Animal Health Res. Rev. 2012, 13, 129–141. [Google Scholar] [CrossRef]
- Swartz, J.D.; Lachman, M.; Westveer, K.; O’Neill, T.; Geary, T.; Kott, R.W.; Berardinelli, J.G.; Hatfield, P.G.; Thomson, J.M.; Roberts, A. Characterization of the vaginal microbiota of ewes and cows reveals a unique microbiota with low levels of lactobacilli and near-neutral ph. Front. Vet. Sci. 2014, 1, 19. [Google Scholar] [CrossRef]
- Francino, M.P. Early development of the gut microbiota and immune health. Pathogens 2014, 3, 769–790. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef]
- Malmuthuge, N.; Griebel, P.J.; Le, L.G. The gut microbiome and its potential role in the development and function of newborn calf gastrointestinal tract. Front. Vet. Sci. 2015, 2, 459–460. [Google Scholar] [CrossRef]
- Pieter, V.D.A.; Tom, V.D.W.; Willy, V.; Sam, P. The host selects mucosal and luminal associations of coevolved gut microorganisms: A novel concept. Fems Microbiol. Rev. 2011, 35, 681–704. [Google Scholar]
- Han, X.; Yang, Y.; Yan, H.; Wang, X.; Qu, L.; Chen, Y. Rumen bacterial diversity of 80 to 110-day-old goats using 16s rrna sequencing. PLoS ONE 2015, 10, e0117811. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Huang, J.; Zhou, C.; Tan, Z. Taxonomic identification of ruminal epithelial bacterial diversity during rumen development in goats. Appl. Environ. Microbiol. 2015, 81, 3502–3509. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Zhang, K.; Guo, M.; Li, G.; Li, C.; Li, B.; Yang, Y.; Chen, Y.; Wang, X. Exploring the spatial-temporal microbiota of compound stomachs in a pre-weaned goat model. Front. Microbiol. 2018, 9, 1846. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhang, K.; Li, C.; Wang, X.; Chen, Y.; Yang, Y. Characterization and comparison of microbiota in the gastrointestinal tracts of the goat (capra hircus) during preweaning development. Front. Microbiol. 2019, 10, 2125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Teng, Y.; Zhang, Y.; Ford, R.; Xu, Z. Effects of nitrification inhibitor 3, 4-dimethylpyrazole phosphate and fungicide iprodione on soil fungal biomass and community: Based on internal transcribed spacer region. J. Soils Sediments 2017, 17, 1021–1029. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I. Qiime allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C. Uparse: Highly accurate otu sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Blaalid, R.; Kumar, S.; Nilsson, R.H.; Abarenkov, K.; Kirk, P.; Kauserud, H. Its1 versus its2 as DNA metabarcodes for fungi. Mol. Ecol. Resour. 2013, 13, 218–224. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer Publishing Company, Inc.: Beilin, Germany, 2009; pp. 180–185. [Google Scholar]
- Wilkinson, T.J.; Huws, S.A.; Edwards, J.E.; Kingston-Smith, A.; Siu Ting, K.; Hughes, M.; Rubino, F.; Friedersdorff, M.; Creevey, C. Cowpi: A rumen microbiome focussed version of the picrust functional inference software. Front. Microbiol. 2018, 9, 1095. [Google Scholar] [CrossRef]
- Dowd, S.E.; Callaway, T.R.; Wolcott, R.D.; Sun, Y.; McKeehan, T.; Hagevoort, R.G.; Edrington, T.S. Evaluation of the bacterial diversity in the feces of cattle using 16s rdna bacterial tag-encoded flx amplicon pyrosequencing (btefap). BMC Microbiol. 2008, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Lettat, A.; Nozière, P.; Silberberg, M.; Morgavi, D.P.; Berger, C.; Martin, C. Rumen microbial and fermentation characteristics are affected differently by bacterial probiotic supplementation during induced lactic and subacute acidosis in sheep. BMC Microbiol. 2012, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Van Baale, M.; Sargeant, J.; Gnad, D.; DeBey, B.; Lechtenberg, K.; Nagaraja, T. Effect of forage or grain diets with or without monensin on ruminal persistence and fecal escherichia coli o157: H7 in cattle. Appl. Environ. Microbiol. 2004, 70, 5336–5342. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Lozupone, C.A.; Hamady, M.; Knight, R.; Gordon, J.I. Worlds within worlds: Evolution of the vertebrate gut microbiota. Nat. Rev. Microbiol. 2008, 6, 776–788. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Singh, K.M.; Ahir, V.B.; Tripathi, A.K.; Ramani, U.V.; Sajnani, M.; Koringa, P.G.; Jakhesara, S.; Pandya, P.R.; Rank, D.N.; Murty, D.S. Metagenomic analysis of surti buffalo (bubalus bubalis) rumen: A preliminary study. Mol. Biol. Rep. 2012, 39, 4841–4848. [Google Scholar] [CrossRef]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T.; et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef]
- Hsu, B.B.; Gibson, T.E.; Yeliseyev, V.; Liu, Q.; Lyon, L.; Bry, L.; Silver, P.A.; Gerber, G.K. Dynamic modulation of the gut microbiota and metabolome by bacteriophages in a mouse model. Cell Host Microbe 2019, 25, 803–814.e5. [Google Scholar] [CrossRef]
- Gritz, E.C.; Bhandari, V. The human neonatal gut microbiome: A brief review. Front. Pediatrics 2015, 3, 17. [Google Scholar]
- O’Sullivan, A.; Farver, M.; Smilowitz, J.T. The influence of early infant-feeding practices on the intestinal microbiome and body composition in infants. Nutr. Metab. Insights 2015, 8, NMI-S29530. [Google Scholar]
- Mshvildadze, M.; Neu, J.; Shuster, J.; Theriaque, D.; Li, N.; Mai, V. Intestinal microbial ecology in premature infants assessed with non–culture-based techniques. J. Pediatrics 2010, 156, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Gosalbes, M.; Llop, S.; Valles, Y.; Moya, A.; Ballester, F.; Francino, M. Meconium microbiota types dominated by lactic acid or enteric bacteria are differentially associated with maternal eczema and respiratory problems in infants. Clin. Exp. Allergy 2013, 43, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Ardissone, A.N.; Diomel, M.; Davis-Richardson, A.G.; Rechcigl, K.T.; Li, N.; Drew, J.C.; Murgas-Torrazza, R.; Sharma, R.; Hudak, M.L.; Triplett, E.W. Meconium microbiome analysis identifies bacteria correlated with premature birth. PLoS ONE 2014, 9, e90784. [Google Scholar] [CrossRef] [PubMed]
- DiGiulio, D.B. Diversity of microbes in amniotic Fluid. In Seminars in Fetal and Neonatal Medicine; Elsevier: Amsterdam, The Netherlands, 2012; pp. 2–11. [Google Scholar]
- DiGiulio, D.B.; Romero, R.; Amogan, H.P.; Kusanovic, J.P.; Bik, E.M.; Gotsch, F.; Kim, C.J.; Erez, O.; Edwin, S.; Relman, D.A. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: A molecular and culture-based investigation. PLoS ONE 2008, 3, e3056. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhou, X.; Zhong, Z.; Wang, C.; Zhang, H.; Li, D.; He, T.; Li, C.; Liu, X.; Yuan, H. Investigation of antibacterial activity of bacillus spp. Isolated from the feces of giant panda and characterization of their antimicrobial gene distributions. World J. Microbiol. Biotechnol. 2014, 30, 3129–3136. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, X.; Li, J.; Zhong, Z.; Li, W.; Liu, X.; Liu, F.; Su, H.; Luo, Y.; Gu, W. Transcriptional regulation and adaptation to a high-fiber environment in bacillus subtilis hh2 isolated from feces of the giant panda. PLoS ONE 2015, 10, e0116935. [Google Scholar] [CrossRef]
- Xu, J.; Gordon, J.I. Honor thy symbionts. Proc. Natl. Acad. Sci. USA 2003, 100, 10452–10459. [Google Scholar] [CrossRef]
- Toscano, M.; de Grandi, R.; Peroni, D.G.; Grossi, E.; Facchin, V.; Comberiati, P.; Drago, L. Impact of delivery mode on the colostrum microbiota composition. BMC Microbiol. 2017, 17, 205. [Google Scholar] [CrossRef]
- Biasucci, G.; Benenati, B.; Morelli, L.; Bessi, E.; Boehm, G. Cesarean delivery may affect the early biodiversity of intestinal bacteria. J. Nutr. 2008, 138, 1796S–1800S. [Google Scholar] [CrossRef]
- Hunt, K.M.; Foster, J.A.; Forney, L.J.; Schütte, U.M.; Beck, D.L.; Abdo, Z.; Fox, L.K.; Williams, J.E.; McGuire, M.K.; McGuire, M.A. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS ONE 2011, 6, e21313. [Google Scholar] [CrossRef]
- Cabrera-Rubio, R.; Collado, M.C.; Laitinen, K.; Salminen, S.; Isolauri, E.; Mira, A. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am. J. Clin. Nutr. 2012, 96, 544–551. [Google Scholar] [CrossRef] [PubMed]
- LaTuga, M.S.; Stuebe, A.; Seed, P.C. A review of the source and function of microbiota in breast milk. In Seminars in Reproductive Medicine; Thieme Medical Publishers: New York, NY, USA, 2014; Volume 32, pp. 68–73. [Google Scholar] [CrossRef]
- Abecia, L.; Jiménez, E.; Martínez-Fernandez, G.; Martín-García, A.I.; Ramos-Morales, E.; Pinloche, E.; Denman, S.E.; Newbold, C.J.; Yáñez-Ruiz, D.R. Natural and artificial feeding management before weaning promote different rumen microbial colonization but not differences in gene expression levels at the rumen epithelium of newborn goats. PLoS ONE 2017, 12, e0182235. [Google Scholar] [CrossRef] [PubMed]
- De Barbieri, I.; Hegarty, R.; Silveira, C.; Gulino, L.; Oddy, V.; Gilbert, R.; Klieve, A.; Ouwerkerk, D. Programming rumen bacterial communities in newborn merino lambs. Small Rumin. Res. 2015, 129, 48–59. [Google Scholar] [CrossRef]
- Erickson, A.R.; Cantarel, B.L.; Lamendella, R.; Darzi, Y.; Mongodin, E.F.; Pan, C.; Shah, M.; Halfvarson, J.; Tysk, C.; Henrissat, B. Integrated metagenomics/metaproteomics reveals human host-microbiota signatures of crohn’s disease. PLoS ONE 2012, 7, e49138. [Google Scholar] [CrossRef]
- Mao, S.; Zhang, M.; Liu, J.; Zhu, W. Characterising the bacterial microbiota across the gastrointestinal tracts of dairy cattle: Membership and potential function. Sci. Rep. 2015, 5, 16116. [Google Scholar] [CrossRef]
- Berry, D.; Schwab, C.; Milinovich, G.; Reichert, J.; Mahfoudh, K.B.; Decker, T.; Engel, M.; Hai, B.; Hainzl, E.; Heider, S. Phylotype-level 16s rrna analysis reveals new bacterial indicators of health state in acute murine colitis. ISME J. 2012, 6, 2091–2106. [Google Scholar] [CrossRef]
- Kumar, S.; Indugu, N.; Vecchiarelli, B.; Pitta, D.W. Associative patterns among anaerobic fungi, methanogenic archaea, and bacterial communities in response to changes in diet and age in the rumen of dairy cows. Front. Microbiol. 2015, 6, 781. [Google Scholar] [CrossRef]
- Chaucheyras-Durand, F.; Ameilbonne, A.; Bichat, A.; Mosoni, P.; Ossa, F.; Forano, E. Live yeasts enhance fibre degradation in the cow rumen through an increase in plant substrate colonization by fibrolytic bacteria and fungi. J. Appl. Microbiol. 2016, 120, 560–570. [Google Scholar] [CrossRef]
- Kusanovic, J.P.; Espinoza, J.; Romero, R.; Gonçalves, L.F.; Nien, J.K.; Soto, E.; Khalek, N.; Camacho, N.; Hendler, I.; Mittal, P. Clinical significance of the presence of amniotic fluid ‘sludge’in asymptomatic patients at high risk for spontaneous preterm delivery. Ultrasound Obstet. Gynecol. 2007, 30, 706–714. [Google Scholar] [CrossRef]
- Romero, R.; Kusanovic, J.; Espinoza, J.; Gotsch, F.; Nhan-Chang, C.; Erez, O.; Kim, C.; Khalek, N.; Mittal, P.; Goncalves, L. What is amniotic fluid ‘sludge’? Ultrasound Obstet. Gynecol. 2007, 30, 793–798. [Google Scholar] [CrossRef]
- Fernando, B.R. Metagenomic Analysis of Microbial Communities in the Bovine Rumen. Ph.D. Thesis, Oklahoma State University, Stillwater, OK, USA, 2012. [Google Scholar]
- Sirohi, S.K.; Choudhury, P.K.; Dagar, S.S.; Puniya, A.K.; Singh, D. Isolation, characterization and fibre degradation potential of anaerobic rumen fungi from cattle. Ann. Microbiol. 2013, 63, 1187–1194. [Google Scholar] [CrossRef]
- Kittelmann, S.; Naylor, G.E.; Koolaard, J.P.; Janssen, P.H. A proposed taxonomy of anaerobic fungi (class neocallimastigomycetes) suitable for large-scale sequence-based community structure analysis. PLoS ONE 2012, 7, e36866. [Google Scholar] [CrossRef] [PubMed]
- Boots, B.; Lillis, L.; Clipson, N.; Petrie, K.; Kenny, D.; Boland, T.; Doyle, E. Responses of anaerobic rumen fungal diversity (phylum neocallimastigomycota) to changes in bovine diet. J. Appl. Microbiol. 2013, 114, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Lima, F.S.; Oikonomou, G.; Lima, S.F.; Bicalho, M.L.; Ganda, E.K.; de Oliveira Filho, J.C.; Lorenzo, G.; Trojacanec, P.; Bicalho, R.C. Prepartum and postpartum rumen fluid microbiomes: Characterization and correlation with production traits in dairy cows. Appl. Environ. Microbiol. 2015, 81, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
| Days | Bacterial | Fungi | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reads | OTU | Ace | Chao | Shannon | Simpson | Reads | OTU | Ace | Chao | Shannon | Simpson | |
| D0 | 23,242 | 364.33 abc | 493.67 abc | 498.33 ab | 2.40 d | 0.33 a | 28,995 | 123.67 b | 129.00 c | 132.33 c | 2.8 | 0.18 |
| D3 | 23,242 | 151.00 d | 207.00 d | 196.33 c | 3.05 cd | 0.13 b | 28,995 | 176.00 ab | 191.00 c | 190.00 c | 2.79 | 0.15 |
| D7 | 23,242 | 183.00 d | 226.00 d | 226.67 c | 2.87 cd | 0.12 b | 28,995 | 227.00 ab | 260.33 bc | 258.67 abc | 2.46 | 0.25 |
| D14 | 23,242 | 269.67 cd | 368.67 cd | 357.00 bc | 3.13 bcd | 0.10 b | 28,995 | 206.33 ab | 245.33 bc | 253.67 abc | 2.44 | 0.25 |
| D21 | 23,242 | 519.33 a | 626.67 ab | 637.67 a | 4.06 ab | 0.05 b | 28,995 | 285.00 a | 360.67 ab | 359.67 ab | 3.11 | 0.11 |
| D28 | 23,242 | 503.33 ab | 656.00 a | 641.67 a | 4.07 ab | 0.05 b | 28,995 | 314.33 a | 431.33 a | 403.67 a | 2.9 | 0.13 |
| D42 | 23,242 | 348.67 bc | 447.67 bc | 457.33 ab | 3.64 abc | 0.06 b | 28,995 | 179.00 ab | 234.00 bc | 217.00 bc | 2.38 | 0.22 |
| D56 | 23,242 | 512.00 a | 590.67 ab | 595.67 a | 4.42 a | 0.04 b | 28,995 | 202.33 ab | 260.00 bc | 251.67 abc | 2.37 | 0.22 |
| SEM | 32.28 | 38.26 | 38.95 | 0.16 | 0.02 | 18.31 | 22.11 | 22.577 | 0.145 | 0.025 | ||
| p-value | 0.24 | 0.24 | 0.21 | 0.04 | 0.01 | 0.02 | 0.09 | 0.1 | 0.16 | 0.15 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Li, B.; Guo, M.; Liu, G.; Yang, Y.; Wang, X.; Chen, Y.; Zhang, E. Maturation of the Goat Rumen Microbiota Involves Three Stages of Microbial Colonization. Animals 2019, 9, 1028. https://doi.org/10.3390/ani9121028
Zhang K, Li B, Guo M, Liu G, Yang Y, Wang X, Chen Y, Zhang E. Maturation of the Goat Rumen Microbiota Involves Three Stages of Microbial Colonization. Animals. 2019; 9(12):1028. https://doi.org/10.3390/ani9121028
Chicago/Turabian StyleZhang, Ke, Bibo Li, Mengmeng Guo, Gongwei Liu, Yuxin Yang, Xiaolong Wang, Yulin Chen, and Enping Zhang. 2019. "Maturation of the Goat Rumen Microbiota Involves Three Stages of Microbial Colonization" Animals 9, no. 12: 1028. https://doi.org/10.3390/ani9121028
APA StyleZhang, K., Li, B., Guo, M., Liu, G., Yang, Y., Wang, X., Chen, Y., & Zhang, E. (2019). Maturation of the Goat Rumen Microbiota Involves Three Stages of Microbial Colonization. Animals, 9(12), 1028. https://doi.org/10.3390/ani9121028





