Evaluation of Oxidative Stress in Dairy Cows with Left Displacement of Abomasum
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Management
2.2. Blood Collection and Analyses
2.3. Statistical Analysis
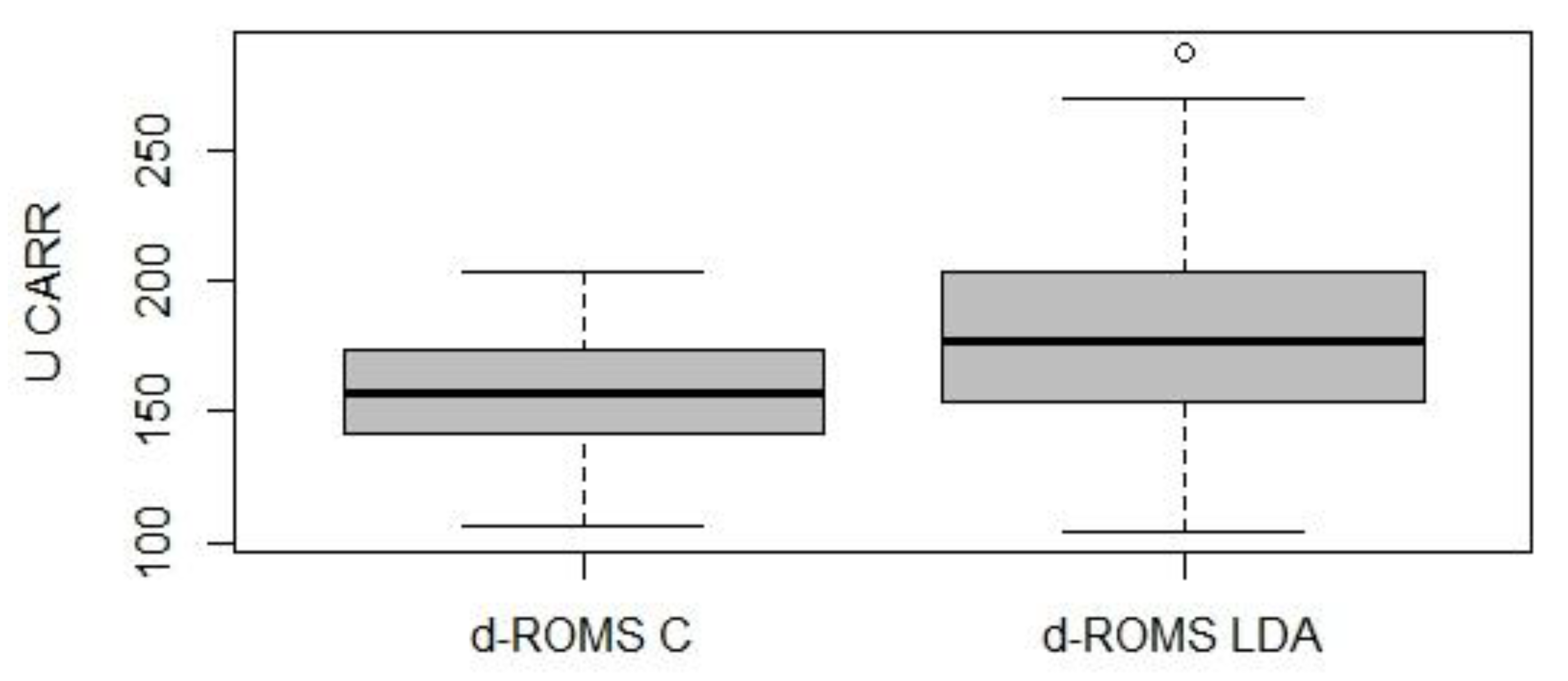
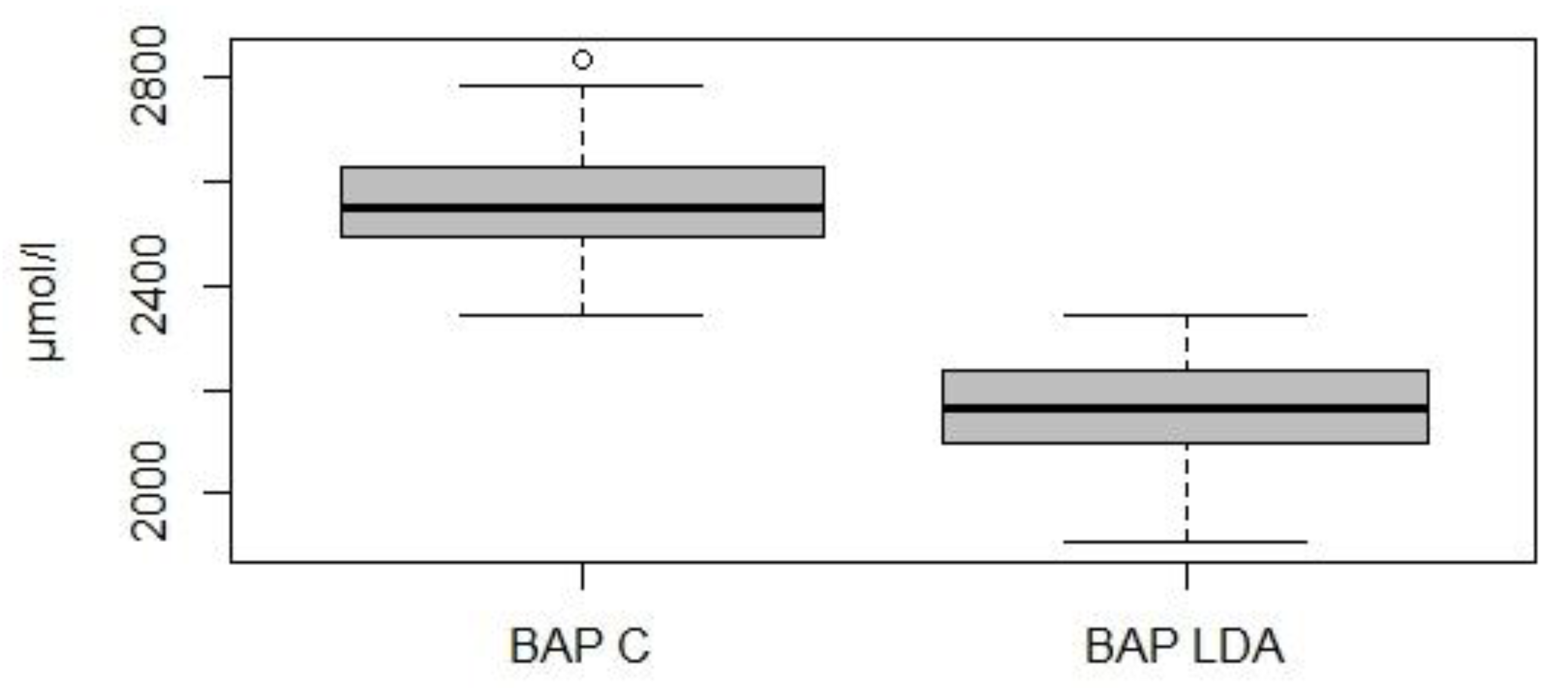
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Geishauser, T.; Leslie, K.; Duffield, T. Metabolic aspects in the etiology of displaced abomasum. Vet. Clin. N. Am. Food Anim. Pract. 2000, 16, 255–265. [Google Scholar] [CrossRef]
- McArt, J.A.; Nydam, D.V.; Overton, M.W. Hyperketonemia in early lactation dairy cattle: A deterministic estimate of component and total cost per case. J. Dairy Sci. 2015, 98, 2043–2054. [Google Scholar] [CrossRef] [PubMed]
- Geishauser, T. Abomasal displacement in the bovine—A review on character, occurrence, aetiology and pathogenesis. Zent. Vet. A 1995, 42, 229–251. [Google Scholar] [CrossRef]
- Sexton, M.; Buckley, W.; Ryan, E. A study of 54 cases of left displacement of the abomasum: February to July 2005. Ir. Vet. J. 2007, 60, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Jorritsma, R.; Westerlaan, B.; Bierma, M.P.; Frankena, K. Milk yield and survival of Holstein-Friesian dairy cattle after laparoscopic correction of left-displaced abomasum. Vet. Rec. 2008, 162, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Fiore, F.; Musina, D.; Cocco, R.; Di Cerbo, A.; Spissu, N. Association between left-displaced abomasum corrected with 2-step laparoscopic abomasopexy and milk production in a commercial dairy farm in Italy. Ir. Vet. J. 2018, 71, 1322. [Google Scholar] [CrossRef]
- Ingvartsen, K.L.; Andersen, J.B. Symposium: Dry matter intake of lactating dairy cattle. Integration of metabolism and intake regulation: A review focusing on periparturient animals. J. Dairy Sci. 2000, 83, 1573–1597. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Raphael, W. Significance of metabolic stress, lipid mobilization, and inflammation on transition cow disorders. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 267–278. [Google Scholar] [CrossRef]
- Abuelo, A.; Hernandez, J.; Benedito, J.L.; Castillo, C. The importance of the oxidative status of dairy cattle in the periparturient period: Revisiting antioxidant supplementation. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1003–1016. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Mavangira, V. The nexus between nutrient metabolism, oxidative stress and inflammation in transition cows. Anim. Prod. Sci. 2014, 54, 1204–1214. [Google Scholar] [CrossRef]
- Castillo, C.; Hernandez, J.; Valverde, I.; Pereira, V.; Sotillo, J.; Alonso, M.L.; Benedito, J.L. Plasma malonaldehyde (MDA) and total antioxidant status (TAS) during lactation in dairy cows. Res. Vet. Sci. 2006, 80, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M.; Aitken, S.L. Impact of oxidative stress on the health and immune function of dairy cattle. Vet. Immunol. Immunopathol. 2009, 128, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J.; Svendsen, O. Oxidants and antioxidants in disease: Oxidative stress in farm animals. Vet. J. 2007, 173, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Merlo, M.; Celi, P.; Barbato, O.; Gabai, G. Relationships between oxidative status and pregnancy outcome in dairy cows. Anim. Prod. Aust. 2008, 27, 84. [Google Scholar] [CrossRef]
- Celi, P. Biomarkers of oxidative stress in ruminant medicine. Immunopharmacol. Immunotoxicol. 2011, 33, 233–240. [Google Scholar] [CrossRef]
- Abuelo, A.; Hernandez, J.; Benedito, J.L.; Castillo, C. Oxidative stress index (OSi) as a new tool to assess redox status in dairy cattle during the transition period Animal. Anim. Consort. 2013, 7, 1374–1378. [Google Scholar] [CrossRef]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. Redox biology in transition periods of dairy cattle: Role in the health of periparturient and neonatal animals. Antioxidants 2019, 8, 20. [Google Scholar] [CrossRef]
- Hasanpour, A.; Saranjam, N.; Amuoghli Tabrizi, B. Antioxidant Concentration Status in the Serum of Cows with Left Displacement Abomasom. Glob. Vet. 2011, 7, 478–481. [Google Scholar]
- Aly, M.A.; Saleh, N.S.; Allam, T.S.; Keshta, H.G. Evaluation of Clinical, Serum Biochemical and Oxidantantioxidant Profiles in Dairy Cows with Left Abomasal Displacement. Asian. J. Anim. Vet. Adv. 2016, 11, 242–247. [Google Scholar] [CrossRef]
- Qu, Y.; Lytle, K.; Traber, M.G.; Bobe, G. Depleted serum vitamin E concentrations precede left displaced abomasum in early-lactation dairy cows. J. Dairy Sci. 2013, 96, 3012–3022. [Google Scholar] [CrossRef]
- Mamak, N.; Devrim, A.K.; Aksit, H.; Aytekin, I.; Yıldız, R. Levels of antioxidant substances, acute phase response and lipid peroxidation in the left and right abomasum displacement in cows. Pol. J. Vet. Sci. 2013, 4, 731–733. [Google Scholar] [CrossRef]
- Maden, M.; Ozturk, A.S.; Bulbul, A.; Avci, G.E.; Yazar, E. Acute-Phase Proteins, Oxidative Stress, and Enzyme Activities of Blood Serum and Peritoneal Fluid in Cattle with Abomasal Displacement. J. Vet. Intern. Med. 2012, 26, 1470–1475. [Google Scholar] [CrossRef] [PubMed]
- Durgut, R.; Sagkan Ozturk, A.; Ozturk, O.H.; Guzel, M. Evaluation of oxidative stress, antioxidant status and lipid profile in cattle with displacement of the abomasum. Ank. Üniv. Vet. Fak. Derg. 2016, 63, 137–141. [Google Scholar]
- National Research Council. Nutrient Requirements of Dairy Cattle, 7th ed.; The National Academies Press: Washington, DC, USA, 2001. [Google Scholar]
- Sprecher, D.J.; Hostetler, D.E.; Kaneene, J.B. A lameness scoring system that uses posture and gait to predict dairy cattle reproductive performance. Theriogenology 1997, 47, 1179–1187. [Google Scholar] [CrossRef]
- Edmonson, A.J.; Lean, I.J.; Weaver, L.D.; Farver, T.; Webster, G. A body condition scoring chart for Holstein dairy cows. J. Dairy Sci. 1989, 72, 68–78. [Google Scholar] [CrossRef]
- Celi, P. Oxidative Stress in Ruminants, in: Studies on Veterinary Medicine; Mandelker, L., Vajdovich, P., Eds.; Humana Press: New York, NY, USA, 2011; Volume 5, pp. 191–231. [Google Scholar]
- Celi, P.; Raadsma, H.W. Effects of Yerba Mate (Ilex paraguariensis) supplementation on the productive performance of dairy cows during mid-lactation. Anim. Sci. 2010, 50, 339–344. [Google Scholar] [CrossRef]
- Pedernera, M.; Celi, P.; García, S.C.; Salvin, H.E.; Barchia, I.; Fulkerson, W.J. Effect of diet, energy balance and milk production on oxidative stress in early-lactating dairy cows grazing pasture. Vet. J. 2010, 186, 352–357. [Google Scholar] [CrossRef]
- Mirzad, A.N.; Tada, T.; Ano, H.; Kobayashi, I.; Yamauchi, T.; Katamoto, H. Seasonal changes in serum oxidative stress biomarkers in dairy and beef cows in a daytime grazing system. J. Vet. Med. Sci. 2018, 80, 20–27. [Google Scholar] [CrossRef]
- Castillo, C.; Hernandez, J.; Bravo, A.; Lopez-Alonso, M.; Pereira, V.; Benedito, J.L. Oxidative status during late pregnancy and early lactation in dairy cows. Vet. J. 2005, 169, 286–292. [Google Scholar] [CrossRef]
- Mudron, P.; Rehage, J.; Sallmann, H.P.; Mertens, M.; Scholz, H.; Kovac, G. Plasma and liver α-tocoferol in dairy cows with left abomasal displacement and fatty liver. J. Vet. Med. A 1997, 44, 91–97. [Google Scholar] [CrossRef]
- Ismael, M.M.; Elshahawy, I.I.; Abdullaziz, I.A. New Insights on Left Displaced Abomasum in Dairy Cows. Alex. J. Vet. Sci. 2018, 56, 127–136. [Google Scholar] [CrossRef]
- Mudron, P.; Herzog, K.; Höltershinken, M.; Rehage, J. Effects of Abdominal Surgery on Thiobarbituric acid Reactive Substances and Plasma Anti-oxidative Capacity in Dairy Cows. J. Vet. Med. 2007, 54, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Meglia, G.E.; Jensen, S.K.; Lauridsen, C.; Persson Waller, K. α-Tocopherol concentration and stereoisomer composition in plasma and milk from dairy cows fed natural or synthetic vitamin E around calving. J. Dairy Res. 2006, 73, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Weiss, W.P.; Hogan, J.S.; Wyatt, D.J. Relative bioavailability of all-rac and RRR vitamin E based on neutrophil function and total α-tocopherol and isomer concentrations in periparturient dairy cows and their calves. J. Dairy Sci. 2009, 92, 720–731. [Google Scholar] [CrossRef]
- Baldi, A. Vitamin E in dairy cows. Livest. Prod. Sci. 2005, 98, 117–122. [Google Scholar] [CrossRef]
- Goff, J.P.; Kimura, K.; Horst, R.L. Effect of mastectomy on milk fever, energy, and vitamins A, E, and β-carotene. J. Dairy Sci. 2002, 85, 1427–1436. [Google Scholar] [CrossRef]
| Ingredients | |
|---|---|
| Grass hay | 34.7% |
| Steam flaked corn | 19.0% |
| Cane-beet molasses blend | 3.8% |
| Grain mix: | 42.5% |
| - Wheat bran | 29.6% |
| - Sorghum grain | 29.4% |
| - Soybean meal | 21.6% |
| - Flaked soybean | 14.7% |
| - Calcium carbonate | 2.2% |
| - Sodium chloride | 1% |
| - Magnesium oxide | 0.4% |
| - Sodium bentonite | 0.9% |
| - Vitamin and premix: | 0.3% |
| Vitamin A | 40,000 IU |
| Vitamin D3 | 4000 IU |
| Vitamin E (α-tocopherol 92%) | 30 mg |
| Vitamin B1 | 5 mg |
| Vitamin B2 | 3 mg |
| Vitamin B6 | 1.5 mg |
| Vitamin B12 | 0.06 mg |
| Vitamin K | 5 mg |
| Para-aminobenzoic acid | 5 mg |
| Vitamin PP (niacin) | 150 mg |
| Choline chloride | 50 mg |
| Fe | 100 mg |
| Co | 1 mg |
| I | 5 mg |
| Mn | 120 mg |
| Cu | 10 mg |
| Zn | 130 mg |
| Item | TMR | Hay |
|---|---|---|
| DM, % | 85.77 ± 0.71 | 88.51 ± 2.89 |
| Ether extract, % of DM | 2.48 ± 0.34 | 1.70 ± 0.18 |
| Ash, % of DM | 9.80 ± 0.37 | 8.97 ± 0.66 |
| ADF, % of DM | 20.73 ± 4.18 | 42.21 ± 1.71 |
| ADL, % of DM | 2.89 ± 0.63 | 6.74 ± 0.76 |
| Starch, % of DM | 23.60 ± 4.74 | 1.89 ± 2.17 |
| CP, % of DM | 14.31 ± 0.88 | 8.63 ± 1.17 |
| Soluble protein, % of DM | 3.89 ± 0.41 | 3.21 ± 0.42 |
| Lactation Number | No. of Cases | Percentage of Cases (%) |
|---|---|---|
| 2 | 20 | 27.0 |
| 3 | 24 | 32.4 |
| 4 | 30 | 40.5 |
| Time Interval (days) | No. of Cases | Percentage of Cases (%) |
|---|---|---|
| ≤7 | 21 | 28.4 |
| 8–14 | 24 | 32.4 |
| 15–21 | 20 | 27.0 |
| 22–30 | 9 | 12.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiore, F.; Spissu, N.; Sechi, S.; Cocco, R. Evaluation of Oxidative Stress in Dairy Cows with Left Displacement of Abomasum. Animals 2019, 9, 966. https://doi.org/10.3390/ani9110966
Fiore F, Spissu N, Sechi S, Cocco R. Evaluation of Oxidative Stress in Dairy Cows with Left Displacement of Abomasum. Animals. 2019; 9(11):966. https://doi.org/10.3390/ani9110966
Chicago/Turabian StyleFiore, Filippo, Nicoletta Spissu, Sara Sechi, and Raffaella Cocco. 2019. "Evaluation of Oxidative Stress in Dairy Cows with Left Displacement of Abomasum" Animals 9, no. 11: 966. https://doi.org/10.3390/ani9110966
APA StyleFiore, F., Spissu, N., Sechi, S., & Cocco, R. (2019). Evaluation of Oxidative Stress in Dairy Cows with Left Displacement of Abomasum. Animals, 9(11), 966. https://doi.org/10.3390/ani9110966





