Effects of Dietary Apple Polyphenols Supplementation on Hepatic Fat Deposition and Antioxidant Capacity in Finishing Pigs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Sample Collection
2.3. Fat Deposition Analysis
2.4. Serum Antioxidant and Biochemical Analysis
2.5. Hepatic Antioxidant and Biochemical Analysis
2.6. Analysis of the Fatty Acid Profile
2.7. Real-Time Quantitative PCR
2.8. Statistical Analyses
3. Results
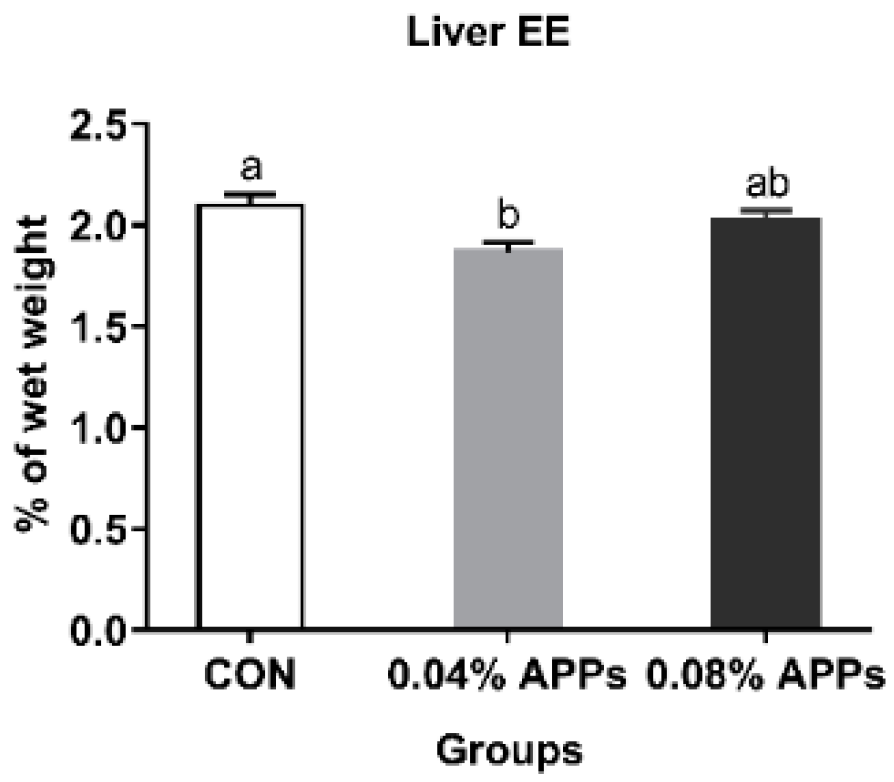
3.1. Fat Deposition
3.2. Serum Parameters
3.3. Hepatic Parameters
3.4. Hepatic Antioxidant-Related Gene mRNA Levels
3.5. Hepatic Lipid Metabolism-Related Gene mRNA Levels
3.6. Hepatic Fatty Acid Profiles
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reilly, S.M.; Saltiel, A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017, 13, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Z.; Xin, Y.N.; Geng, N.; Jiang, M.; Zhang, D.D.; Xuan, S.Y. PNPLA3 I148M variant in nonalcoholic fatty liver disease: Demographic and ethnic characteristics and the role of the variant in nonalcoholic fatty liver fibrosis. World J. Gastroenterol. 2015, 21, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qi, Y.; Aluo, Z.; Liu, S.; Zhang, Z.; Zhou, L. Betaine increases mitochondrial content and improves hepatic lipid metabolism. Food Funct. 2019, 10, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Biedrzycka, E.; Amarowicz, R. Diet and health: Apple polyphenols as antioxidants. Food Rev. Int. 2008, 24, 235–251. [Google Scholar] [CrossRef]
- Silvina, B.L.; Balz, F. Relevance of apple polyphenols as antioxidants in human plasma: Contrasting in vitro and in vivo effects. Free Radic. Biol. Med. 2004, 36, 201–211. [Google Scholar] [CrossRef]
- Jung, M.; Triebel, S.; Anke, T.; Richling, E.; Erkel, G.; Schrenk, D. Influence of apple polyphenols on inflammatory gene expression. Mol. Nutr. Food Res. 2010, 53, 1263–1280. [Google Scholar] [CrossRef]
- Manzano, M.; Giron, M.D.; Vilchez, J.D.; Sevillano, N.; El-Azem, N.; Rueda, R.; Salto, R.; Lopez-Pedrosa, J.M. Apple polyphenol extract improves insulin sensitivity in vitro and in vivo in animal models of insulin resistance. Nutr. Metab. 2016, 13, 32. [Google Scholar] [CrossRef]
- He, R.R.; Wang, M.; Wang, C.Z. Protective effect of apple polyphenols against stress-provoked influenza viral infection in restraint mice. J. Agric. Food Chem. 2011, 59, 3730–3737. [Google Scholar] [CrossRef]
- Bolea, G.; Philouze, C.; Dubois, M.; Humberclaude, A.; Ginies, C.; Arnaud, C.; Meyer, G.; Dufour, C. Apple polyphenols decrease endothelial dysfunction and atherosclerosis after chronic Western diet in a ApoE mouse model. Arch. Cardiovasc. Dis. Suppl. 2018, 10, 181. [Google Scholar] [CrossRef]
- Sylvain, A.; Mathieu, S.; Elyett, G.; Christine, M.; Andrzej, M.; Dragan, M.; Augustin, S. Apple polyphenols and fibers attenuate atherosclerosis in apolipoprotein E-deficient mice. J. Agric. Food Chem. 2008, 56, 5558–5563. [Google Scholar] [CrossRef]
- Shoji, T.; Akazome, Y.; Kanda, T.; Ikeda, M. The toxicology and safety of apple polyphenol extract. Food Chem. Toxicol. 2004, 42, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.R.; Li, J.Y.; Dong, X.W.; Tan, Z.J.; Wu, W.Z.; Xie, Q.M.; Yang, Y.M. Apple polyphenols decrease atherosclerosis and hepatic steatosis in ApoE−/− mice through the ROS/MAPK/NF-κB pathway. Nutrients 2015, 7, 7085–7105. [Google Scholar] [CrossRef] [PubMed]
- Yuki, Y.; Arata, T.; Yuki, T.; Karina, K.; Koichi, S.; Shohei, N.; Motoyuki, T.; Ryuichi, T.; Koichi, N. Dietary apple polyphenols increase skeletal muscle capillaries in Wistar rats. Physiol. Rep. 2018, 6, e13866. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Pelicano, H.; Carney, D.; Huang, P. ROS stress in cancer cells and therapeutic implications. Drug Resist. Update 2004, 7, 97–110. [Google Scholar] [CrossRef]
- Paravicini, T.M.; Touyz, R.M. Redox signaling in hypertension. Cardiovasc. Res. 2006, 71, 247–258. [Google Scholar] [CrossRef]
- Haigis, M.C.; Yankner, B.A. The Aging Stress Response. Mol. Cell 2010, 40, 333–344. [Google Scholar] [CrossRef]
- Ogino, Y.; Osada, K.; Nakamura, S.; Ohta, Y.; Kanda, T.; Sugano, M. Absorption of dietary cholesterol oxidation products and their downstream metabolic effects are reduced by dietary apple polyphenols. Lipids 2007, 42, 151–161. [Google Scholar] [CrossRef]
- Fang, X.D.; Mu, Y.L.; Huang, Z.Y.; Li, Y.; Han, L.J.; Zhang, Y.F.; Feng, Y.; Chen, Y.X.; Jiang, X.T.; Zhao, W.; et al. The sequence and analysis of a Chinese pig genome. Gigascience 2012, 1. [Google Scholar] [CrossRef]
- Groenen, M.A.M.; Archibald, A.L.; Uenishi, H.; Tuggle, C.K.; Takeuchi, Y.; Rothschild, M.F.; Rogel-Gaillard, C.; Park, C.; Milan, D.; Megens, H.J.; et al. Analyses of pig genomes provide insight into porcine demography and evolution. Nature 2012, 491, 393–398. [Google Scholar] [CrossRef]
- Cheng, Z.; Luo, J.; Bing, Y.; Chen, J.; Chen, D. Effects of resveratrol on lipid metabolism in muscle and adipose tissues: A reevaluation in a pig model. J. Funct. Food. 2015, 14, 590–595. [Google Scholar] [CrossRef]
- Wan, J.; Jiang, F.; Zhang, J.; Xu, Q.; Chen, D.; Yu, B.; Mao, X.; Yu, J.; Luo, Y.; He, J. Amniotic fluid metabolomics and biochemistry analysis provides novel insights into the diet-regulated foetal growth in a pig model. Sci. Rep. 2017, 7, 44782. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Zhang, Y.; Yu, B.; Yu, J.; Zheng, P.; Huang, Z.Q.; Luo, Y.H.; Luo, J.Q.; Mao, X.B.; Yan, H.L.; He, J.; et al. Butyrate promotes slow-twitch myofiber formation and mitochondrial biogenesis in finishing pigs via inducing specific microRNAs and PGC-1α expression. J. Anim. Sci. 2019. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef]
- Numa, S.; Nakanishi, S.; Hashimoto, T.; Iritani, N.; Okazaki, T. Role of acetyl coenzyme A carboxylase in the control of fatty acid synthesis. Vitam. Horm. 1970, 28, 213–243. [Google Scholar] [CrossRef]
- Smith, S.; Witkowski, A.; Joshi, A.K. Structural and functional organization of the animal fatty acid synthase. Prog. Lipid Res. 2003, 42, 289–317. [Google Scholar] [CrossRef]
- Langfort, J.; Donsmark, M.; Ploug, T.; Holm, C.; Galbo, H. Hormone-sensitive lipase in skeletal muscle: Regulatory mechanisms. Acta Physiol. Scand. 2003, 178, 397–403. [Google Scholar] [CrossRef]
- Zachary, G.H.; Joseph, T.R.; Olivia, B.; Carles, L.; Seung, H.K.; Raul, M.; Frederick, W.A.; Wu, Z.; Pere, P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J. 2007, 26, 1913–1923. [Google Scholar] [CrossRef]
- Petter, P.L.; Lin, X.; Odle, J. Hepatic β-oxidation and carnitine palmitoyltransferase I in neonatal pigs after dietary treatments of clofibric acid, isoproterenol, and medium-chain triglycerides. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R1518–R1524. [Google Scholar] [CrossRef]
- Huang, Q.C.; Han, X.Y.; Xu, Z.R.; Yang, X.Y.; Cheng, T.; Zheng, X.T. Betaine suppresses carnitine palmitoyltransferase I in skeletal muscle but not in liver of finishing pigs. Livest. Sci. 2009, 126, 130–135. [Google Scholar] [CrossRef]
- Lee, K.E.; Youm, J.K.; Lee, W.J.; Kang, S.; Kim, Y.J. Polyphenol-rich apple extract inhibits dexamethasone-induced sebaceous lipids production by regulating SREBP1 expression. Exp. Dermatol. 2017, 26, 958–960. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; He, R.-r.; Zeng, X.-h.; Huang, X.-j.; Du, T.-l.; Cui, J.-c.; Hiroshi, K. Hypotriglyceridemic effects of apple polyphenols extract via up-regulation of lipoprotein lipase in triton WR-1339-induced mice. Chin. J. Integr. Med. 2014, 20, 31–35. [Google Scholar] [CrossRef]
- Azuma, T.; Osada, K.; Aikura, E.; Imasaka, H.; Handa, M. Anti-obesity effect of dietary polyphenols from unripe apple in rats. J. Jpn. Soc. Food Sci. Technol.-Nippon Shokuhin Kagaku Kogaku Kaishi 2013, 60, 184–192. [Google Scholar] [CrossRef]
- Warensjo, E.; Ohrvall, M.B. Fatty acid composition and estimated desaturase activities are associated with obesity and lifestyle variables in men and women. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 128–136. [Google Scholar] [CrossRef]
- Ntambi, J.M. The regulation of stearoyl-CoA desaturase (SCD). Prog. Lipid Res. 1995, 34, 139–150. [Google Scholar] [CrossRef]
- Ntambi, J.M.; Makoto, M.; Stoehr, J.P.; Hong, L.; Kendziorski, C.M.; Yandell, B.S.; Yang, S.; Paul, C.; Friedman, J.M.; Attie, A.D. Loss of stearoyl-CoA desaturase-1 function protects mice against adiposity. Proc. Natl. Acad. Sci. USA 2002, 99, 11482–11486. [Google Scholar] [CrossRef]
- Zhou, Y.; Ruan, Z.; Wen, Y.; Yang, Y.; Mi, S.; Zhou, L.; Wu, X.; Ding, S.; Deng, Z.; Wu, G. Chlorogenic acid from honeysuckle improves hepatic lipid dysregulation and modulates hepatic fatty acid composition in rats with chronic endotoxin infusion. J. Clin. Biochem. Nutr. 2016, 58, 146–155. [Google Scholar] [CrossRef]
- Pelikanova, T.; Kazdova, L.; Chvojkova, S.; Bas, J. Serum phospholipid fatty acid composition and insulin action in type 2 diabetic patients. Metabolism 2001, 50, 1472–1478. [Google Scholar] [CrossRef]
- Vessby, B. Dietary fat, fatty acid composition in plasma and the metabolic syndrome. Curr. Opin. Lipidol. 2003, 14, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Pikuleva, I.A. Cholesterol-metabolizing cytochromes P450: Implications for cholesterol lowering. Expert Opin. Drug Metab. Toxicol. 2008, 4, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.C.; Calabrese, G.; Stiuso, P.; Ritieni, A.; Giannetti, D.; Novellino, E. Effects of Annurca apple polyphenols on lipid metabolism in HepG2 cell lines: A source of nutraceuticals potentially indicated for the metabolic syndrome. Food Res. Int. 2014, 63, 252–257. [Google Scholar] [CrossRef]
- Goedeke, L.; Fernandez-Hernando, C. Regulation of cholesterol homeostasis. Cell. Mol. Life Sci. 2012, 69, 915–930. [Google Scholar] [CrossRef]
- Osada, K.; Suzuki, T.; Kawakami, Y.; Senda, M.; Kasai, A.; Sami, M.; Ohta, Y.; Kanda, T.; Ikeda, M. Dose-dependent hypocholesterolemic actions of dietary apple polyphenol in rats fed cholesterol. Lipids 2006, 41, 133–139. [Google Scholar] [CrossRef]
- Wakabayashi, N.; Itoh, K.; Wakabayashi, J.; Motohashi, H.; Noda, S.; Takahashi, S.; Imakado, S.; Kotsuji, T.; Otsuka, F.; Roop, D.R.; et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat. Genet. 2003, 35, 238–245. [Google Scholar] [CrossRef]
- Keum, Y.S.; Choi, B.Y. Molecular and chemical regulation of the Keap1-Nrf2 signaling pathway. Molecules 2014, 19, 10074–10089. [Google Scholar] [CrossRef]
- Motohashi, H.; Yamamoto, M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004, 10, 549–557. [Google Scholar] [CrossRef]
| Ingredient | Content (%) | Nutrient Levels 3 | Content |
|---|---|---|---|
| Maize | 79.18 | Digestible energy (Mcal/kg) | 3.40 |
| Soybean meal | 16.02 | Crude protein (%) | 13.76 |
| Soybean oil | 1.97 | Calcium (%) | 0.49 |
| Maize starch | 0.15 | Total P (%) | 0.41 |
| L-Lysine·HCl | 0.34 | Available P (%) | 0.23 |
| DL-Methionine | 0.10 | Digestible lysine (%) | 0.78 |
| L-Threonine | 0.15 | Digestible Met + Cys (%) | 0.47 |
| L-Tryptophan | 0.02 | Digestible Thr (%) | 0.53 |
| Limestone | 0.76 | Digestible Thr (%) | 0.14 |
| CaHPO4 | 0.60 | ||
| NaCl | 0.30 | ||
| Choline chloride | 0.10 | ||
| Vitamin premix 1 | 0.015 | ||
| Mineral premix 2 | 0.30 | ||
| Total | 100.00 |
| Genes | Primer Sequence (5′–3′) | Product Size (bp) | GeneBank ID Accession No. |
|---|---|---|---|
| GAPDH | F: ACTCACTCTTCTACCTTTGATGCT R: TGTTGCTGTAGCCAAATTCA | 100 | NM_001206359 |
| ACC | F: ACCGAATTGGTTCCTTTGGAC R: CCAGTCCGATTCTTGCTCCA | 123 | AF175308 |
| FAS | F: ACACCTTCGTGCTGGCCTAC R: ATGTCGGTGAACTGCTGCAC | 112 | NM_001099930 |
| HSL | F: CCCATCCTCTCCATCGACT R: CAGCAGTAGGCGTAGAAGCAC | 83 | NM_214315 |
| PPARα | F: GAGTTCGCCAAGTCCATCC R: CCGTCCTTGTTCATCACAGAG | 122 | NM_001044526 |
| CPT1b | F: TGACTCGAATGTTCCGGGAG R: AGATCTTGCAGGTCTGCTTTCA | 118 | NM_001007191 |
| HMG-CoAR | F: GGTCAGGATGCGGCACAGAACG R: GCCCCACGGTCCCGATCTCTATG | 127 | NM_001122988 |
| CYP7A1 | F: TATAGGGCACGATGCACAGA R: ACCTGACCAGTTCCGAGATG | 200 | NM_001005352 |
| LDL-R | F: AGAACTGGAGGCTTAAGAGCATC R: GAGGGGTAGGTGTAGCCGTCCTG | 115 | NM_001206354 |
| SOD1 | F: AGACCTGGGCAATGTGACTG R: GTGCGGCCAATGATGGAATG | 102 | NM_001190422 |
| CAT | F: CAGATGAAGCATTGGAAGGAGC R: TTGTCTCCTATCGGATTCCCAG | 83 | NM_214301 |
| GPX1 | F: GTGAATGGCGCAAATGCTCA R: ATTGCGACACACTGGAGACC | 126 | NM_214201 |
| GST | F: CCAACCCAGAAGACTGCTCA R: CATTCAGGTGGGCTCTTCGT | 102 | AB000884 |
| Nrf2 | F: GCCCCTGGAAGCGTTAAAC | 67 | XM_003133500 |
| R: GGACTGTATCCCCAGAAGGTTGT | |||
| Keap1 | F: ACGACGTGGAGACAGAAACGT | 56 | NM_001114671 |
| R: GCTTCGCCGATGCTTCA |
| Items | CON | 0.04% APPs | 0.08% APPs |
|---|---|---|---|
| Antioxidant capacity | |||
| MDA, nmol/mL | 0.65 ± 0.09 | 0.57 ± 0.07 | 0.48 ± 0.10 |
| T-AOC, U/mL | 0.81 ± 0.07 b | 1.15 ± 0.09 a | 1.15 ± 0.10 a |
| T-SOD, U/mL | 102.77 ± 1.19 | 99.36 ± 1.93 | 100.28 ± 1.81 |
| GSH-PX, U/mL | 416.51 ± 21.53 | 500.78 ± 42.09 | 486.06 ± 25.30 |
| CAT, U/mL | 11.09 ± 0.77 b | 13.15 ± 1.34 ab | 14.23 ± 0.77 a |
| Biochemistry parameters | |||
| T-CHO, mmol/L | 3.04 ± 0.10 a | 2.69 ± 0.07 b | 2.86 ± 0.10 ab |
| TG, mmol/L | 0.46 ± 0.01 a | 0.36 ± 0.02 b | 0.36 ± 0.01 b |
| LDL-C, mmol/L | 1.34 ± 0.06 | 1.18 ± 0.06 | 1.30 ± 0.07 |
| HDL-C, mmol/L | 1.70 ± 0.06 | 1.72 ± 0.08 | 1.79 ± 0.09 |
| Items | CON | 0.04% APPs | 0.08% APPs |
|---|---|---|---|
| Antioxidant capacity | |||
| MDA, nmol/mg prot | 2.29 ± 0.19 a | 1.11 ± 0.17 b | 1.11 ± 0.20 b |
| T-AOC, U/mg prot | 1.07 ± 0.06 | 1.18 ± 0.05 | 1.19 ± 0.08 |
| T-SOD, U/mg prot | 1.89 ± 0.08 | 1.78 ± 0.06 | 1.69 ± 0.11 |
| GSH-PX, U/mg prot | 411.58 ± 23.10 b | 412.41 ± 11.92 b | 470.95 ± 18.24 a |
| CAT, U/mg prot | 19.37 ± 0.51 | 18.49 ± 0.47 | 18.56 ± 0.27 |
| Biochemistry parameters | |||
| T-CHO, mmol/mg prot | 58.55 ± 3.96 a | 42.48 ± 1.56 b | 43.98 ± 4.27 b |
| TG, mmol/mg prot | 106.34 ± 6.27 a | 83.61 ± 5.12 b | 79.86 ± 2.06 b |
| Items | CON | 0.04% APPs | 0.08% APPs |
|---|---|---|---|
| SOD1 | 1.00 ± 0.03 c | 1.25 ± 0.05 b | 1.49 ± 0.06 a |
| CAT | 1.00 ± 0.02 b | 1.37 ± 0.05 a | 1.28 ± 0.04 a |
| GPX1 | 1.00 ± 0.02 c | 1.60 ± 0.05 b | 1.97 ± 0.05 a |
| GST | 1.00 ± 0.02 b | 1.21 ± 0.04 b | 1.65 ± 0.10 a |
| Keap-1 | 1.00 ± 0.04 a | 0.80 ± 0.03 b | 0.77 ± 0.03 b |
| Nrf2 | 1.00 ± 0.08 b | 1.76 ± 0.05 a | 1.86 ± 0.21 a |
| Items | CON | 0.04% APPs | 0.08% APPs |
|---|---|---|---|
| ACC | 1.00 ± 0.06 | 0.85 ± 0.11 | 1.03 ± 0.15 |
| FAS | 1.00 ± 0.02 | 1.00 ± 0.04 | 1.10 ± 0.03 |
| HSL | 1.00 ± 0.12 b | 1.67 ± 0.12 a | 1.37 ± 0.14 a |
| CPT1b | 1.00 ± 0.07 b | 1.57 ± 0.08 a | 1.75 ± 0.16 a |
| PPARα | 1.00 ± 0.06 b | 0.82 ± 0.06 b | 1.75 ± 0.17 a |
| HMG-CoAR | 1.00 ± 0.29 | 0.78 ± 0.08 | 0.81 ± 0.05 |
| CYP7A1 | 1.00 ± 0.08 b | 1.39 ± 0.09 a | 1.64 ± 0.10 a |
| LDL-R | 1.00 ± 0.09 b | 1.16 ± 0.13 b | 2.05 ± 0.12 a |
| Items | CON | 0.04% APPs | 0.08% APPs |
|---|---|---|---|
| C14:0 | 0.25 ± 0.02 | 0.29 ± 0.06 | 0.19 ± 0.02 |
| C16:0 | 17.40 ± 0.63 a | 15.03 ± 0.68 b | 15.10 ± 0.51 b |
| C17:0 | 1.05 ± 0.22 | 1.21 ± 0.28 | 1.93 ± 0.45 |
| C18:0 | 27.70 ± 0.77 | 29.92 ± 1.16 | 27.61 ± 0.53 |
| C16:1 | 0.41 ± 0.02 | 0.35 ± 0.06 | 0.38 ± 0.04 |
| C17:1 | 0.28 ± 0.01 | 0.25 ± 0.05 | 0.33 ± 0.07 |
| C18:1n9 | 12.46 ± 0.47 | 14.58 ± 2.50 | 10.91 ± 0.38 |
| C18:2n6 | 20.16 ± 0.81 | 20.18 ± 0.94 | 19.76 ± 0.41 |
| C18:3n6 | 0.24 ± 0.01 | 0.21 ± 0.02 | 0.19 ± 0.02 |
| C18:3n3 | 0.49 ± 0.04 | 0.52 ± 0.07 | 0.42 ± 0.04 |
| C20:1n9 | 0.23 ± 0.01 | 0.22 ± 0.01 | 0.23 ± 0.01 |
| C20:3n6 | 0.65 ± 0.04 | 0.64 ± 0.07 | 0.80 ± 0.06 |
| C20:4n6 | 0.11 ± 0.01 a | 0.09 ± 0.01 b | 0.09 ± 0.01 b |
| C20:5n3 | 0.71 ± 0.02 | 0.67 ± 0.05 | 0.67 ± 0.08 |
| C22:6n3 | 0.94 ± 0.25 | 1.18 ± 0.21 | 1.11 ± 0.15 |
| SFA 1 | 61.10 ± 0.36 | 61.80 ± 0.65 | 61.15 ± 0.29 |
| MUFA 2 | 13.53 ± 0.48 | 13.27 ± 0.87 | 12.01 ± 0.46 |
| PUFA 3 | 23.71 ± 0.75 | 23.26 ± 0.81 | 24.22 ± 0.57 |
| Δ9-16 desaturase activity 4 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 |
| Δ9-18 desaturase activity 5 | 0.46 ± 0.02 a | 0.41 ± 0.03 ab | 0.39 ± 0.02 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Chen, X.; Huang, Z.; Chen, D.; He, J.; Zheng, P.; Chen, H.; Luo, J.; Luo, Y.; Yu, B.; et al. Effects of Dietary Apple Polyphenols Supplementation on Hepatic Fat Deposition and Antioxidant Capacity in Finishing Pigs. Animals 2019, 9, 937. https://doi.org/10.3390/ani9110937
Xu X, Chen X, Huang Z, Chen D, He J, Zheng P, Chen H, Luo J, Luo Y, Yu B, et al. Effects of Dietary Apple Polyphenols Supplementation on Hepatic Fat Deposition and Antioxidant Capacity in Finishing Pigs. Animals. 2019; 9(11):937. https://doi.org/10.3390/ani9110937
Chicago/Turabian StyleXu, Xiaojiao, Xiaoling Chen, Zhiqing Huang, Daiwen Chen, Jun He, Ping Zheng, Hong Chen, Junqiu Luo, Yuheng Luo, Bing Yu, and et al. 2019. "Effects of Dietary Apple Polyphenols Supplementation on Hepatic Fat Deposition and Antioxidant Capacity in Finishing Pigs" Animals 9, no. 11: 937. https://doi.org/10.3390/ani9110937
APA StyleXu, X., Chen, X., Huang, Z., Chen, D., He, J., Zheng, P., Chen, H., Luo, J., Luo, Y., Yu, B., & Yu, J. (2019). Effects of Dietary Apple Polyphenols Supplementation on Hepatic Fat Deposition and Antioxidant Capacity in Finishing Pigs. Animals, 9(11), 937. https://doi.org/10.3390/ani9110937






