Study on Hematological and Biochemical Characters of Cloned Duroc Pigs and Their Progeny
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
2.3. Blood Sample Collection and Analyses
2.4. Statistical Analysis
3. Results
3.1. Hematology
3.2. Blood Biochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wilmut, I.; Schnieke, A.E.; McWhir, J.; Kind, A.J.; Campbell, K.H. Viable offspring derived from fetal and adult mammalian cells. Nature 1997, 385, 810–813. [Google Scholar] [CrossRef]
- Galli, C.; Lagutina, I.; Perota, A.; Colleoni, S.; Duchi, R.; Lucchini, F.; Lazzari, G. Somatic cell nuclear transfer and transgenesis in large animals: Current and future insights. Reprod. Domest. Anim. 2012, 47, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Prather, R.S.; Hawley, R.J.; Carter, D.B.; Lai, L.; Greenstein, J.L. Transgenic swine for biomedicine and agriculture. Theriogenology 2003, 59, 115–123. [Google Scholar] [CrossRef]
- Tachibana, M.; Amato, P.; Sparman, M.; Gutierrez, N.M.; Tippner-Hedges, R.; Ma, H.; Kang, E.; Fulati, A.; Lee, H.S.; Sritanaudomchai, H.; et al. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell 2013, 153, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.J. A study on current risk assessments and guidelines on the use of food animal products derived from cloned animals. Food Chem. Toxicol. 2017, 108, 85–92. [Google Scholar] [CrossRef]
- Schmidt, M.; Winther, K.D.; Secher, J.O.; Callesen, H. Postmortem findings in cloned and transgenic piglets dead before weaning. Theriogenology 2015, 84, 1014–1023. [Google Scholar] [CrossRef]
- Yamanaka, K.; Kaneda, M.; Inaba, Y.; Saito, K.; Kubota, K.; Sakatani, M.; Sugimura, S.; Imai, K.; Watanabe, S.; Takahashi, M. DNA methylation analysis on satellite I region in blastocysts obtained from somatic cell cloned cattle. Anim. Sci. J. 2011, 82, 523–530. [Google Scholar] [CrossRef]
- Ruan, Z.; Zhao, X.; Qin, X.; Luo, C.; Liu, X.; Deng, Y.; Zhu, P.; Li, Z.; Huang, B.; Shi, D.; et al. DNA methylation and expression of imprinted genes are associated with the viability of different sexual cloned buffaloes. Reprod. Domest. Anim. 2018, 53, 203–212. [Google Scholar] [CrossRef]
- Shen, C.J.; Lin, C.C.; Shen, P.C.; Cheng, W.T.; Chen, H.L.; Chang, T.C.; Liu, S.S.; Chen, C.M. Imprinted genes and satellite loci are differentially methylated in bovine somatic cell nuclear transfer clones. Cell. Reprogram. 2013, 15, 413–424. [Google Scholar] [CrossRef]
- Watanabe, S. Effect of calf death loss on cloned cattle herd derived from somatic cell nuclear transfer: Clones with congenital defects would be removed by the death loss. Anim. Sci. J. 2013, 84, 631–638. [Google Scholar] [CrossRef]
- Curran, E.K.; Godfrey, J.; Kline, J. Mechanisms of Immune Tolerance in Leukemia and Lymphoma. Trends Immunol. 2017, 38, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.M.; Rowland, A.; Kichenadasse, G.; Wiese, M.D.; Gurney, H.; McKinnon, R.A.; Karapetis, C.S.; Sorich, M.J. Predicting response and toxicity to immune checkpoint inhibitors using routinely available blood and clinical markers. Br. J. Cancer 2017, 117, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B. Innate immunity: An overview. Mol. Immunol. 2004, 40, 845–859. [Google Scholar] [CrossRef] [PubMed]
- van de Vosse, E.; van Dissel, J.T.; Ottenhoff, T.H. Genetic deficiencies of innate immune signalling in human infectious disease. Lancet Infect. Dis. 2009, 9, 688–698. [Google Scholar] [CrossRef]
- Arosa, F.A.; Pereira, C.F.; Fonseca, A.M. Red blood cells as modulators of T cell growth and survival. Curr. Pharm. Des. 2004, 10, 191–201. [Google Scholar] [CrossRef]
- Nikinmaa, M. Oxygen and carbon dioxide transport in vertebrate erythrocytes: An evolutionary change in the role of membrane transport. J. Exp. Biol. 1997, 200, 369–380. [Google Scholar]
- Zhang, F.; Zhang, Z.; Yan, X.; Chen, H.; Zhang, W.; Hong, Y.; Huang, L. Genome-wide association studies for hematological traits in Chinese Sutai pigs. BMC Genet. 2014, 15, 41. [Google Scholar] [CrossRef]
- Lam, F.W.; Vijayan, K.V.; Rumbaut, R.E. Platelets and Their Interactions with Other Immune Cells. Compr. Physiol. 2015, 5, 1265–1280. [Google Scholar]
- Qiu, Y.; Ciciliano, J.; Myers, D.R.; Tran, R.; Lam, W.A. Platelets and physics: How platelets “feel” and respond to their mechanical microenvironment. Blood Rev. 2015, 29, 377–386. [Google Scholar] [CrossRef]
- Maged, A.M.; Aid, G.; Bassiouny, N.; Eldin, D.S.; Dahab, S.; Ghamry, N.K. Association of biochemical markers with the severity of pre-eclampsia. Int. J. Gynaecol. Obstet. 2017, 136, 138–144. [Google Scholar] [CrossRef]
- Wang, H.; Fang, K.; Zhang, J.; Jiang, Y.; Wang, G.; Zhang, H.; Chen, T.; Shi, X.; Li, Y.; Duan, F.; et al. The significance of De Ritis (aspartate transaminase/alanine transaminase) ratio in predicting pathological outcomes and prognosis in localized prostate cancer patients. Int. Urol. Nephrol. 2017, 49, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Diao, S.; Huang, S.; Xu, Z.; Ye, S.; Yuan, X.; Chen, Z.; Zhang, H.; Zhang, Z.; Li, J. Genetic Diversity of Indigenous Pigs from South China Area Revealed by SNP Array. Animals 2019, 9, 361. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Xu, G.; Jiang, C.; Hou, L.; Wu, Z.; Wang, C. PRDM16 Represses the Pig White Lipogenesis through Promoting Lipolysis Activity. BioMed Res. Int. 2019, 2019, 1969413. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhou, R.; Luo, L.; Mai, R.; Zeng, H.; He, X.; Liu, D.; Zeng, F.; Cai, G.; Ji, H.; et al. Influence of embryo handling and transfer method on pig cloning efficiency. Anim. Reprod. Sci. 2015, 154, 121–127. [Google Scholar] [CrossRef]
- Tang, X.; Zhu, J.; Zhong, Z.; Luo, M.; Li, G.; Gong, Z.; Zhang, C.; Fei, F.; Ruan, X.; Zhou, J.; et al. Mobilization and removing of cadmium from kidney by GMDTC utilizing renal glucose reabsorption pathway. Toxicol. Appl. Pharmacol. 2016, 305, 143–152. [Google Scholar] [CrossRef]
- Tang, X.; Li, N.; Kang, L.; Dubois, A.M.; Gong, Z.; Wu, B.; Lai, G.; Yang, A.; Ruan, X.; Gao, H.; et al. Chronic low level trimethyltin exposure and the risk of developing nephrolithiasis. Occup. Environ. Med. 2013, 70, 561–567. [Google Scholar] [CrossRef]
- Ponsuksili, S.; Trakooljul, N.; Basavaraj, S.; Hadlich, F.; Murani, E.; Wimmers, K. Epigenome-wide skeletal muscle DNA methylation profiles at the background of distinct metabolic types and ryanodine receptor variation in pigs. BMC Genom. 2019, 20, 492. [Google Scholar] [CrossRef]
- Jang, D.; Yoon, J.; Taye, M.; Lee, W.; Kwon, T.; Shim, S.; Kim, H. Multivariate genome-wide association studies on tenderness of Berkshire and Duroc pig breeds. Genes Genom. 2018, 40, 701–705. [Google Scholar] [CrossRef]
- Jin, J.X.; Lee, S.; Taweechaipaisankul, A.; Kim, G.A.; Lee, B.C. The HDAC Inhibitor LAQ824 Enhances Epigenetic Reprogramming and In Vitro Development of Porcine SCNT Embryos. Cell. Physiol. Biochem. 2017, 41, 1255–1266. [Google Scholar] [CrossRef]
- Silveira, M.M.; Salgado Bayao, H.X.; Dos Santos Mendonca, A.; Borges, N.A.; Vargas, L.N.; Caetano, A.R.; Rumpf, R.; Franco, M.M. DNA methylation profile at a satellite region is associated with aberrant placentation in cloned calves. Placenta 2018, 70, 25–33. [Google Scholar] [CrossRef]
- Hirose, M.; Hada, M.; Kamimura, S.; Matoba, S.; Honda, A.; Motomura, K.; Ogonuki, N.; Shawki, H.H.; Inoue, K.; Takahashi, S.; et al. Aberrant imprinting in mouse trophoblast stem cells established from somatic cell nuclear transfer-derived embryos. Epigenetics 2018, 13, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Ao, Z.; Li, Z.; Wang, X.; Zhao, C.; Gan, Y.; Wu, X.; Zeng, F.; Shi, J.; Gu, T.; Hong, L.; et al. Identification of amniotic fluid metabolomic and placental transcriptomic changes associated with abnormal development of cloned pig fetuses. Mol. Reprod. Dev. 2019, 86, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Mir, B.; Zaunbrecher, G.; Archer, G.S.; Friend, T.H.; Piedrahita, J.A. Progeny of somatic cell nuclear transfer (SCNT) pig clones are phenotypically similar to non-cloned pigs. Cloning Stem Cells 2005, 7, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Heyman, Y.; Chavatte-Palmer, P.; Fromentin, G.; Berthelot, V.; Jurie, C.; Bas, P.; Dubarry, M.; Mialot, J.P.; Remy, D.; Richard, C.; et al. Quality and safety of bovine clones and their products. Animal 2007, 1, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Archer, G.S.; Dindot, S.; Friend, T.H.; Walker, S.; Zaunbrecher, G.; Lawhorn, B.; Piedrahita, J.A. Hierarchical phenotypic and epigenetic variation in cloned swine. Biol. Reprod. 2003, 69, 430–436. [Google Scholar] [CrossRef]
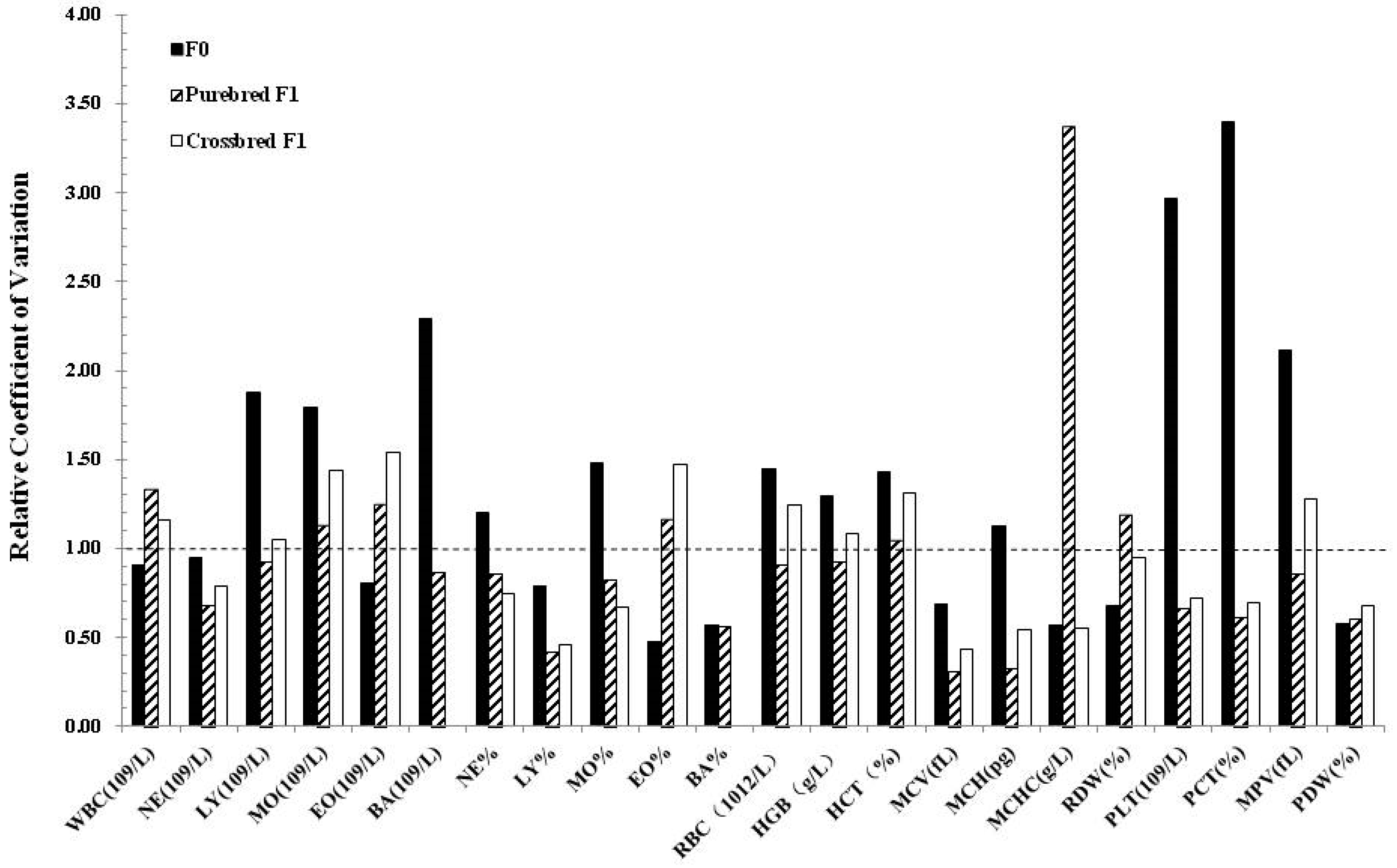
| Hematological Variables | Clones (n = 4) | Non-clones (n = 4) | F1 Clone x Non-clone (n = 4) | F1 Non-clone x Non-clone (n = 4) | Clone x Landrace Crossbreeds Cloned (n = 6) | Non-clone x Landrace Crossbreeds (n = 6) |
|---|---|---|---|---|---|---|
| WBC (109/L) | 13.53 ± 2.64 | 13.24 ± 2.83 | 22.87 ± 4.40 | 22.14 ± 3.19 | 17.08 ± 1.79 | 20.67 ± 1.86 |
| NE (109/L) | 5.35 ± 2.05 | 7.07 ± 2.84 | 6.45 ± 1.31 | 9.61 ± 2.86 | 4.63 ± 0.52 a | 7.93 ± 1.13 b |
| LY (109/L) | 7.53 ± 1.44 | 5.74 ± 0.58 | 15.64 ± 3.81 | 11.91 ± 3.11 | 12.03 ± 1.32 | 12.35 ± 1.30 |
| MO (109/L) | 0.27 ± 0.33 | 0.11 ± 0.07 | 0.33 ± 0.32 | 0.08 ± 0.06 | 0.22 ± 0.04 | 0.20 ± 0.03 |
| EO (109/L) | 0.31 ± 0.08 | 0.28 ± 0.08 | 0.22 ± 0.12 | 0.46 ± 0.21 | 0.20 ± 0.05 | 0.18 ± 0.03 |
| BA (109/L) | 0.08 ± 0.02 a | 0.04 ± 0.01 b | 0.23 ± 0.13 | 0.08 ± 0.05 | 0 | 0 |
| NE% | 38.85 ± 9.69 | 51.85 ± 10.7 | 28.75 ± 6.76 | 43.50 ± 11.84 | 27.50 ± 2.22 a | 38.12 ± 4.11 b |
| LY% | 56.13 ± 9.61 | 44.75 ± 9.69 | 68.00 ± 6.32 | 53.68 ± 11.83 | 70.12 ± 2.19 | 60.02 ± 4.07 |
| MO% | 2.18 ± 2.67 | 0.875 ± 0.72 | 1.38 ± 1.15 | 0.33 ± 0.33 | 1.30 ± 0.11 a | 0.90 ± 0.11 b |
| EO% | 2.28 ± 0.49 | 2.20 ± 1.00 | 0.95 ± 0.53 | 2.13 ± 1.01 | 1.08 ± 0.20 | 0.97 ± 0.12 |
| BA% | 0.58 ± 0.13 a | 0.33 ± 0.12 b | 0.92 ± 0.39 | 0.38 ± 0.28 | 0 | 0 |
| RBC (1012/L) | 7.88 ± 0.68 | 7.59 ± 0.45 | 7.61 ± 0.37 | 7.51 ± 0.41 | 6.12 ± 0.33 | 6.33 ± 0.27 |
| HGB (g/L) | 153.75 ± 13.23 | 141.00 ± 9.38 | 126.00 ± 5.16 | 121.00 ± 5.35 | 114.17 ± 5.31 | 112.50 ± 4.81 |
| HCT (%) | 49.90 ± 4.66 | 46.48 ± 3.03 | 43.18 ± 1.74 | 41.10 ± 1.59 | 32.62 ± 1.51 | 32.62 ± 1.14 |
| MCV (fL) | 63.33 ± 1.25 | 61.25 ± 1.76 | 56.85 ± 1.08 | 54.83 ± 3.29 | 53.35 ± 0.60 | 51.67 ± 1.37 |
| MCH (pg) | 19.53 ± 0.32 A | 18.60 ± 0.27 B | 16.63 ± 0.34 | 16.13 ± 0.99 | 18.68 ± 0.29 | 17.80 ± 0.51 |
| MCHC (g/L) | 308.25 ± 5.32 | 304.25 ± 9.17 | 292.50 ± 5.80 | 294.50 ± 1.73 | 350.00 ± 2.13 | 344.50 ± 3.79 |
| RDW (%) | 18.45 ± 0.51 | 18.53 ± 0.74 | 19.83 ± 1.49 | 20.80 ± 1.32 | 16.05 ± 0.25 | 15.43 ± 0.25 |
| PLT (109/L) | 151.25 ± 82.66 | 204.75 ± 37.73 | 252.50 ± 21.39 | 218.25 ± 27.67 | 439.17 ± 0.396 | 367.33 ± 61.34 |
| PCT (%) | 0.16 ± 0.09 | 0.21 ± 0.03 | 0.24 ± 0.03 | 0.20 ± 0.04 | 0.21 ± 0.03 | 0.19 ± 0.03 |
| MPV (fL) | 10.40 ± 0.74 | 10.30 ± 0.34 | 9.45 ± 0.70 | 9.28 ± 0.80 | 4.82 ± 0.15 | 4.98 ± 0.12 |
| PDW (%) | 15.65 ± 0.17 | 15.30 ± 0.29 | 15.23 ± 0.22 | 14.90 ± 0.36 | 16.93 ± 0.30 | 16.80 ± 0.45 |
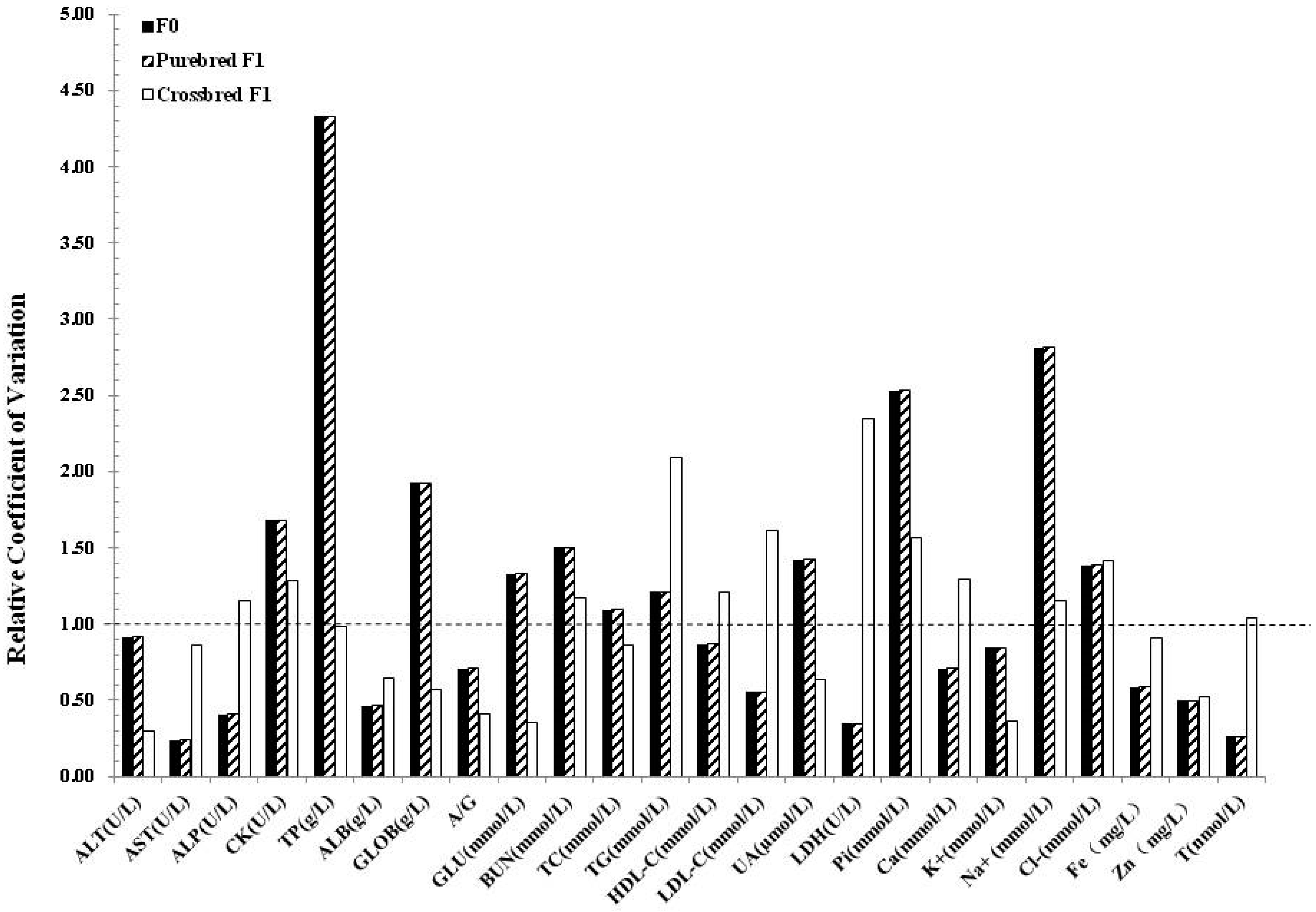
| Biochemical Parameters | Clones (n = 4) | Non-clones (n = 4) | F1 Clone x Non-clone (n = 4) | F1 Non-clone x Non-clone (n = 4) | Clone x Landrace Crossbreeds (n = 6) | Non-clone x Landrace Crossbreeds (n = 6) |
|---|---|---|---|---|---|---|
| ALT (U/L) | 43.75 ± 7.14 | 43.75 ± 7.80 | 39.50 ± 8.35 | 35.75 ± 6.95 | 41.33 ± 1.43 | 35.67 ± 4.17 |
| AST (U/L) | 39.25 ± 4.50 | 54.50 ± 26.38 | 27.50 ± 5.45 | 35.75 ± 12.89 | 44.83 ± 6.17 | 46.83 ± 7.49 |
| ALP (U/L) | 68.00 ± 4.76 | 52.25 ± 8.99 | 128.25 ± 21.36 | 150.75 ± 33.01 | 63.83 ± 5.55 | 81.33 ± 6.15 |
| CK (U/L) | 1777.25 ± 866.34 | 1206.75 ± 350.27 | 1190.00 ± 470.34 | 1379.00 ± 963.95 | 1641.17 ± 620.39 | 1395.33 ± 411.63 |
| TP (g/L) | 82.70 ± 4.20 | 76.38 ± 0.90 | 67.25 ± 4.86 | 66.00 ± 2.29 | 72.87 ± 1.92 | 71.87 ± 1.93 |
| ALB (g/L) | 40.98 ± 0.92 | 40.08 ± 1.96 | 34.18 ± 3.14 | 34.40 ± 2.24 | 29.62 ± 0.90 | 28.25 ± 1.34 |
| GLOB (g/L) | 41.73 ± 3.37 a | 36.30 ± 1.52b | 33.08 ± 2.39 | 31.60 ± 2.10 | 43.25 ± 1.63 | 43.62 ± 2.86 |
| A/G | 0.99 ± 0.06 | 1.11 ± 0.09 | 1.03 ± 0.09 | 1.09 ± 0.12 | 0.63 ± 0.03 | 0.67 ± 0.07 |
| GLU (mmol/L) | 3.19 ± 0.66 | 3.35 ± 0.52 | 4.52 ± 0.40a | 5.31 ± 0.27 b | 3.02 ± 0.34 | 3.62 ± 0.15 |
| BUN (mmol/L) | 5.48 ± 0.95 | 5.37 ± 0.62 | 4.00 ± 1.39 | 3.57 ± 1.09 | 4.61 ± 0.27 | 5.21 ± 0.35 |
| TC (mmol/L) | 1.30 ± 0.11 | 1.37 ± 0.11 | 2.02 ± 0.13 | 1.99 ± 0.18 | 3.57 ± 0.19 | 3.23 ± 0.20 |
| TG (mmol/L) | 0.28 ± 0.12 | 0.22 ± 0.07 | 0.38 ± 0.06 | 0.29 ± 0.08 | 0.45 ± 0.08 | 0.45 ± 0.04 |
| HDL-C (mmol/L) | 0.65 ± 0.08 | 0.58 ± 0.08 | 0.85 ± 0.09 a | 0.68 ± 0.08 b | 1.21 ± 0.08 | 1.23 ± 0.07 |
| LDL-C (mmol/L) | 0.51 ± 0.04 | 0.63 ± 0.09 | 1.00 ± 0.04 | 1.13 ± 0.15 | 1.56 ± 0.20 | 1.38 ± 0.11 |
| UA (µmol/L) | 1.75 ± 0.95 | 1.50 ± 0.57 | 1.25 ± 0.50 | 1.50 ± 1.00 | 5.67 ± 0.48 | 5.17 ± 0.48 |
| LDH (U/L) | 435.50 ± 65.23 | 493.50 ± 212.64 | 491.25 ± 48.33 | 464.25 ± 81.88 | 851.33 ± 97.83 | 675.00 ± 33.02 |
| Pi (mmol/L) | 2.34 ± 0.08 A | 2.15 ± 0.03 B | 3.21 ± 0.17 | 2.72 ± 0.34 | 3.31 ± 0.09 | 3.17 ± 0.05 |
| Ca (mmol/L) | 2.68 ± 0.02 | 2.65 ± 0.03 | 2.72 ± 0.09 | 2.69 ± 0.13 | 2.61 ± 0.05 | 2.50 ± 0.04 |
| K+ (mmol/L) | 4.56 ± 0.23 | 4.54 ± 0.27 | 5.29 ± 0.77 | 5.30 ± 1.01 | 4.78 ± 0.13 | 4.75 ± 0.34 |
| Na+ (mmol/L) | 142.25 ± 6.19 | 145.00 ± 2.24 | 145.48 ± 4.70 | 145.33 ± 3.78 | 140.90 ± 2.62 | 137.98 ± 2.22 |
| Cl− (mmol/L) | 97.00 ± 3.12 | 95.10 ± 2.21 | 99.08 ± 2.56 | 100.70 ± 4.20 | 96.95 ± 1.73 | 98.73 ± 1.25 |
| Fe (mg/L) | 5.45 ± 2.69 | 5.10 ± 4.28 | 3.80 ± 1.98 | 6.43 ± 4.01 | 17.56 ± 1.06 | 19.66 ± 1.31 |
| Zn (mg/L) | 0.76 ± 0.10 | 0.65 ± 0.18 | 0.41 ± 0.14 | 0.46 ± 0.30 | 9.14 ± 0.32 | 9.84 ± 0.65 |
| T (nmol/L) | 24.44 ± 2.12 | 27.54 ± 9.13 | 38.59 ± 5.57 | 26.65 ± 7.72 | 23.07 ± 2.88 | 20.19 ± 2.88 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, T.; Shi, J.; Luo, L.; Li, Z.; Yang, J.; Cai, G.; Zheng, E.; Hong, L.; Wu, Z. Study on Hematological and Biochemical Characters of Cloned Duroc Pigs and Their Progeny. Animals 2019, 9, 912. https://doi.org/10.3390/ani9110912
Gu T, Shi J, Luo L, Li Z, Yang J, Cai G, Zheng E, Hong L, Wu Z. Study on Hematological and Biochemical Characters of Cloned Duroc Pigs and Their Progeny. Animals. 2019; 9(11):912. https://doi.org/10.3390/ani9110912
Chicago/Turabian StyleGu, Ting, Junsong Shi, Lvhua Luo, Zicong Li, Jie Yang, Gengyuan Cai, Enqin Zheng, Linjun Hong, and Zhenfang Wu. 2019. "Study on Hematological and Biochemical Characters of Cloned Duroc Pigs and Their Progeny" Animals 9, no. 11: 912. https://doi.org/10.3390/ani9110912
APA StyleGu, T., Shi, J., Luo, L., Li, Z., Yang, J., Cai, G., Zheng, E., Hong, L., & Wu, Z. (2019). Study on Hematological and Biochemical Characters of Cloned Duroc Pigs and Their Progeny. Animals, 9(11), 912. https://doi.org/10.3390/ani9110912






