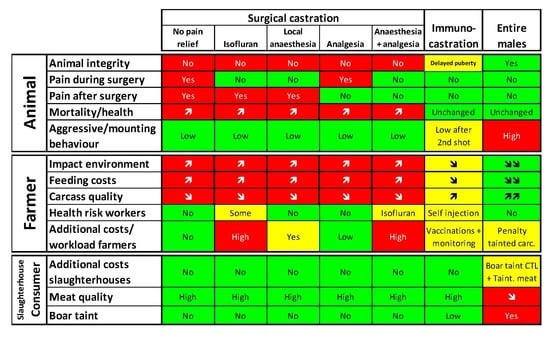
Pros and Cons of Alternatives to Piglet Castration: Welfare, Boar Taint, and Other Meat Quality Traits
Abstract
Simple Summary
Abstract
1. Introduction
2. Why Are Piglets Castrated?
3. Consequences of Surgical Castration
3.1. Unfavorable and Favorable Consequences of Surgical Castration for Animal Welfare
3.2. Consequences of Surgical Castration for Feed Efficiency, Carcass Content, and Meat Quality
4. What Are the Alternatives?
5. Entire Male Pigs
5.1. Pros and Cons of Raising Entire Male Pigs
5.2. Management of the Boar Taint Problem
5.2.1. Decreasing the Occurrence of Animals Exhibiting Boar Taint
5.2.2. Detection of Boar Taint on the Slaughter Line
5.2.3. Processing Tainted Meat to Reduce Boar Taint Perception
6. Immunocastration
6.1. The Effects of Immunocastration
6.2. Pros and Cons of Immunocastration
7. Surgical Castration with Anesthesia and/or Analgesia
7.1. Anaesthesia
7.1.1. General Anesthesia
7.1.2. Local Anesthesia
7.2. Analgesia
7.3. Pros and Cons of Surgical Castration with Pain Relief
8. The Current Situation in Europe
8.1. Entire Male Production
8.2. Immunocastration
8.3. Continuation of Surgical Castration
9. Conclusions
Funding
Conflicts of Interest
References
- Prunier, A.; Bonneau, M.; Von Borell, E.H.; Cinotti, S.; Gunn, M.; Fredriksen, B.; Giersing, M.; Morton, D.B.; Tuyttens, F.A.M.; Velarde, A. A review of the welfare consequences of surgical castration in piglets and evaluation of non-surgical methods. Anim. Welf. 2006, 15, 277–289. [Google Scholar]
- Von Borell, E.; Baumgartner, J.; Giersing, M.; Jäggin, N.; Prunier, A.; Tuyttens, F.A.M.; Edwards, S.A. Animal welfare implications of surgical castration and its alternatives in pigs. Animal 2009, 3, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- Backus, G.; Higuera, M.; Juul, N.; Nalon, E.; De Briyne, N. Second Progress Report 2015–2017 on the European Declaration on Alternatives to Surgical Castration of Pigs. Available online: https://www.boarsontheway.com/wp-content/uploads/2018/08/Second-progress-report-2015-2017-final-1.pdf (accessed on 10 June 2019).
- De Briyne, N.; Berg, C.; Blaha, T.; Temple, D. Pig castration: Will the EU manage to ban pig castration by 2018? Porcine Health Manag. 2016, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- IPEMA. Available online: www.ca-ipema.eu/ (accessed on 10 June 2019).
- Claus, R.; Weiler, U.; Herzog, U. Physiological aspects of androstenone and skatole formation in the boar—A review with experimental data. Meat Sci. 1994, 38, 289–305. [Google Scholar] [CrossRef]
- Font-I-Furnols, M.; Gispert, M.; Diestre, A.; Oliver, M.A. Acceptability of boar meat by consumers depending on their age, gender, culinary habits, and sensitivity and appreciation of androstenone odour. Meat Sci. 2003, 64, 433–440. [Google Scholar] [CrossRef]
- Patterson, R.L.S. 5α-androst-16-ene-3-one: Compound responsible for taint in boar fat. J. Sci. Food Agric. 1968, 19, 31–38. [Google Scholar] [CrossRef]
- Gower, D.B. 16-Unsaturated C19 steroids a review of their chemistry, biochemistry and possible physiological role. J. Steroid Biochem. 1972, 3, 45–64. [Google Scholar] [CrossRef]
- Booth, W.D. Factors affecting the pheromone composition of voided boar saliva. J. Reprod. Fertil. 1987, 81, 427–431. [Google Scholar] [CrossRef]
- Walstra, P.; Claudi-Magnussen, C.; Chevillon, P.; Von Seth, G.; Diestre, A.; Matthews, K.R.; Homer, D.B.; Bonneau, M. An international study on the importance of androstenone and skatole for boar taint: Levels of androstenone and skatole by country and season. Livest. Prod. Sci. 1999, 62, 15–28. [Google Scholar] [CrossRef]
- Lunde, K.; Egelandsdal, B.; Skuterud, E.; Mainland, J.D.; Lea, T.; Hersleth, M.; Matsunami, H. Genetic Variation of an Odorant Receptor OR7D4 and Sensory Perception of Cooked Meat Containing Androstenone. PLoS ONE 2012, 7, e35259. [Google Scholar] [CrossRef]
- Weiler, U.; Fischer, K.; Kemmer, H.; Dobrowolski, A.; Claus, R. Influence of androstenone sensitivity on consumer reactions to boar meat. In Boar Taint in Entire Male Pigs, Proceedings of a Meeting of the EAAP Working Group Production and Utilisation of Meat from Entire Male Pigs, Stockholm, Sweden, 1–3 October 1997; Bonneau, M., Lundstrom, K., Malmfors, B., Eds.; EAAP Publication: Rome, Italy, 1997; pp. 147–151. [Google Scholar]
- Annor-Frempong, I.; Nute, G.; Whittington, F.; Wood, J. The problem of taint in pork: I. Detection thresholds and odour profiles of androstenone and skatole in a model system. Meat Sci. 1997, 46, 45–55. [Google Scholar] [CrossRef]
- Vold, E. Fleischproduktionseigenschaften bei Ebern und Kastraten. IV. Organoleptische und gaschromatografische Untersuchungen wasserdampfflüchtiger Stoffe des Rückenspeckes von Ebern. Meldinger fra Norges Landbrukshøgskole 1970, 49, 1–25. [Google Scholar]
- Doran, E.; Whittington, F.W.; Wood, J.D.; McGivan, J.D. Cytochrome P450IIE1 (CYP2E1) is induced by skatole and this induction is blocked by androstenone in isolated pig hepatocytes. Chem. Biol. Interact. 2002, 140, 81–92. [Google Scholar] [CrossRef]
- Zamaratskaia, G.; Gilmore, W.J.; Lundström, K.; Squires, E.J. Effect of testicular steroids on catalytic activities of cytochrome P450 enzymes in porcine liver microsomes. Food Chem. Toxicol. 2007, 45, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Wiercinska, P.; Lou, Y.; Squires, E.J. The roles of different porcine cytochrome P450 enzymes and cytochrome b5A in skatole metabolism. Animal 2012, 6, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Degawa, M. Serum androgen level is determined by autosomal dominant inheritance and regulates sex-related CYP genes in pigs. Biochem. Biophys. Res. Commun. 2013, 430, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Weiler, U.; Font-i-Furnols, M.; Fischer, K.; Kemmer, H.; Oliver, M.A.; Gispert, M.; Dobrowolski, A.; Claus, R. Influence of differences in sensitivity of Spanish and German consumers to perceive androstenone on the acceptance of boar meat differing in skatole and androstenone concentrations. Meat Sci. 2000, 54, 297–304. [Google Scholar] [CrossRef]
- Morales, J.; Dereu, A.; Manso, A.; De Frutos, L.; Piñeiro, C.; Manzanilla, E.G.; Wuyts, N. Surgical castration with pain relief affects the health and productive performance of pigs in the suckling period. Porcine Health Manag. 2017, 3, 18. [Google Scholar] [CrossRef]
- Rydhmer, L.; Zamaratskaia, G.; Andersson, H.K.; Algers, B.; Guillemet, R.; Lundström, K. Aggressive and sexual behaviour of growing and finishing pigs reared in groups, without castration. Acta Agric. Scand. Sect. A Anim. Sci. 2006, 56, 109–119. [Google Scholar] [CrossRef]
- Weiler, U.; Isernhagen, M.; Stefanski, V.; Ritzmann, M.; Kress, K.; Hein, C.; Zöls, S. Penile Injuries in Wild and Domestic Pigs. Animals 2016, 6, 25. [Google Scholar] [CrossRef]
- Reiter, S.; Zöls, S.; Ritzmann, M.; Stefanski, V.; Weiler, U. Penile Injuries in Immunocastrated and Entire Male Pigs of One Fattening Farm. Animals 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Lundström, K.; Matthews, K.R.; Haugen, J.-E. Pig meat quality from entire males. Animal 2009, 3, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Pauly, C.; Luginbühl, W.; Ampuero, S.; Bee, G. Expected effects on carcass and pork quality when surgical castration is omitted—Results of a meta-analysis study. Meat Sci. 2012, 92, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Nautrup, B.P.; Vlaenderen, I.V.; Aldaz, A.; Mah, C.K. The effect of immunization against gonadotropin-releasing factor on growth performance, carcass characteristics and boar taint relevant to pig producers and the pork packing industry: A meta-analysis. Res. Vet. Sci. 2018, 119, 182–195. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Welfare aspects of the castration of piglets. EFSA J. 2004, 91, 19–118. Available online: https://ec.europa.eu/food/sites/food/files/animals/docs/aw_prac_farm_pigs_cast-alt_sci_efsa_opinion_welfare-aspects.pdf (accessed on 10 June 2019).
- Bee, G.; Chevillon, P.; Bonneau, M. Entire male pig production in Europe. Anim. Prod. Sci. 2015, 55. [Google Scholar] [CrossRef]
- European Commission. Establishing Best Practices on the Production, the Processing and the Marketing of Meat from Uncastrated Pigs or Pigs Vaccinated against Boar Taint (Immunocastrated). 2019. Available online: https://ec.europa.eu/food/sites/food/files/animals/docs/aw_prac_farm_pigs_cast-alt_establishing-best-practices.pdf (accessed on 10 June 2019).
- Bonneau, M.; Čandek-Potokar, M.; Škrlep, M.; Font-i-Furnols, M.; Aluwé, M.; Fontanesi, L. Potential sensitivity of pork production situations aiming at high-quality products to the use of entire male pigs as an alternative to surgical castrates. Animal 2018, 12, 1287–1295. [Google Scholar] [CrossRef]
- Font-i-Furnols, M. Consumer studies on sensory acceptability of boar taint: A review. Meat Sci. 2012, 92, 319–329. [Google Scholar] [CrossRef]
- Larzul, C.; Fontanesi, L.; Tholen, E.; Van Son, M. Genetic approaches for rearing entire males. Adv. Anim. Biosci. 2018, 9, S01. [Google Scholar]
- Schiavo, G.; Bovo, S.; Cheloni, S.; Ribani, A.; Geraci, C.; Gallo, M.; Etherington, G.; Palma, F.D.; Fontanesi, L. Mining whole genome resequencing data to identify functional mutations in boar taint-candidate genes. Adv. Anim. Biosci. 2018, 9, S04. [Google Scholar]
- Van Son, M.; Agarwal, R.; Grindflek, E.; Grove, H.; Kent, M.P.; Lien, S. Fine mapping of QTL regions for boar taint using whole genome re-sequencing data. Adv. Anim. Biosci. 2018, 9, S06. [Google Scholar]
- Wesoly, R.; Weiler, U. Nutritional Influences on Skatole Formation and Skatole Metabolism in the Pig. Animals 2012, 2, 221–242. [Google Scholar] [CrossRef] [PubMed]
- Zamaratskaia, G.; Rasmussen, M.K. Is it possible to reduce androstenone by dietary means? Adv. Anim. Biosci. 2018, 9, S22. [Google Scholar]
- Wesoly, R.; Jungbluth, I.; Stefanski, V.; Weiler, U. Pre-slaughter conditions influence skatole and androstenone in adipose tissue of boars. Meat Sci. 2015, 99, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.K.; Ten Napel, J.; Bloemhof, S.; Heres, L.; Knol, E.F.; Mulder, H.A. A human nose scoring system for boar taint and its relationship with androstenone and skatole. Meat Sci. 2012, 91, 414–422. [Google Scholar] [CrossRef]
- Haugen, J.E.; Brunius, C.; Zamaratskaia, G. Review of analytical methods to measure boar taint compounds in porcine adipose tissue: The need for harmonised methods. Meat Sci. 2012, 90, 9–19. [Google Scholar] [CrossRef]
- Hart, J.; Crew, A.; McGuire, N.; Doran, O. Sensor and Method for Detecting Androstenone or Skatole in Boar. Taint. Patent EP2966441 A1, 1 January 2016. Available online: https://google.com/patents/EP2966441A1?cl=en. (accessed on 19 June 2019).
- Verplanken, K. Rapid evaporative ionization mass spectrometry for high-throughput screening in food analysis: The case of boar taint. Talanta 2017, 169, 30–36. [Google Scholar] [CrossRef]
- Birkler, R.I.D.; Borggaard, C.; Støier, S.; Lund, B.L.W. Fully automated and rapid at-line method for measuring boar taint related compounds in back fat. Adv. Anim. Biosci. 2018, 9, S33. [Google Scholar]
- Caraty, A.; Bonneau, M. Active immunisation of male pig against gonadoliberine: Effects on gonadotropic hormone secretion and 5-alpha-androst-16-ene-3-one levels in adipose tissue. Comptes Rendus de l’Académie des Sciences 1986, 303, 673–676. [Google Scholar]
- Dunshea, F.R.; Colantoni, C.; Howard, K.; McCauley, I.; Jackson, P.; Long, K.A.; Lopaticki, S.; Nugent, E.A.; Simons, J.A.; Walker, J.; et al. Vaccination of boars with a GnRH vaccine (Improvac) eliminates boar taint and increases growth performance. J. Anim. Sci. 2001, 79, 2524–2535. [Google Scholar] [CrossRef]
- Čandek-Potokar, M.; Škrlep, M.; Zamaratskaia, G. Immunocastration as Alternative to Surgical Castration in Pigs. In Theriogenology; Payan-Carreira, R., Ed.; IntechOpen: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Kress, K.; Millet, S.; Labussière, É.; Weiler, U.; Stefanski, V. Sustainability of Pork Production with Immunocastration in Europe. Sustainability 2019, 11, 3335. [Google Scholar] [CrossRef]
- Batorek, N.; Čandek-Potokar, M.; Bonneau, M.; Van Milgen, J. Meta-analysis of the effect of immunocastration on production performance, reproductive organs and boar taint compounds in pigs. Animal 2012, 6, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Zamaratskaia, G.; Rydhmer, L.; Andersson, H.K.; Chen, G.; Lowagie, S.; Andersson, K.; Lundström, K. Long-term effect of vaccination against gonadotropin-releasing hormone, using Improvac, on hormonal profile and behaviour of male pigs. Anim. Reprod. Sci. 2008, 108, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Rydhmer, L.; Lundström, K.; Andersson, K. Immunocastration reduces aggressive and sexual behaviour in male pigs. Animal 2010, 4, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Karaconji, B.; Lloyd, B.; Campbell, N.; Meaney, D.; Ahern, T. Effect of an anti-gonadotropinreleasing factor vaccine on sexual and aggressive behaviour in male pigs during the finishing period under Australian field conditions. Aust. Vet. J. 2015, 93, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Cronin, G.M.; Dunshea, F.R.; Butler, K.L.; McCauly, I.; Branett, J.L.; Hemsworth, P.H. The effects of immuno- and surgical castration on the behaviour and consequently growth of group-housed, male finisher pigs. Appl. Anim. Behav. Sci. 2003, 81, 111–126. [Google Scholar] [CrossRef]
- EPA (European Medicines Agency). Improvac EPAR—Scientific Discussion. 2010. Available online: https://www.ema.europa.eu/en/documents/scientific-discussion/improvac-epar-scientific-discussion_en.pdf (accessed on 10 June 2019).
- Andersson, K.; Brunius, C.; Zamaratskaia, G.; Lundström, K. Early vaccination with Improvac®: Effects on performance and behaviour of male pigs. Animal 2012, 6, 87–95. [Google Scholar] [CrossRef]
- Izquierdo, M.; Pérez, M.A.; Del Rosario, A.I.; Rodriguez, P.; García, J.; Duarte, J.L.; Dalmau, A.; Hernández-García, F.I. The effect of immunocastration on carcass and meat cut yields in extensively reared Iberian gilts. Acta Agric. Slov. 2013, 4, 205–209. [Google Scholar]
- Pinna, A.; Schivazappa, C.; Virgili, R.; Parolari, G. Effect of vaccination against gonadotropin-reeasing hormone (GnRH) in heavy male pigs for Italian typical dry-cured ham production. Meat Sci. 2015, 110, 153–169. [Google Scholar] [CrossRef]
- Martinez-Macipe, M.; Rodríguez, P.; Izquierdo, M.; Gispert, M.; Manteca, X.; Mainauc, E.; Hernández, F.I. Comparison of meat quality parameters in surgical castrated versus vaccinated against gonadotropin-releasing factor male and female Iberian pigs reared in free ranging conditions. Meat Sci. 2016, 111, 116–121. [Google Scholar] [CrossRef]
- Einarsson, S. Vaccination against GnRH: Pros and cons. Acta Vet. Scand. 2006, 48, S10. [Google Scholar] [CrossRef]
- Spoolder, H.; Bracke, M.; Mueller-Graf, C.; Edwards, S. Preparatory Work for the Future Development of Animal Based Measures for Assessing the Welfare of Sow, Boar and Piglet including Aspects Related to Pig Castration. Technical Report Submitted to EFSA 2011; EFSA: Parma, Italy, 2011; pp. 1–108. Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/sp.efsa.2011.EN-178 (accessed on 10 June 2019).
- Castrum consortium. CASTRUM—Pig Castration for Traditional and Conventional Products: A Report on Methods and their Impacts on Animal Welfare, Meat Quality and Sustainability of European Pork Production Systems. Final Report. 2016. Available online: https://publications.europa.eu/en/publication-detail/-/publication/5fe8db00-dbb8-11e6-ad7c-01aa75ed71a1 (accessed on 10 June 2019).
- Haga, H.A.; Ranheim, B. Castration of piglets: The analgesic effects of intratesticular and intrafunicular lidocaine injection. Vet. Anaesth. Analg. 2005, 32, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kluivers-Poodt, M.; Robben, S.R.M.; Van Nes, A.; Houx, B.B. The effect of anaesthesia and/or analgesia on the response of piglets during castration. In Castration under Anaesthesia and/or Analgesia in Commercial Pig Production; Kluivers-Poodt, M., Hopster, H., Spoolder, H.A.M., Eds.; Report 85; Animal Science Group: Wageningen, The Netherlands, 2007; pp. 3–15. [Google Scholar]
- Hofmann, K.; Rauh, A.; Harlizius, J.; Weiß, C.; Scholz, T.; Schulze-Horsel, T.; Escribano, D.; Ritzmann, M.; Zöls, S. Pain and distress responses of suckling piglets to injection and castration under local anaesthesia with procaine and lidocaine—Part 1: Cortisol, chromogranin A, wound healing, weights, losses. Tierärztliche Praxis Ausgabe G: Großtiere/Nutztiere 2019, 47, 87–96. [Google Scholar] [CrossRef]
- Fredriksen, B.; Nafstad, O. Surveyed attitudes, perceptions and practices in Norway regarding the use of local anaesthesia in piglet castration. Res. Vet. Sci. 2006, 81, 293–295. [Google Scholar] [CrossRef]
- De Roest, K.; Montanari, C.; Fowler, T.; Baltussen, W. Resource efficiency and economic implications of alternatives to surgical castration without anaesthesia. Animal 2009, 3, 1522–1531. [Google Scholar] [CrossRef]
- Study and economic analysis of the costs and benefits of ending surgical castration of pigs. Available online: https://ec.europa.eu/food/sites/food/files/animals/docs/aw_prac_farm_pigs_cast-alt_research_civic_pt1-synthesis_20131202.pdf (accessed on 30 October 2019).
- Fredriksen, B.; Font-I-Furnols, M.; Lunström, K.; Migdal, W.; Prunier, A.; Tuyttens, F.; Bonneau, M. Practice on castration of piglets in Europe. Animal 2008, 3, 1480–1487. [Google Scholar] [CrossRef]
- Clarke, I.J.; Walker, J.S.; Hennessy, D.; Kreeger, J.; Nappier, J.M.; Crane, J.S. Inherent Food Safety of a Synthetic Gonadotropin-Releasing Factor (GnRF) Vaccine for the Control of Boar Taint in Entire Male Pigs. Int. J. Appl. Res. Vet. Med. 2008, 6, 7–14. [Google Scholar]
- Tomasevic, I.; Bahelka, I.; Čandek-Potokar, M.; Čítek, J.; Djekić, I.; Djurkin Kušec, I.; Getya, A.; Guerrero, A.; Iordachescu, G.; Nakov, D.; et al. Attitudes and beliefs of Eastern European consumers towards piglet castration and meat from castrated pigs. Meat Sci. 2019, 160, 107965. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonneau, M.; Weiler, U. Pros and Cons of Alternatives to Piglet Castration: Welfare, Boar Taint, and Other Meat Quality Traits. Animals 2019, 9, 884. https://doi.org/10.3390/ani9110884
Bonneau M, Weiler U. Pros and Cons of Alternatives to Piglet Castration: Welfare, Boar Taint, and Other Meat Quality Traits. Animals. 2019; 9(11):884. https://doi.org/10.3390/ani9110884
Chicago/Turabian StyleBonneau, Michel, and Ulrike Weiler. 2019. "Pros and Cons of Alternatives to Piglet Castration: Welfare, Boar Taint, and Other Meat Quality Traits" Animals 9, no. 11: 884. https://doi.org/10.3390/ani9110884
APA StyleBonneau, M., & Weiler, U. (2019). Pros and Cons of Alternatives to Piglet Castration: Welfare, Boar Taint, and Other Meat Quality Traits. Animals, 9(11), 884. https://doi.org/10.3390/ani9110884