Early Pregnancy Induces Expression of STAT1, OAS1 and CXCL10 in Ovine Spleen
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Tissue Collection
2.2. RNA Extraction and RT-QPCR Assay
2.3. Western Blot Analysis
2.4. Immunohistochemistry Analysis
2.5. Statistical Analysis
3. Results
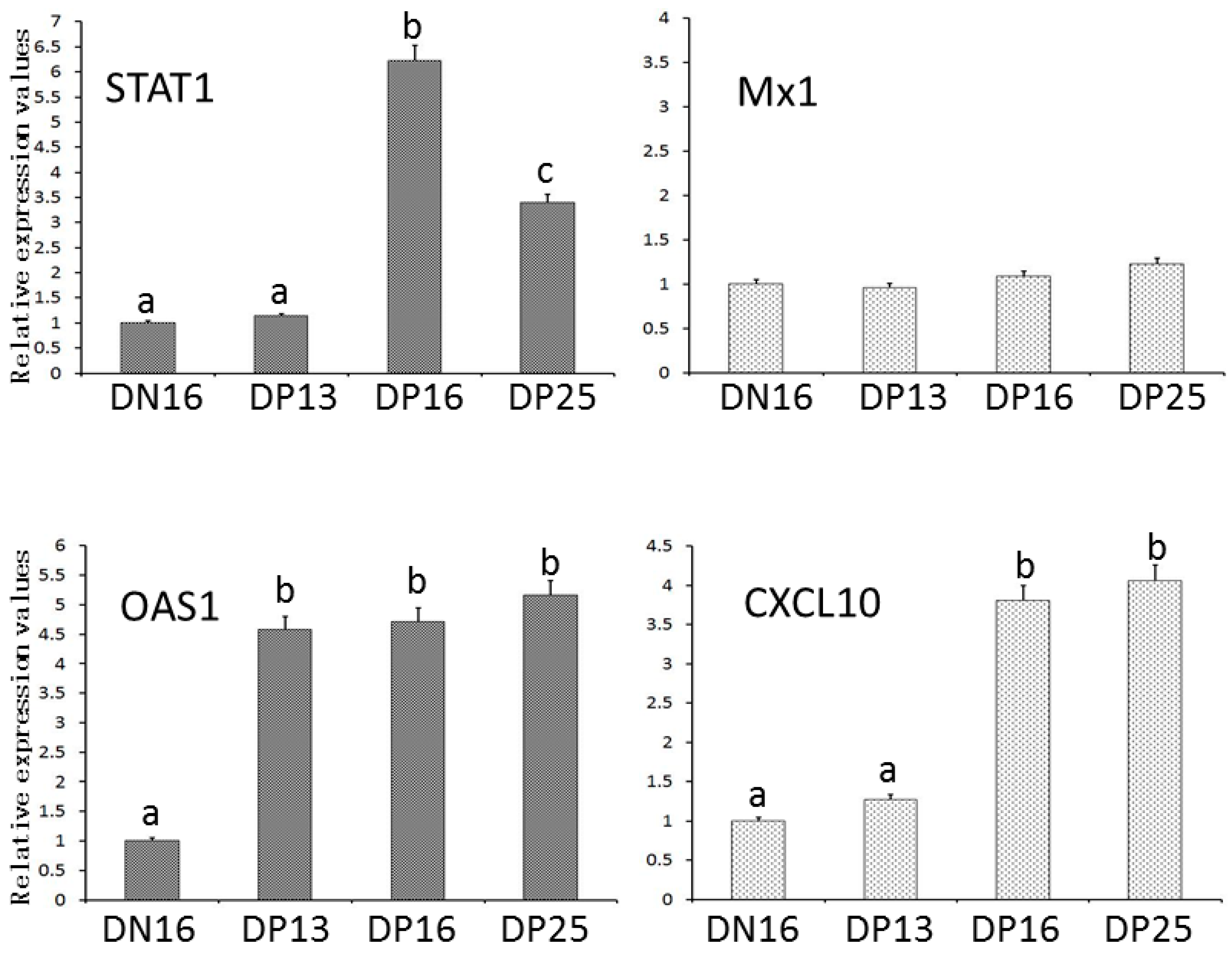
3.1. Expression of STAT1, OAS1, MX1 and CXCL10 mRNA in the Spleens
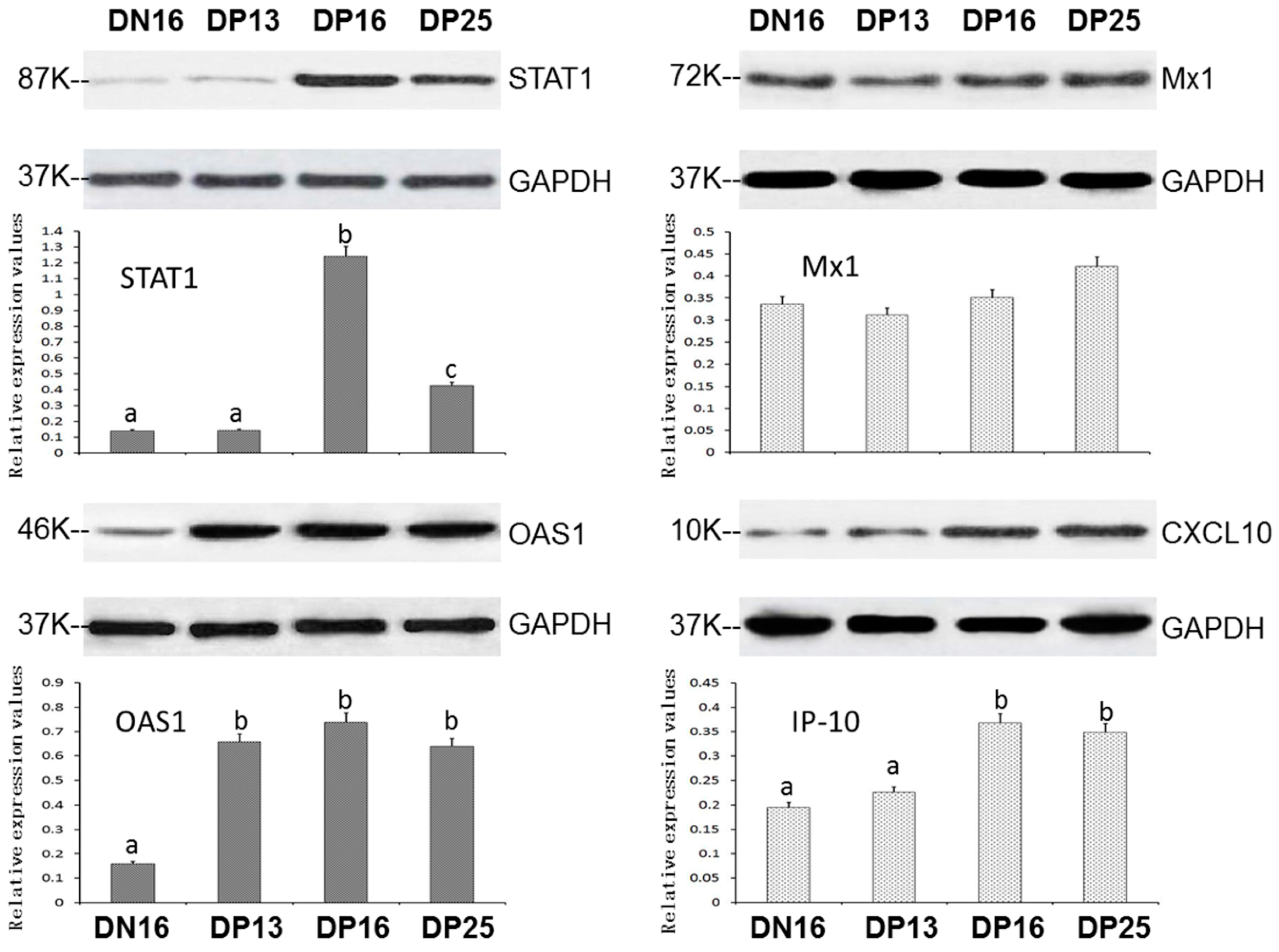
3.2. Expression of STAT1, OAS1, Mx1 and CXCL10 Proteins in the Spleens
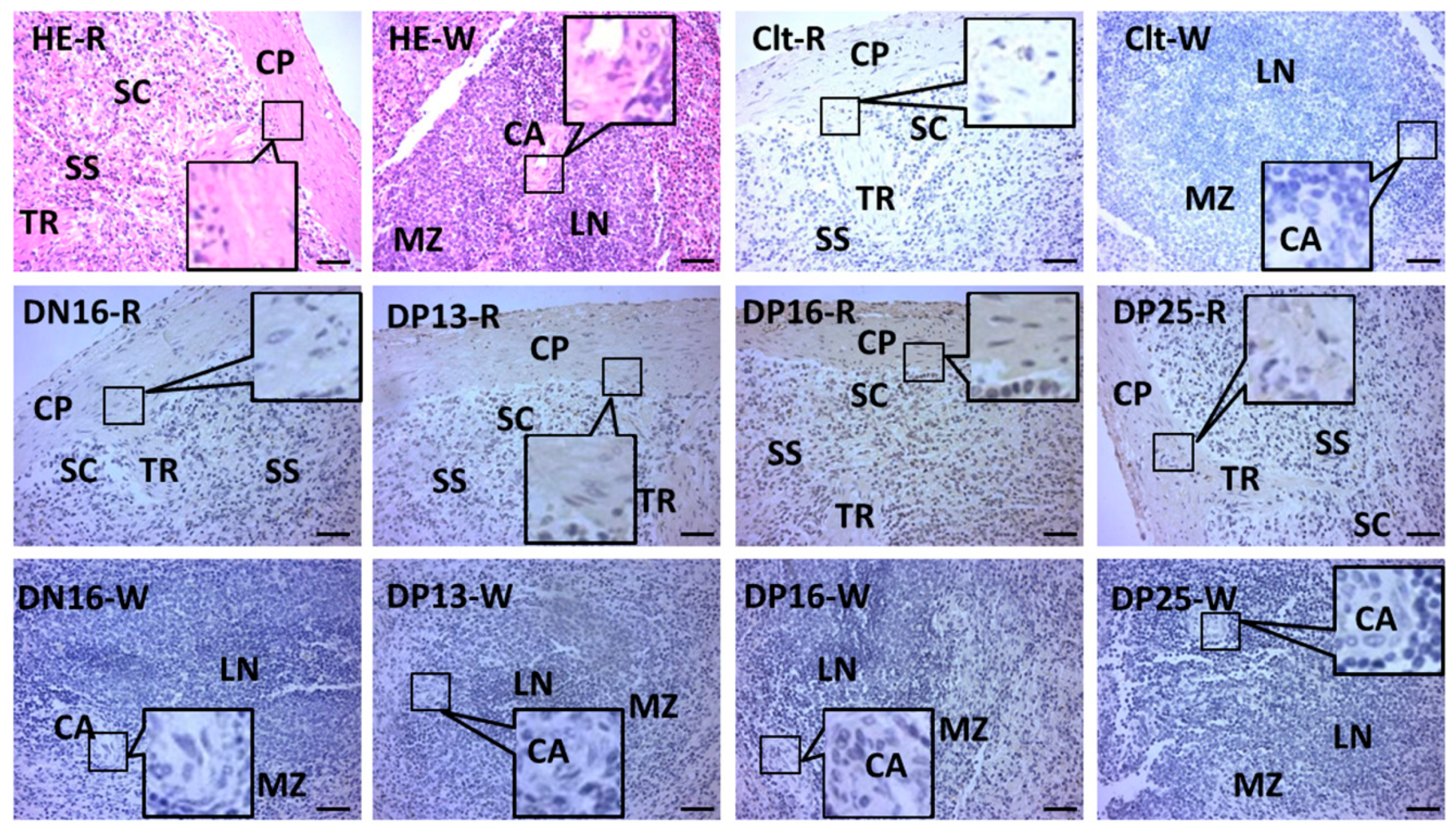
3.3. Immunohistochemistry for STAT1 Protein in the Spleens
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bazer, F.W.; Burghardt, R.C.; Johnson, G.A.; Spencer, T.E.; Wu, G. Mechanisms for the establishment and maintenance of pregnancy: Synergies from scientific collaborations. Biol. Reprod. 2018, 99, 225–241. [Google Scholar] [CrossRef] [PubMed]
- McCracken, J.A.; Einer-Jensen, N.; Fried, J. Prostaglandin F2 alpha and its 13-dehydro analogs: Comparative luteolytic effects in vivo. Adv. Exp. Med. Biol. 1979, 112, 577–601. [Google Scholar] [CrossRef] [PubMed]
- Bott, R.C.; Ashley, R.L.; Henkes, L.E.; Antoniazzi, A.Q.; Bruemmer, J.E.; Niswender, G.D.; Bazer, F.W.; Spencer, T.E.; Smirnova, N.P.; Anthony, R.V.; et al. Uterine vein infusion of interferon tau (IFNT) extends luteal life span in ewes. Biol. Reprod. 2010, 82, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.A.; Spencer, T.E.; Hansen, T.R.; Austin, K.J.; Burghardt, R.C.; Bazer, F.W. Expression of the interferon tau inducible ubiquitin cross-reactive protein in the ovine uterus. Biol. Reprod. 1999, 61, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Mirando, M.A.; Short, E.C., Jr.; Geisert, R.D.; Vallet, J.L.; Bazer, F.W. Stimulation of 2′,5′-oligoadenylate synthetase activity in sheep endometrium during pregnancy, by intrauterine infusion of ovine trophoblast protein-1, and by intramuscular administration of recombinant bovine interferon-alpha I1. J. Reprod. Fertil. 1991, 93, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Ott, T.L.; Yin, J.; Wiley, A.A.; Kim, H.T.; Gerami-Naini, B.; Spencer, T.E.; Bartol, F.F.; Burghardt, R.C.; Bazer, F.W. Effects of the estrous cycle and early pregnancy on uterine expression of Mx protein in sheep (Ovis aries). Biol. Reprod. 1998, 59, 784–794. [Google Scholar] [CrossRef]
- Oliveira, J.F.; Henkes, L.E.; Ashley, R.L.; Purcell, S.H.; Smirnova, N.P.; Veeramachaneni, D.N.; Anthony, R.V.; Hansen, T.R. Expression of interferon (IFN)-stimulated genes in extrauterine tissues during early pregnancy in sheep is the consequence of endocrine IFN-tau release from the uterine vein. Endocrinology 2008, 149, 1252–1259. [Google Scholar] [CrossRef]
- Yang, L.; Liu, B.; Yan, X.; Zhang, L.; Gao, F.; Liu, Z. Expression of ISG15 in bone marrow during early pregnancy in ewes. Kafkas Univ. Vet. Fak. Derg. 2017, 23, 767–772. [Google Scholar] [CrossRef]
- Zhang, L.; Xue, J.; Wang, Q.; Lv, W.; Mi, H.; Liu, Y.; Yang, L. Changes in expression of ISG15, progesterone receptor and progesterone-induced blocking factor in ovine thymus during early pregnancy. Theriogenology 2018, 121, 153–159. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Y.; Lv, W.; Wang, P.; Wang, B.; Xue, J.; Zhang, L. Expression of interferon-stimulated gene 15-kDa protein, cyclooxygenase (COX) 1, COX-2, aldo-keto reductase family 1, member B1, and prostaglandin E synthase in the spleen during early pregnancy in sheep. Anim. Sci. J. 2018, 89, 1540–1548. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Q.; Liu, Y.; Zhang, L.; Lv, W.; Liu, B. Expression profiles of interferon-stimulated gene 15 and prostaglandin synthases in the ovine lymph nodes during early pregnancy. Mol. Reprod. Dev. 2019, 86, 100–108. [Google Scholar] [CrossRef] [PubMed]
- De Porto, A.P.; Lammers, A.J.; Bennink, R.J.; ten Berge, I.J.; Speelman, P.; Hoekstra, J.B. Assessment of splenic function. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Mebius, R.E.; Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 2005, 5, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Chelmonska-Soyta, A.; Ozgo, M.; Lepczynski, A.; Herosimczyk, A.; Buska-Pisarek, K.; Kedzierska, A.; Nowak, D.; Mazur, A.J. Proteome of spleen CD4 lymphocytes in mouse preimplantation pregnancy. J. Physiol. Pharmacol. 2014, 65, 719–731. Available online: http://www.jpp.krakow.pl/journal/archive/10_14/pdf/719_10_14_article.pdf (accessed on 22 May 2019).
- Yang, L.; Guo, R.; Yao, X.; Yan, J.; Bai, Y.; Zhang, L. Expression of progesterone receptor and progesterone-induced blocking factor in the spleen during early pregnancy in ewes. Livest. Sci. 2018, 209, 14–19. [Google Scholar] [CrossRef]
- Li, N.; Zhao, Z.; Bai, J.; Liu, B.; Mi, H.; Zhang, L.; Li, G.; Yang, L. Characterization of the Th cytokines profile in ovine spleen during early pregnancy. J. Appl. Anim. Res. 2019, 47, 386–393. [Google Scholar] [CrossRef]
- Li, J.; Roberts, R.M. Interferon-tau and interferon-alpha interact with the same receptors in bovine endometrium. Use of a readily iodinatable form of recombinant interferon-tau for binding studies. J. Biol. Chem. 1994, 269, 13544–13550. [Google Scholar] [CrossRef]
- Binelli, M.; Subramaniam, P.; Diaz, T.; Johnson, G.; Hansen, T.R.; Badinga, L.; Thatcher, W.W. Bovine interferon-tau stimulates the Janus kinase-signal transducer and activator of transcription pathway in bovine endometrial epithelial cells. Biol. Reprod. 2001, 64, 654–665. [Google Scholar] [CrossRef]
- Choi, Y.; Johnson, G.A.; Burghardt, R.C.; Berghman, L.R.; Joyce, M.M.; Taylor, K.M.; Stewart, M.D.; Bazer, F.W.; Spencer, T.E. Interferon regulatory factor-two restricts expression of interferon-stimulated genes to the endometrial stroma and glandular epithelium of the ovine uterus. Biol. Reprod. 2001, 65, 1038–1049. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Davis, W.H.; Beer, J.R.; Cook, E.F. Effects of pregnancy on the spleen in mice. J. Mammal. 1961, 42, 53–56. [Google Scholar] [CrossRef]
- Mattsson, R.; Ocklind, G.; Andersson, M. Splenic macrophages during pregnancy in the mouse. Dev. Comp. Immunol. 1984, 8, 443–450. [Google Scholar] [CrossRef]
- Pan, H.; Ma, Y.; Wang, D.; Wang, J.; Jiang, H.; Pan, S.; Zhao, B.; Wu, Y.; Xu, D.; Sun, X.; et al. Effect of IFN-α on KC and LIX expression: Role of STAT1 and its effect on neutrophil recruitment to the spleen after lipopolysaccharide stimulation. Mol. Immunol. 2013, 56, 12–22. [Google Scholar] [CrossRef]
- Stewart, M.D.; Johnson, G.A.; Vyhlidal, C.A.; Burghardt, R.C.; Safe, S.H.; Yu-Lee, L.Y.; Bazer, F.W.; Spencer, T.E. Interferon-tau activates multiple signal transducer and activator of transcription proteins and has complex effects on interferon-responsive gene transcription in ovine endometrial epithelial cells. Endocrinology 2001, 142, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Care, M.A.; Westhead, D.R.; Tooze, R.M. Gene expression meta-analysis reveals immune response convergence on the IFNγ-STAT1-IRF1 axis and adaptive immune resistance mechanisms in lymphoma. Genome Med. 2015, 7, 96. [Google Scholar] [CrossRef]
- Seif, F.; Khoshmirsafa, M.; Aazami, H.; Mohsenzadegan, M.; Sedighi, G.; Bahar, M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun. Signal. 2017, 15, 23. [Google Scholar] [CrossRef]
- Sheikh, A.A.; Hooda, O.K.; Kalyan, A.; Kamboj, A.; Mohammed, S.; Alhussien, M.; Reddi, S.; Shimray, P.G.; Rautela, A.; Pandita, S.; et al. Interferon-tau stimulated gene expression: A proxy to predict embryonic mortality in dairy cows. Theriogenology 2018, 120, 61–67. [Google Scholar] [CrossRef]
- Pugliesi, G.; Miagawa, B.T.; Paiva, Y.N.; França, M.R.; Silva, L.A.; Binelli, M. Conceptus-induced changes in the gene expression of blood immune cells and the ultrasound-accessed luteal function in beef cattle: How early can we detect pregnancy? Biol. Reprod. 2014, 91, 1–12. [Google Scholar] [CrossRef]
- Ruhmann, B.; Giller, K.; Hankele, A.K.; Ulbrich, S.E.; Schmicke, M. Interferon-τ induced gene expression in bovine hepatocytes during early pregnancy. Theriogenology 2017, 104, 198–204. [Google Scholar] [CrossRef]
- Leisching, G.; Cole, V.; Ali, A.T.; Baker, B. OAS1, OAS2 and OAS3 restrict intracellular M. tb replication and enhance cytokine secretion. Int. J. Infect. Dis. 2019, 80, S77–S84. [Google Scholar] [CrossRef]
- Pillai, P.S.; Molony, R.D.; Martinod, K.; Dong, H.; Pang, I.K.; Tal, M.C.; Solis, A.G.; Bielecki, P.; Mohanty, S.; Trentalange, M.; et al. Mx1 reveals innate pathways to antiviral resistance and lethal influenza disease. Science 2016, 352, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Gifford, C.A.; Racicot, K.; Clark, D.S.; Austin, K.J.; Hansen, T.R.; Lucy, M.C.; Davies, C.J.; Ott, T.L. Regulation of interferon-stimulated genes in peripheral blood leukocytes in pregnant and bred, nonpregnant dairy cows. J. Dairy Sci. 2007, 90, 274–280. [Google Scholar] [CrossRef]
- Romero, J.J.; Antoniazzi, A.Q.; Smirnova, N.P.; Webb, B.T.; Yu, F.; Davis, J.S.; Hansen, T.R. Pregnancy-associated genes contribute to antiluteolytic mechanisms in ovine corpus luteum. Physiol. Genom. 2013, 45, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Toyokawa, K.; Carling, S.J.; Ott, T.L. Cellular localization and function of the antiviral protein, ovine Mx1 (oMx1): I. Ovine Mx1 is secreted by endometrial epithelial cells via an ‘unconventional’ secretory pathway. Am. J. Reprod. Immunol. 2007, 57, 13–22. [Google Scholar] [CrossRef]
- Meyerholz, M.M.; Mense, K.; Knaack, H.; Sandra, O.; Schmicke, M. Pregnancy-induced ISG-15 and MX-1 gene expression is detected in the liver of Holstein-Friesian heifers during late peri-implantation period. Reprod. Domest. Anim. 2016, 51, 175–177. [Google Scholar] [CrossRef]
- Simon, R.; Heithoff, D.M.; Mahan, M.J.; Samuel, C.E. Comparison of tissue-selective proinflammatory gene induction in mice infected with wild-type, DNA adenine methylase-deficient, and flagellin-deficient Salmonella enterica. Infect. Immun. 2007, 75, 5627–5669. [Google Scholar] [CrossRef]
- Lalić, I.M.; Bichele, R.; Repar, A.; Despotović, S.Z.; Petričević, S.; Laan, M.; Peterson, P.; Westermann, J.; Milićević, Ž.; Mirkov, I.; et al. Lipopolysaccharide induces tumor necrosis factor receptor-1 independent relocation of lymphocytes from the red pulp of the mouse spleen. Ann. Anat. 2018, 216, 125–134. [Google Scholar] [CrossRef]
- Sakumoto, R.; Hayashi, K.G.; Fujii, S.; Kanahara, H.; Hosoe, M.; Furusawa, T.; Kizaki, K. Possible Roles of CC- and CXC-Chemokines in Regulating Bovine Endometrial Function during Early Pregnancy. Int. J. Mol. Sci. 2017, 18, E742. [Google Scholar] [CrossRef]
- Sakumoto, R.; Iga, K.; Hayashi, K.G.; Fujii, S.; Kanahara, H.; Hosoe, M.; Furusawa, T. Gene expression of CCL8 and CXCL10 in peripheral blood leukocytes during early pregnancy in cows. J. Anim. Sci. Biotechnol. 2018, 9, 46. [Google Scholar] [CrossRef]
- Gray, C.A.; Abbey, C.A.; Beremand, P.D.; Choi, Y.; Farmer, J.L.; Adelson, D.L.; Thomas, T.L.; Bazer, F.W.; Spencer, T.E. Identification of endometrial genes regulated by early pregnancy, progesterone, and interferon tau in the ovine uterus. Biol. Reprod. 2006, 74, 383–394. [Google Scholar] [CrossRef]
- Mauffré, V.; Grimard, B.; Eozenou, C.; Inghels, S.; Silva, L.; Giraud-Delville, C.; Capo, D.; Sandra, O.; Constant, F. Interferon stimulated genes as peripheral diagnostic markers of early pregnancy in sheep: A critical assessment. Animal 2016, 10, 1856–1863. [Google Scholar] [CrossRef] [PubMed]
- Imakawa, K.; Imai, M.; Sakai, A.; Suzuki, M.; Nagaoka, K.; Sakai, S.; Lee, S.R.; Chang, K.T.; Echternkamp, S.E.; Christenson, R.K. Regulation of conceptus adhesion by endometrial CXC chemokines during the implantation period in sheep. Mol. Reprod. Dev. 2006, 73, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Gu, M.J.; Yoo, I.; Choi, Y.; Jang, H.; Kim, M.; Yun, C.H.; Ka, H. Analysis of cysteine-X-cysteine motif chemokine ligands 9, 10, and 11, their receptor CXCR3, and their possible role on the recruitment of immune cells at the maternal-conceptus interface in pigs. Biol. Reprod. 2017, 97, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Steiniger, B.S. Human spleen microanatomy: Why mice do not suffice. Immunology 2015, 145, 334–346. [Google Scholar] [CrossRef]
- Den Haan, J.M.; Kraal, G. Innate immune functions of macrophage subpopulations in the spleen. J. Innate Immun. 2012, 4, 437–445. [Google Scholar] [CrossRef]
- Swirski, F.K.; Nahrendorf, M.; Etzrodt, M.; Wildgruber, M.; Cortez-Retamozo, V.; Panizzi, P.; Figueiredo, J.L.; Kohler, R.H.; Chudnovskiy, A.; Waterman, P.; et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009, 325, 612–616. [Google Scholar] [CrossRef]
- Godkin, J.D.; Bazer, F.W.; Moffatt, J.; Sessions, F.; Roberts, R.M. Purification and properties of a major, low molecular weight protein released by the trophoblast of sheep blastocysts at day 13–21. J. Reprod. Fertil. 1982, 65, 141–150. [Google Scholar] [CrossRef]
- Mcnatty, K.P.; Revefeim, K.J.; Young, A. Peripheral plasma progesterone concentrations in sheep during the oestrous cycle. J. Endocrinol. 1973, 58, 219–225. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, L.Y.; Wang, C.; Wang, B.; Wang, X.M.; Zeng, S.M. Differential expression pattern of ISG15 in different tissue explants and cells induced by various interferons. Microbiol. Immunol. 2012, 56, 163–170. [Google Scholar] [CrossRef]
| Gene | Primer | Sequence | Size (bp) | Accession Numbers |
|---|---|---|---|---|
| STAT1 | Forward | GTGGCGGAGAGTCTGCAGCA | 190 | NM_001166203.1 |
| Reverse | GGTGAGTTGGCATGCAGGGC | |||
| OAS1 | Forward | AGCCTTCCTGAAGAGTCGTCCTAC | 88 | XM_012097882.2 |
| Reverse | TCCAAGCTGCTCCTTACACAGTTG | |||
| MX1 | Forward | CCACCACCGACAGCTCCCCT | 147 | NM_001009753.1 |
| Reverse | GCAGGTGTGGGCGTGAAGCA | |||
| CXCL10 | Forward | TCTAGGAACACACGCTGCAC | 108 | NM_001009191.1 |
| Reverse | GACACGTGGGCAGGATTGAC | |||
| GAPDH | Forward | GGGTCATCATCTCTGCACCT | 176 | NM_001190390.1 |
| Reverse | GGTCATAAGTCCCTCCACGA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Han, X.; Zhang, L.; Cao, N.; Cao, L.; Yang, L. Early Pregnancy Induces Expression of STAT1, OAS1 and CXCL10 in Ovine Spleen. Animals 2019, 9, 882. https://doi.org/10.3390/ani9110882
Wang Y, Han X, Zhang L, Cao N, Cao L, Yang L. Early Pregnancy Induces Expression of STAT1, OAS1 and CXCL10 in Ovine Spleen. Animals. 2019; 9(11):882. https://doi.org/10.3390/ani9110882
Chicago/Turabian StyleWang, Yujiao, Xu Han, Leying Zhang, Nan Cao, Lidong Cao, and Ling Yang. 2019. "Early Pregnancy Induces Expression of STAT1, OAS1 and CXCL10 in Ovine Spleen" Animals 9, no. 11: 882. https://doi.org/10.3390/ani9110882
APA StyleWang, Y., Han, X., Zhang, L., Cao, N., Cao, L., & Yang, L. (2019). Early Pregnancy Induces Expression of STAT1, OAS1 and CXCL10 in Ovine Spleen. Animals, 9(11), 882. https://doi.org/10.3390/ani9110882





