Fine Particulate Matter (PM) Effects on Swine Granulosa and Ovarian Endothelial Cells
Simple Summary
Abstract
1. Introduction
- Inhaled PM2.5 particles, along with their adsorbed toxic chemical payload, are deposited in the lung alveoli [5].
- Within the ovarian microenvironment, particles may extravasate from the capillaries and become sequestered in the ovarian stroma or interstitial tissue. From here, they can directly interact with the cells of the follicular unit—including theca cells, granulosa cells, and the oocyte—as well as the endothelial cells of the ovarian vasculature [9].
- This direct, localized exposure to a complex mixture of toxicants, superimposed upon a state of systemic inflammation induced by the pulmonary response [10], initiates the cascade of cellular damage, including oxidative stress, inflammation, disrupted cell replication, and altered endocrine function.
2. Materials and Methods
2.1. Isolation and Culture of Swine Granulosa Cells and Endothelial Cells from Swine Corpus Luteum
2.2. Cell Proliferation
2.3. Cell Metabolic Activity
2.4. Cell Redox Status
2.4.1. Non-Enzymatic Scavenging Activity
2.4.2. Enzymatic Scavenging Activity: Superoxide Dismutase (SOD)
2.5. Nitric Oxide (NO) Production
2.6. Superoxide (O2−) Production
2.7. 8-Hydroxydeoxyguanosine (8-OHdG)
2.8. Granulosa Cell Steroidogenesis
2.9. Autophagy in Granulosa Cells
2.10. Endothelial Cell Vascular Endothelial Growth Factor (VEGF) Production
2.11. Statistical Analysis
3. Results
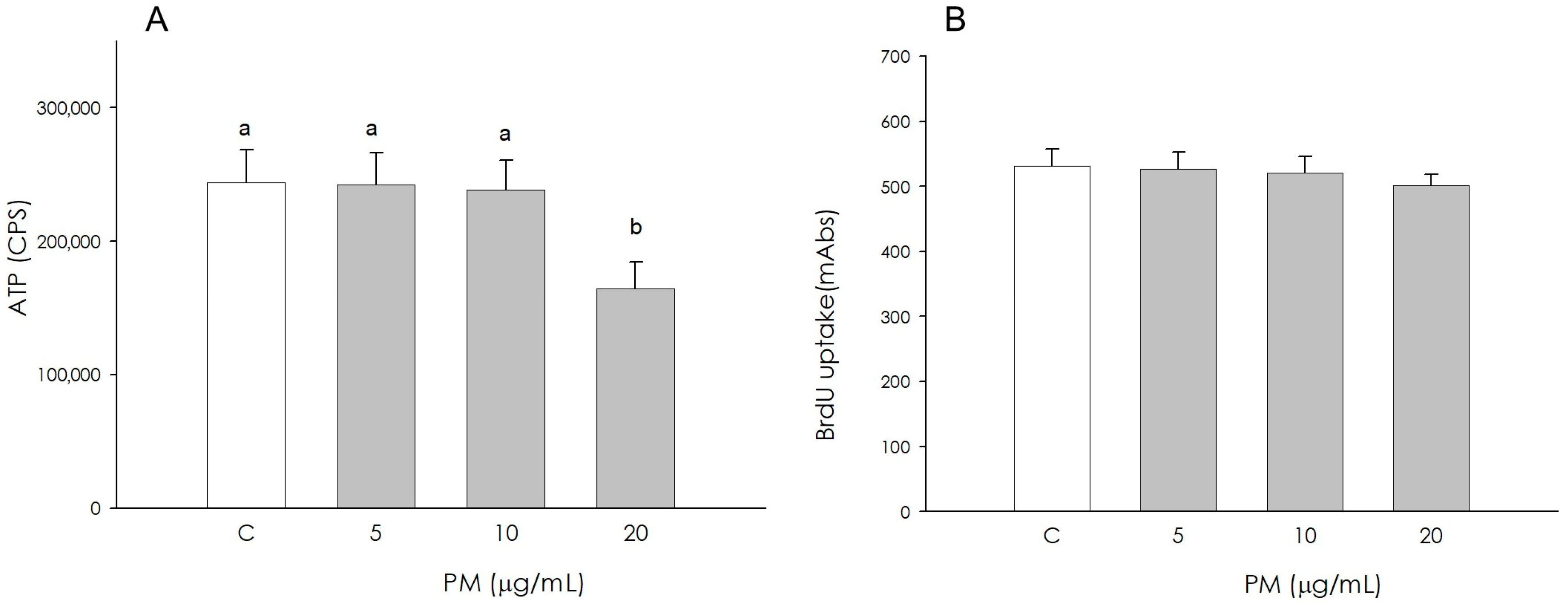
3.1. Granulosa Cell Growth
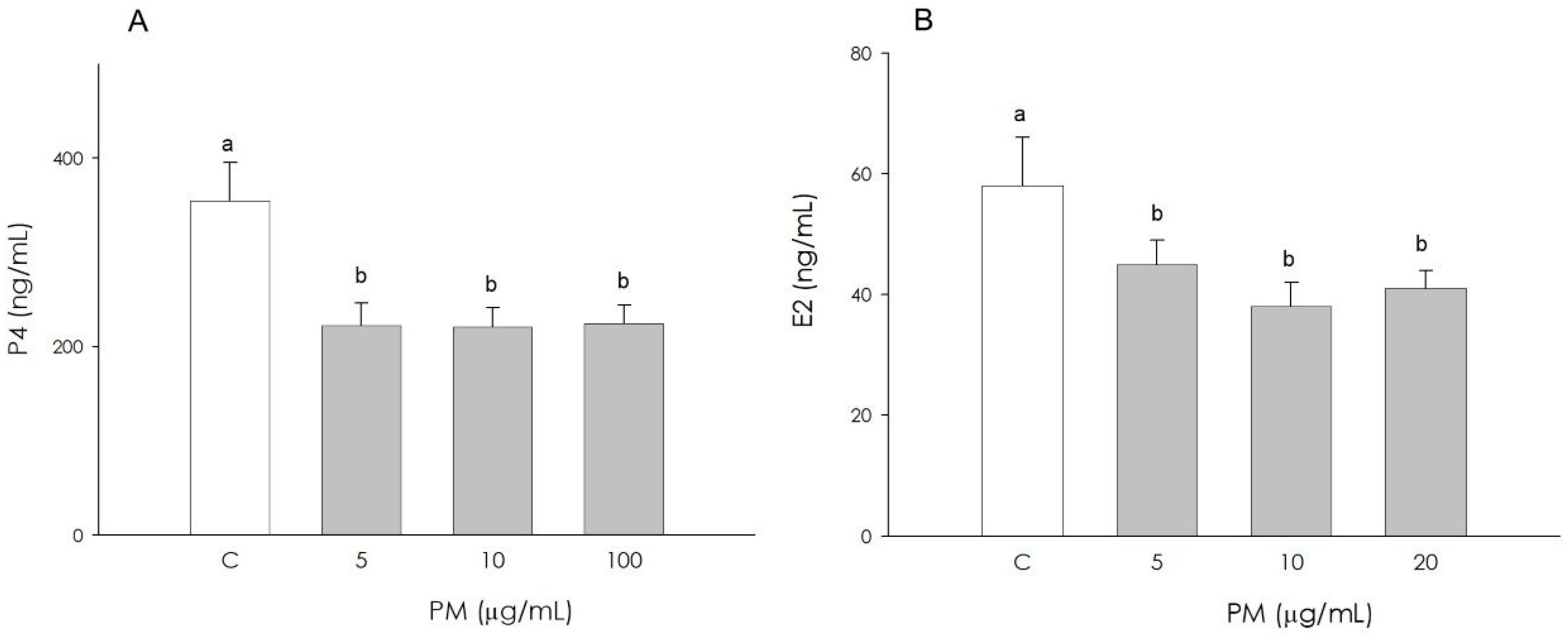
3.2. Granulosa Cell Steroidogenesis
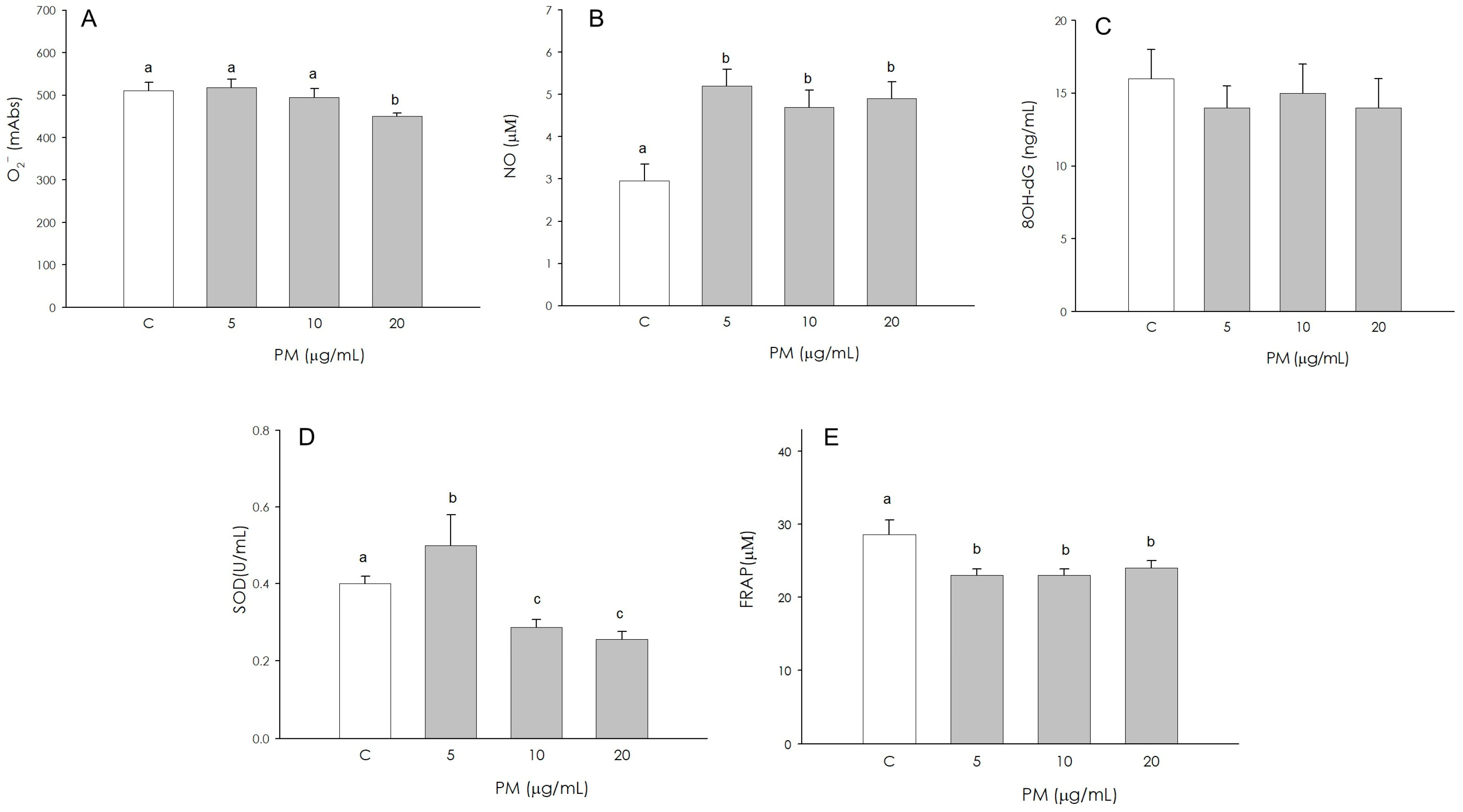
3.3. Granulosa Cell Redox Status
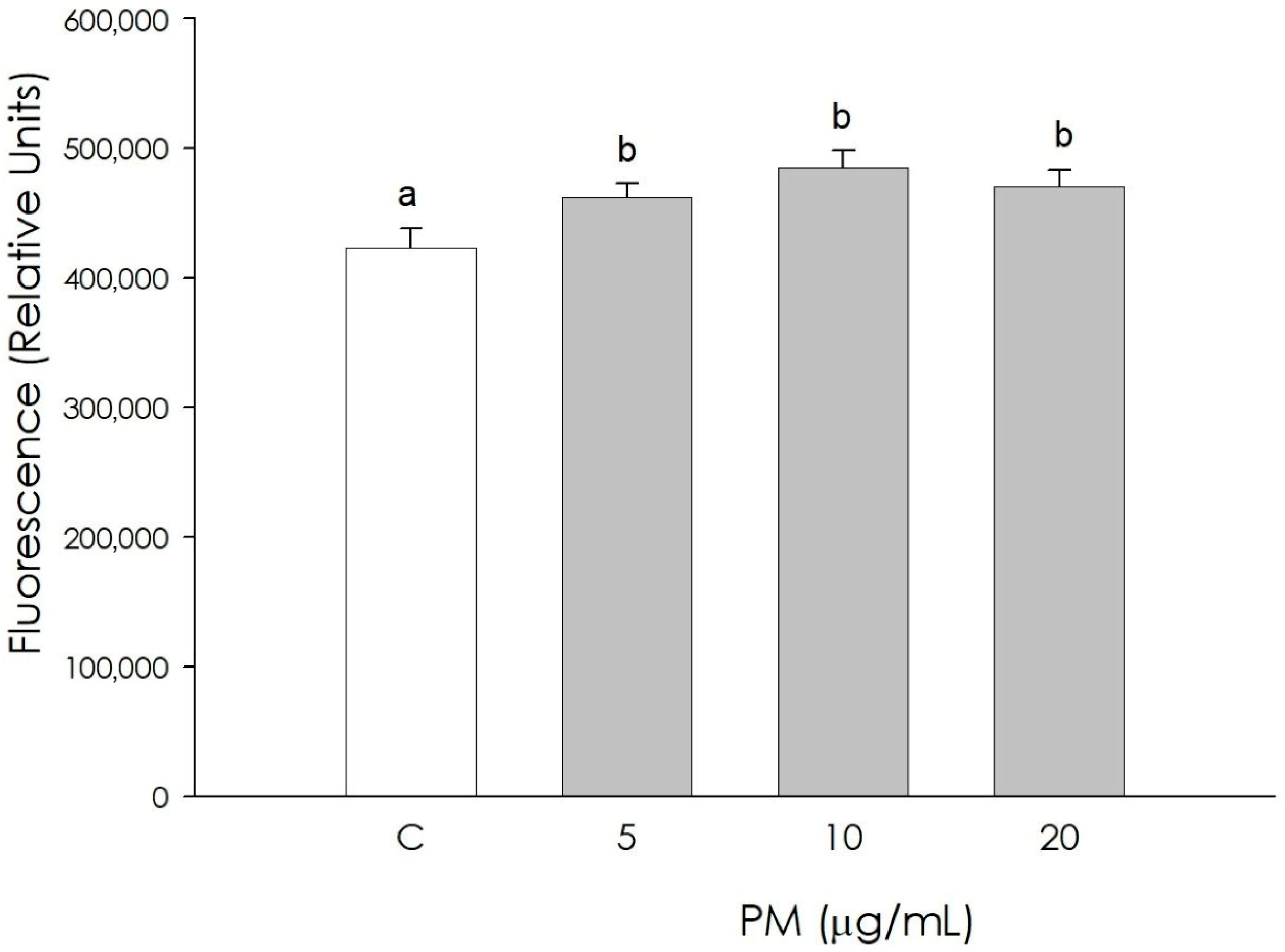
3.4. Granulosa Cell Autophagy
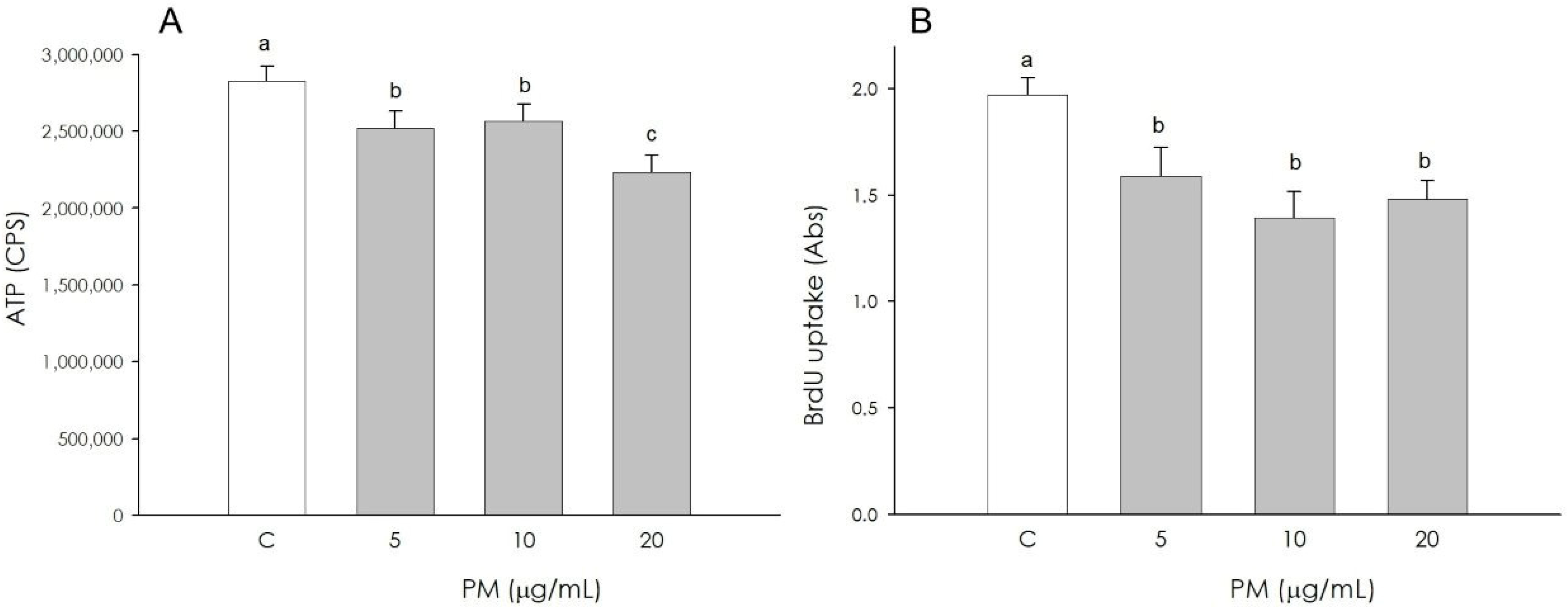
3.5. Endothelial Cell Growth
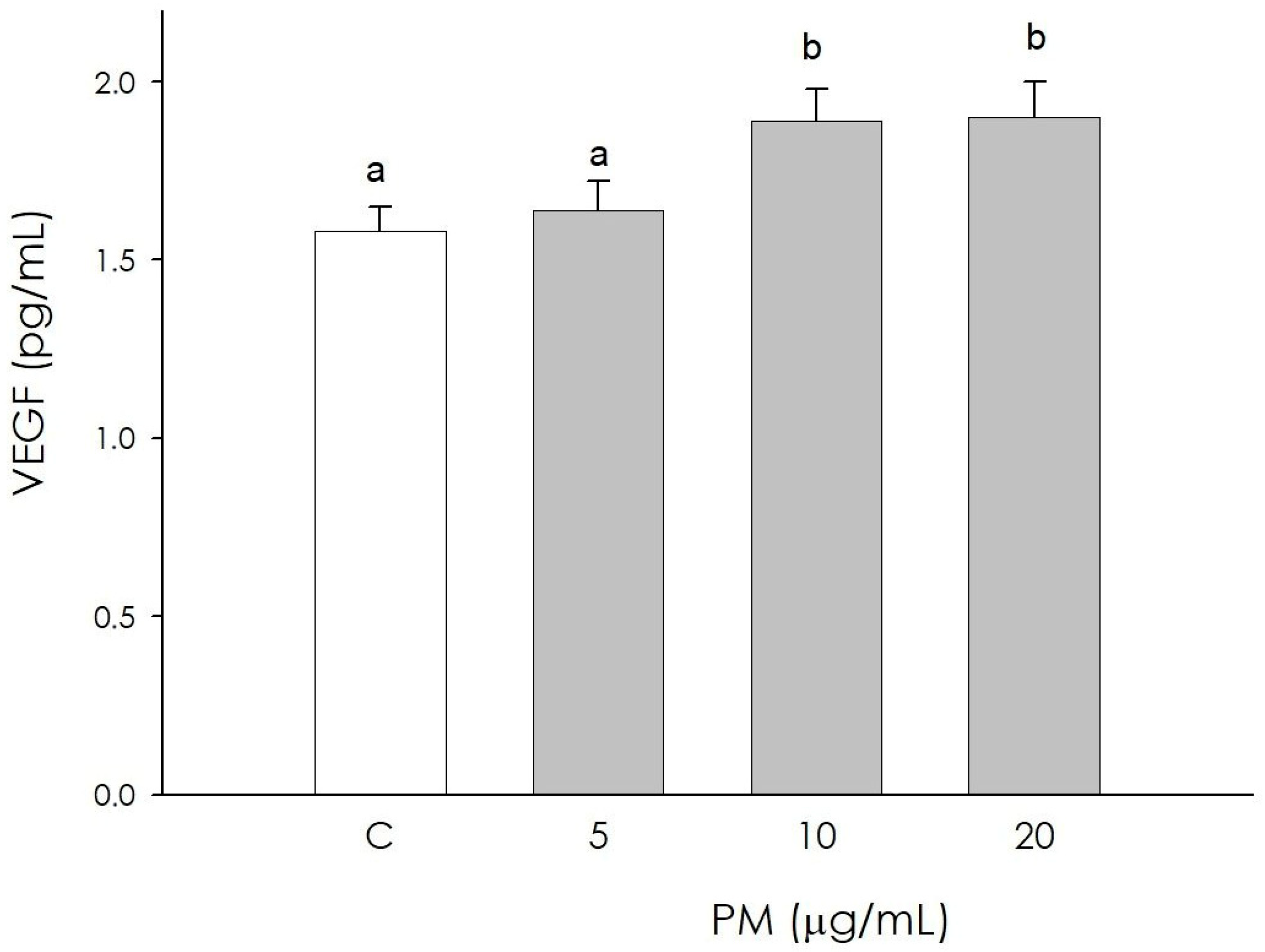
3.6. Endothelial Cell VEGF Production
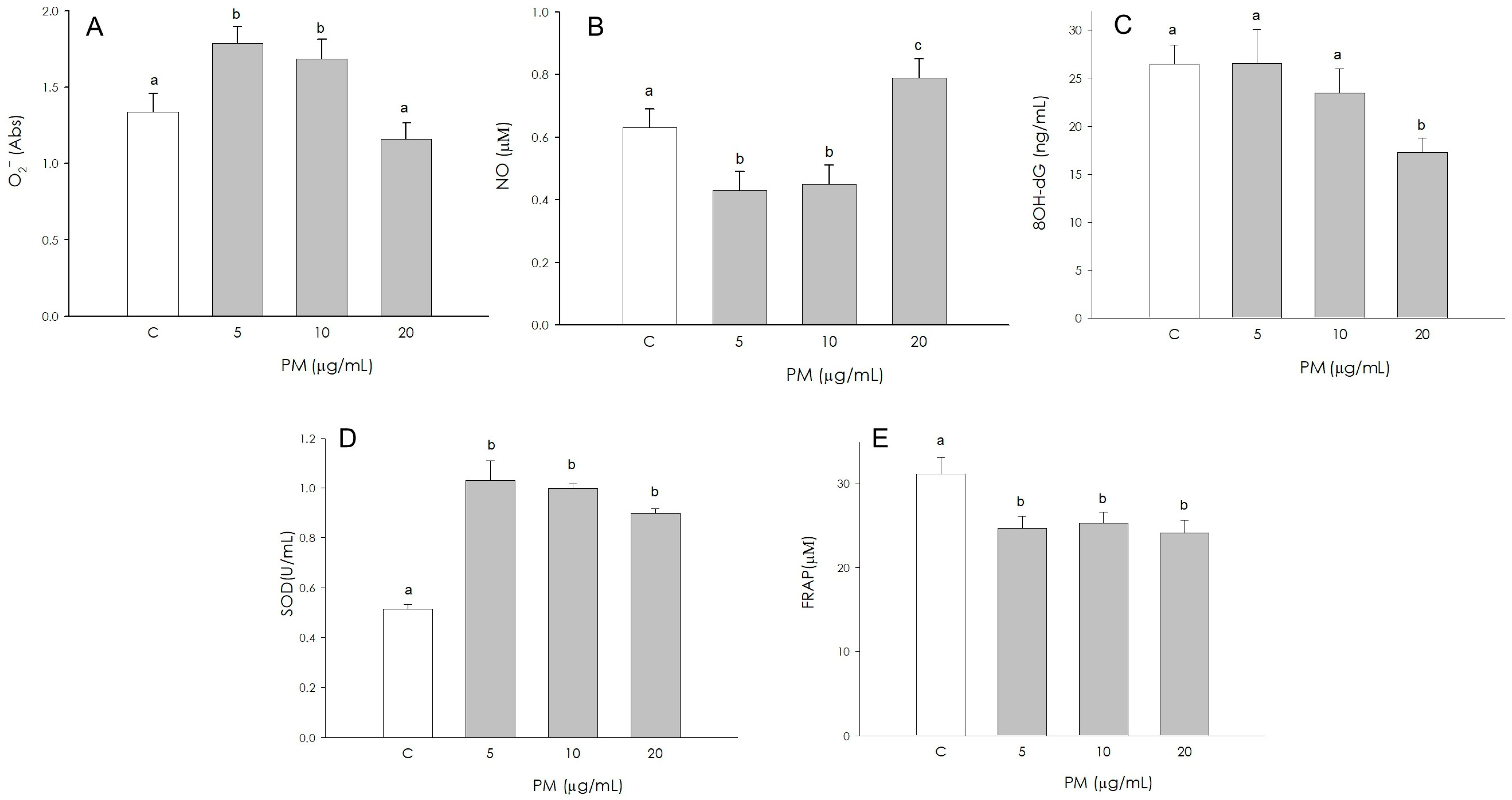
3.7. Endothelial Cell Redox Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Available online: https://www.who.int/health-topics/air-pollution#tab=tab_1 (accessed on 18 November 2025).
- Madureira, J.; Slezakova, K.; Costa, C.; Pereira, M.C.; Teixeira, J.P. Assessment of indoor air exposure among newborns and their mothers: Levels and sources of PM10, PM2.5 and ultrafine particles at 65 home environments. Environ. Pollut. 2020, 264, 114746. [Google Scholar] [CrossRef]
- Li, B.; Ma, Y.; Zhou, Y.; Chai, E. Research progress of different components of PM2.5 and ischemic stroke. Sci. Rep. 2023, 13, 15965. [Google Scholar] [CrossRef]
- Amnuaylojaroen, T.; Parasin, N. Pathogenesis of PM2.5-Related Disorders in Different Age Groups: Children, Adults, and the Elderly. Epigenomes 2024, 8, 13. [Google Scholar] [CrossRef]
- Thangavel, P.; Park, D.; Lee, Y.C. Recent Insights into Particulate Matter (PM2.5)-Mediated Toxicity in Humans: An Overview. Int. J. Environ. Res. Public Health 2022, 19, 7511. [Google Scholar] [CrossRef]
- Elder, A.; Oberdörster, G. Translocation and effects of ultrafine particles outside of the lung. Clin. Occup. Environ. Med. 2006, 54, 785–796. [Google Scholar] [CrossRef]
- Li, D.; Li, Y.; Li, G.; Zhang, Y.; Li, J.; Chen, H. Fluorescent reconstitution on deposition of PM2.5 in lung and extrapulmonary organs. Proc. Natl. Acad. Sci. USA 2019, 116, 2488–2493. [Google Scholar] [CrossRef]
- Gangwar, R.S.; Bevan, G.H.; Palanivel, R.; Das, L.; Rajagopalan, S. Oxidative stress pathways of air pollution mediated toxicity: Recent insights. Redox Biol. 2020, 34, 101545. [Google Scholar] [CrossRef]
- Liu, X.; Ge, P.; Lu, Z.; Yang, R.; Liu, Z.; Zhao, F.; Chen, M. Reproductive toxicity and underlying mechanisms of fine particulate matter (PM2.5) on Caenorhabditis elegans in different seasons. Ecotoxicol. Environ. Saf. 2022, 248, 114281. [Google Scholar] [CrossRef]
- Kurlawala, Z.; Singh, P.; Hill, B.G.; Haberzettl, P. Fine Particulate Matter (PM2.5)-Induced Pulmonary Oxidative Stress Contributes to Changes in the Plasma Lipidome and Liver Transcriptome in Mice. Toxicol. Sci. 2023, 192, 209–222. [Google Scholar] [CrossRef]
- GBD 2021 Fertility and Forecasting Collaborators. Global fertility in 204 countries and territories, 1950–2021, with forecasts to 2100: A comprehensive demographic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2057–2099. [Google Scholar] [CrossRef]
- Bala, R.; Singh, V.; Rajender, S.; Singh, K. Environment, Lifestyle, and Female Infertility. Reprod. Sci. 2021, 28, 617–638. [Google Scholar] [CrossRef]
- Seli, D.A.; Taylor, H.S. The impact of air pollution and endocrine disruptors on reproduction and assisted reproduction. Curr. Opin. Obstet. Gynecol. 2023, 35, 210–215. [Google Scholar] [CrossRef]
- Tao, Q.; Zhao, Z.; Yang, R.; Li, Q.; Qiao, J. Fine particulate matter and ovarian health: A review of emerging risks. Heliyon 2024, 10, e40503. [Google Scholar] [CrossRef]
- Chen, Y.; Xi, Y.; Li, M.; Wu, Y.; Yan, W.; Dai, J.; Wu, M.; Ding, W.; Zhang, J.; Zhang, F.; et al. Maternal exposure to PM2.5 decreases ovarian reserve in neonatal offspring mice through activating PI3K/AKT/FoxO3a pathway and ROS-dependent NF-κB pathway. Toxicology 2022, 481, 153352. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Li, H.; Wang, H.; Du, D.; Huang, H. ATF3 mediates PM2.5-induced apoptosis and inflammation in ovarian granulosa cells. J. Ovarian Res. 2024, 17, 215. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, D.D.; Mbemya, G.T.; Bruno, J.B.; Faustino, L.R.; de Figueiredo, J.R.; Rodrigues, A.P.R. In vitro culture systems as an alternative for female reproductive toxicology studies. Zygote 2019, 27, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Tumbleson, M.E.; Schook, L.B. Advances in Swine in Biomedical Research; Plenum Press: New York, NY, USA, 1996. [Google Scholar]
- Rogers, C.S.; Abraham, W.M.; Brogden, K.A.; Engelhardt, J.F.; Fisher, J.T.; McCray, P.B., Jr.; McLennan, G.; Meyerholz, D.K.; Namati, E.; Ostedgaard, L.S.; et al. The porcine lung as a potential model for cystic fibrosis. Am. J. Physiol. Lung. Cell Mol. Physiol. 2008, 295, L240–L263. [Google Scholar] [CrossRef]
- Joshi, K.; Katam, T.; Hegde, A.; Cheng, J.; Prather, R.S.; Whitworth, K.; Wells, K.; Bryan, J.N.; Hoffman, T.; Telugu, B.P.; et al. Pigs: Large Animal Preclinical Cancer Models. World J. Oncol. 2024, 15, 149–168. [Google Scholar] [CrossRef] [PubMed]
- Mordhorst, B.R.; Prather, R.S. Pig models of reproduction. In Animal Models and Human Reproduction; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 213–234. [Google Scholar]
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the pig as a human biomedical model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef]
- Basini, G.; Bussolati, S.; Bertini, S.; Quintavalla, F.; Grasselli, F. Evaluation of Triclosan Effects on Cultured Swine Luteal Cells. Animals 2021, 11, 606. [Google Scholar] [CrossRef]
- Basini, G.; Bussolati, S.; Torcianti, V.; Grasselli, F. Perfluorooctanoic acid (PFOA) affects steroidogenesis and antioxidant defence in granulosa cells from swine ovary. Environ. Toxicol. Pharmacol. 2023, 101, 104169. [Google Scholar] [CrossRef]
- Basini, G.; Bussolati, S.; Grolli, S.; Berni, P.; Grasselli, F. Are the new phthalates safe? Evaluation of Diisononilphtalate (DINP) effects in porcine ovarian cell cultures. Environ. Toxicol. Pharmacol. 2024, 106, 104384. [Google Scholar] [CrossRef]
- Park, S.R.; Lee, J.W.; Kim, S.K.; Yu, W.J.; Lee, S.J.; Kim, D.; Kim, K.W.; Jung, J.W.; Hong, I.S. The impact of fine particulate matter (PM) on various beneficial functions of human endometrial stem cells through its key regulator SERPINB2. Exp. Mol. Med. 2021, 53, 1850–1865. [Google Scholar] [CrossRef]
- Akins, E.L.; Morrissette, M.C. Gross ovarian changes during estrous cycle of swine. Am. J. Vet. Res. 1968, 29, 1953–1957. [Google Scholar] [PubMed]
- McDonald, L.E. Veterinary Endocrinology and Reproduction, 2nd ed.; Lea & Febiger: Philadelphia, PA, USA, 1975; pp. 283–285. [Google Scholar]
- Babalola, G.O.; Shapiro, B.H. Correlation of follicular steroid hormone profiles with ovarian cyclicity in sows. J. Reprod. Fertil. 1988, 84, 79–87. [Google Scholar] [CrossRef]
- Gregoraszczuk, E.L.; Oblonczyk, K. Effect of a specific aromatase inhibitor on oestradiol secretion by porcine corpora lutea at various stages of the luteal phase. Reprod. Nutr. Dev. 1996, 36, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Nitkiewicz, A.; Smolinska, N.; Przała, J.; Kaminski, T. Expression of orexin receptors 1 (OX1R) and 2 (OX2R) in the porcine ovary during the oestrous cycle. Regul. Pept. 2010, 165, 186–190. [Google Scholar] [CrossRef]
- Basini, G.; Bussolati, S.; Santini, S.E.; Grasselli, F. The impact of the phyto-oestrogen genistein on swine granulosa cell function. J. Anim. Physiol. Anim. Nutr. 2010, 94, e374–e382. [Google Scholar] [CrossRef] [PubMed]
- Basini, G.; Bussolati, S.; Ciccimarra, R.; Grasselli, F. Melatonin potentially acts directly on swine ovary by modulating granulosa cell function and angiogenesis. Reprod. Fertil. Dev. 2017, 29, 2305–2312. [Google Scholar] [CrossRef]
- Basini, G.; Ciccimarra, R.; Bussolati, S.; Grolli, S.; Ragionieri, L.; Ravanetti, F.; Botti, M.; Gazza, F.; Cacchioli, A.; Di Lecce, R.; et al. Orexin A in swine corpus luteum. Domest. Anim. Endocrinol. 2018, 64, 38–48. [Google Scholar] [CrossRef]
- Grasselli, F.; Basini, G.; Tirelli, M.; Cavalli, V.; Bussolati, S.; Tamanini, C. Angiogenic activity of porcine granulosa cells cocultured with endothelial cells in a microcarrier-based three-dimensional fibrin gel. J. Physiol. Pharmacol. 2003, 54, 361–370. [Google Scholar] [PubMed]
- Foxcroft, G.R.; Hunter, M.G. Basic physiology of follicular maturation in the pig. J. Reprod. Fertil. 1985, 33, 1–19. [Google Scholar]
- Basini, G.; Baioni, L.; Bussolati, S.; Grolli, S.; Grasselli, F. Prolactin is a potential physiological modulator of swine ovarian follicle function. Regul. Pept. 2014, 189, 22–30. [Google Scholar] [CrossRef]
- Ciccimarra, R.; Bussolati, S.; Grasselli, F.; Grolli, S.; Ragionieri, L.; Ravanetti, F.; Botti, M.; Gazza, F.; Cacchioli, A.; Di Lecce, R.; et al. Orexin system in swine ovarian follicles. Domest. Anim. Endocrinol. 2018, 62, 49–59. [Google Scholar] [CrossRef]
- Basini, G.; Bussolati, S.; Torcianti, V.; Grasselli, F. Perfluorooctanoic Acid (PFOA) Induces Redox Status Disruption in Swine Granulosa Cells. Vet. Sci. 2022, 9, 254. [Google Scholar] [CrossRef]
- Spanel-Borowski, K.; van der Bosch, J. Different phenotyes of cultured microvessel endothelial cells obtained from bovine corpus luteum. Study by light microscopy and by scanning electron microscopy (SEM). Cell Tissue Res. 1990, 261, 35–47. [Google Scholar] [CrossRef]
- Basini, G.; Falasconi, I.; Bussolati, S.; Grolli, S.; Ramoni, R.; Grasselli, F. Isolation of endothelial cells and pericytes from swine corpus luteum. Domest. Anim. Endocrinol. 2014, 48, 100–109. [Google Scholar] [CrossRef]
- Mun, H.Y.; Prismasari, S.; Hong, J.H.; Lee, H.; Kim, D.; Kim, H.S.; Shin, D.M.; Kang, J.Y. Fine particulate matter induces osteoclast-mediated bone loss in mice. Korean J. Physiol. Pharmacol. 2025, 29, 9–19. [Google Scholar] [CrossRef]
- Chen, Y.; Zhan, N.; Xu, J.; Huang, Y.; Su, Y.; Liu, Z.; Feng, H.; Ji, W.; Liang, J.; Zhao, S.; et al. PM2.5 from biofuel smoke induces inflammatory response through the TRPC6/Ca2+/NLRP3 signaling pathway. Environ. Geochem. Health 2025, 47, 269. [Google Scholar] [CrossRef]
- Liang, S.; Zhang, J.; Ning, R.; Du, Z.; Liu, J.; Batibawa, J.W.; Duan, J.; Sun, Z. The critical role of endothelial function in fine particulate matter-induced atherosclerosis. Part. Fibre Toxicol. 2020, 17, 61. [Google Scholar] [CrossRef] [PubMed]
- Hinderliter, P.M.; Minard, K.R.; Orr, G.; Chrisler, W.B.; Thrall, B.D.; Pounds, J.G.; Teeguarden, J.G. ISDD: A computational model of particle sedimentation, diffusion and target cell dosimetry for in vitro toxicity studies. Part. Fibre Toxicol. 2010, 17, 36. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, Q.; Song, E.; Song, Y. Characterization of blood protein adsorption on PM2.5 and its implications on cellular uptake and cytotoxicity of PM2.5. J. Hazard Mater. 2021, 414, 125499. [Google Scholar] [CrossRef]
- Dodi, A.; Bussolati, S.; Grolli, S.; Grasselli, F.; Di Lecce, R.; Basini, G. Melatonin modulates swine luteal and adipose stromal cell functions. Reprod. Fertil. Dev. 2021, 33, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Basini, G.; Bussolati, S.; Andriani, L.; Grolli, S.; Ramoni, R.; Bertini, S.; Iemmi, T.; Menozzi, A.; Berni, P.; Grasselli, F. Nanoplastics impair in vitro swine granulosa cell functions. Domest. Anim. Endocrinol. 2021, 76, 106611. [Google Scholar] [CrossRef] [PubMed]
- Basini, G.; Bussolati, S.; Baioni, L.; Grasselli, F. Gossypol, a polyphenolic aldehyde from cotton plant, interferes with swine granulosa cell function. Domest. Anim. Endocrinol. 2009, 37, 30–36. [Google Scholar] [CrossRef]
- Basini, G.; Santini, S.E.; Bussolati, S.; Grasselli, F. The phytoestrogen Quercetin Impairs Steroidogenesis and Angiogenesis in swine granulosa cells in vitro. J. Biomed. Biotech. 2009, 2009, 419891. [Google Scholar] [CrossRef]
- Quintavalla, F.; Basini, G.; Fidanzio, F.; Bussolati, S.; Sabetti, M.C.; Crosta, M.C.; Grolli, S.; Ramoni, R. Blood plasma and urinary biomarkers of oxidative stress in cats with urethral obstruction. BMC Vet. Res. 2024, 20, 163. [Google Scholar] [CrossRef] [PubMed]
- Basini, G.; Tamanini, C. Selenium stimulates estradiol production in bovine granulosa cells: Possible involvement of nitric oxide. Domest. Anim. Endocrinol. 2000, 18, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Pacentra, A.; Grasselli, F.; Bussolati, S.; Grolli, S.; Di Lecce, R.; Cantoni, A.M.; Basini, G. The effect of pathogen-associated molecular patterns on the swine granulosa cells. Theriogenology 2020, 145, 207–216. [Google Scholar] [CrossRef]
- Barboni, B.; Turriani, M.; Galeati, G.; Spinaci, M.; Bacci, M.L.; Forni, M.; Mattioli, M. Vascular endothelial growth factor production in growing pig antral follicles. Biol. Reprod. 2000, 63, 858–864. [Google Scholar] [CrossRef]
- Basini, G.; Falasconi, I.; Bussolati, S.; Grolli, S.; Di Lecce, R.; Grasselli, F. Swine Granulosa Cells Show Typical Endothelial Cell Characteristics. Reprod. Sci. 2016, 23, 630–637. [Google Scholar] [CrossRef]
- Liu, R.L.; Xu, Z.L.; Hu, Y.L.; Lv, X.Y.; Yao, Q.Z.; He, J.L.; Fu, L.J.; Geng, L.H.; Wang, T.; Zhong, Z.H.; et al. Association between PM2.5 components and poor ovarian response in assisted reproductive technology patients: A retrospective cohort study identifying sensitive exposure windows in China. Environ Int. 2025, 196, 109321. [Google Scholar] [CrossRef] [PubMed]
- Luderer, U. Impact of real-life environmental exposure on reproduction: Adverse impacts of particulate matter air pollution on female and male reproduction. Reproduction 2025, 169, e240194. [Google Scholar] [CrossRef]
- Sun, H.; Cheng, W.; Zhang, X.; Sun, Z.; Sun, H.; Tian, S.; Tang, J. Measuring Carbon Content in Airway Macrophages Exposed to Carbon-Containing Particulate Matters. J. Vis. Exp. 2024, 12, 209. [Google Scholar] [CrossRef]
- Yefimova, M.G.; Lefevre, C.; Bashamboo, A.; Eozenou, C.; Burel, A.; Lavault, M.T.; Meunier, A.C.; Pimentel, C.; Veau, S.; Neyroud, A.S.; et al. Granulosa cells provide elimination of apoptotic oocytes through unconventional autophagy-assisted phagocytosis. Hum. Reprod. 2020, 35, 1346–1362. [Google Scholar] [CrossRef]
- Buoio, E.; Cialini, C.; Costa, A. Air Quality Assessment in Pig Farming: The Italian Classyfarm. Animals 2023, 13, 2297. [Google Scholar] [CrossRef]
- Okeson, C.D.; Riley, M.R.; Fernandez, A.; Wendt, J.O. Impact of the composition of combustion generated fine particles on epithelial cell toxicity: Influences of metals on metabolism. Chemosphere 2003, 51, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Cao, Z.; Jiao, X.; Bai, D.; Zhang, Y.; Hua, J.; Liu, W.; Teng, X. Pre-pregnancy exposure to fine particulate matter (PM2.5) increases reactive oxygen species production in oocytes and decrease litter size and weight in mice. Environ. Pollut. 2021, 268, 115858. [Google Scholar] [CrossRef]
- Rui, W.; Guan, L.; Zhang, F.; Zhang, W.; Ding, W. PM2.5-induced oxidative stress increases adhesion molecules expression in human endothelial cells through the ERK/AKT/NF-κB-dependent pathway. J. Appl. Toxicol. 2016, 36, 48–59. [Google Scholar] [CrossRef]
- Wang, T.; Chiang, E.T.; Moreno-Vinasco, L.; Lang, G.D.; Pendyala, S.; Samet, J.M.; Geyh, A.S.; Breysse, P.N.; Chillrud, S.N.; Natarajan, V.; et al. Particulate matter disrupts human lung endothelial barrier integrity via ROS- and p38 MAPK-dependent pathways. Am. J. Respir. Cell. Mol. Biol. 2010, 42, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.Y.; Ma, Y.C.; Lin, J.M.; Li, C.Y.; Lin, R.S.; Sung, F.C. Oxidative stress associated with indoor air pollution and sick building syndrome-related symptoms among office workers in Taiwan. Inhal. Toxicol. 2007, 19, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, J.; Ren, L.; Wei, J.; Duan, J.; Zhang, L.; Zhou, X.; Sun, Z. PM2.5 induces male reproductive toxicity via mitochondrial dysfunction, DNA damage and RIPK1 mediated apoptotic signaling pathway. Sci. Total Environ. 2018, 634, 1435–1444. [Google Scholar] [CrossRef]
- Sugino, N. Reactive oxygen species in ovarian physiology. Reprod. Med. Biol. 2005, 4, 31–44. [Google Scholar] [CrossRef]
- Takiguchi, S.; Sugino, N.; Kashida, S.; Yamagata, Y.; Nakamura, Y.; Kato, H. Rescue of the corpus luteum and an increase in luteal superoxide dismutase expression induced by placental luteotropins in the rat: Action of testosterone without conversion to estrogen. Biol. Reprod. 2000, 62, 398–403. [Google Scholar] [CrossRef]
- Long, Y.M.; Yang, X.Z.; Yang, Q.Q.; Clermont, A.C.; Yin, Y.G.; Liu, G.L.; Hu, L.G.; Liu, Q.; Zhou, Q.F.; Liu, Q.S.; et al. PM2.5 induces vascular permeability increase through activating MAPK/ERK signaling pathway and ROS generation. J. Hazard Mater. 2020, 386, 121659. [Google Scholar] [CrossRef]
- Kouassi, K.S.; Billet, S.; Garçon, G.; Verdin, A.; Diouf, A.; Cazier, F.; Djaman, J.; Courcot, D.; Shirali, P. Oxidative damage induced in A549 cells by physically and chemically characterized air particulate matter (PM2.5) collected in Abidjan, Côte d’Ivoire. J. Appl. Toxicol. 2010, 30, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Chirino, Y.I.; Sánchez-Pérez, Y.; Osornio-Vargas, A.R.; Morales-Bárcenas, R.; Gutiérrez-Ruíz, M.C.; Segura-García, Y.; Rosas, I.; Pedraza-Chaverri, J.; García-Cuellar, C.M. PM(10) impairs the antioxidant defense system and exacerbates oxidative stress driven cell death. Toxicol. Lett. 2010, 93, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Q.; Ren, Q.L.; Chen, J.F.; Gao, B.W.; Wang, X.W.; Zhang, Z.J.; Wang, J.; Xu, Z.J.; Xing, B.S. Autophagy Contributes to Oxidative Stress-Induced Apoptosis in Porcine Granulosa Cells. Reprod. Sci. 2021, 28, 2147–2160. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.Q.; Liu, C.B.; Xie, S.J.; Liu, Y.; Deng, Y.B.; He, S.W.; Fu, X.P.; Fu, B.B.; Wang, Y.L.; Chen, M.H.; et al. Effects of fine particulate matter (PM2.5) on ovarian function and embryo quality in mice. Environ. Int. 2020, 135, 105338. [Google Scholar] [CrossRef]
- Graham, J.D.; Clarke, C.L. Physiological action of progesterone in target tissues. Endocr. Rev. 1997, 18, 502–519. [Google Scholar] [CrossRef]
- Wang, C.; Yang, J.; Hao, Z.; Gong, C.; Tang, L.; Xu, Y.; Lu, D.; Li, Z.; Zhao, M. Suppression of progesterone synthesis in human trophoblast cells by fine particulate matter primarily derived from industry. Environ. Pollut. 2017, 231, 1172–1180. [Google Scholar] [CrossRef]
- Pillai, P.; Pandya, C.; Gupta, S.; Gupta, S. Biochemical and molecular effects of gestational and lactational coexposure to lead and cadmium on ovarian steroidogenesis are associated with oxidative stress in F1 generation rats. J. Biochem. Mol. Toxicol. 2010, 24, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Nampoothiri, L.P.; Agarwal, A.; Gupta, S. Effect of co-exposure to lead and cadmium on antioxidant status in rat ovarian granulose cells. Arch. Toxicol. 2007, 81, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Britt, K.L.; Saunders, P.K.; McPherson, S.J.; Misso, M.L.; Simpson, E.R.; Findlay, J.K. Estrogen actions on follicle formation and early follicle development. Biol. Reprod. 2004, 71, 1712–1723. [Google Scholar] [CrossRef]
- Takeda, K.; Tsukue, N.; Yoshida, S. Endocrine-disrupting activity of chemicals in diesel exhaust and diesel exhaust particles. Environ. Sci. 2004, 11, 33–45. [Google Scholar]
- Dang, S.; Ding, D.; Lu, Y.; Su, Q.; Lin, T.; Zhang, X.; Zhang, H.; Wang, X.; Tan, H.; Zhu, Z.; et al. PM2.5 exposure during pregnancy induces hypermethylation of estrogen receptor promoter region in rat uterus and declines offspring birth weights. Environ. Pollut. 2018, 243, 851–861. [Google Scholar] [CrossRef]
- Tomei, G.; Ciarrocca, M.; Fortunato, B.R.; Capozzella, A.; Rosati, M.V.; Cerratti, D.; Tomao, E.; Anzelmo, V.; Monti, C.; Tomei, F. Exposure to traffic pollutants and effects on 17-beta-estradiol (E2) in female workers. Int. Arch. Occup. Environ. Health 2006, 80, 70–77. [Google Scholar] [CrossRef]
- Nam, H.Y.; Choi, B.H.; Lee, J.Y.; Lee, S.G.; Kim, Y.H.; Lee, K.H.; Yoon, H.K.; Song, J.S.; Kim, H.J.; Lim, Y. The role of nitric oxide in the particulate matter (PM2.5)-induced NFkappaB activation in lung epithelial cells. Toxicol. Lett. 2004, 148, 95–102. [Google Scholar] [CrossRef]
- Tamanini, C.; Basini, G.; Grasselli, F.; Tirelli, M. Nitric oxide and the ovary. J. Anim. Sci. 2003, 81, E1–E7. [Google Scholar]
- Weldy, C.S.; Wilkerson, H.W.; Larson, T.V.; Stewart, J.A.; Kavanagh, T.J. DIESEL particulate exposed macrophages alter endothelial cell expression of eNOS, iNOS, MCP1, and glutathione synthesis genes. Toxicol. Vitro 2011, 25, 2064–2073. [Google Scholar] [CrossRef] [PubMed]
- Hongbao, M.; Ma, M.; Yan, Y. Inducible Nitric Oxide Synthase (iNOS) and Kidney Research Literatures. J. Pharmacol. 2006, 147, 966–974. [Google Scholar]
- Xu, X.; Kherada, N.; Hong, X.; Quan, C.; Zheng, L.; Wang, A.; Wold, L.; Lippmann, M.; Chen, L.C.; Rajagopalan, S.; et al. Diesel exhaust exposure induces angiogenesis. Toxicol. Lett. 2009, 191, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Davis-Smyth, T. The biology of vascular endothelial growth factor. Endocr. Rev. 1997, 8, 4–25. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, F.; Basini, G.; Bussolati, S.; Tamanini, C. Effects of VEGF and bFGF on proliferation and production of steroids and nitric oxide in porcine granulosa cells. Reprod. Domest. Anim. 2002, 37, 362–368. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Basini, G.; Ramoni, R.; Grolli, S.; Bussolati, S.; Assogna, L.; Grasselli, F. Fine Particulate Matter (PM) Effects on Swine Granulosa and Ovarian Endothelial Cells. Animals 2026, 16, 81. https://doi.org/10.3390/ani16010081
Basini G, Ramoni R, Grolli S, Bussolati S, Assogna L, Grasselli F. Fine Particulate Matter (PM) Effects on Swine Granulosa and Ovarian Endothelial Cells. Animals. 2026; 16(1):81. https://doi.org/10.3390/ani16010081
Chicago/Turabian StyleBasini, Giuseppina, Roberto Ramoni, Stefano Grolli, Simona Bussolati, Laura Assogna, and Francesca Grasselli. 2026. "Fine Particulate Matter (PM) Effects on Swine Granulosa and Ovarian Endothelial Cells" Animals 16, no. 1: 81. https://doi.org/10.3390/ani16010081
APA StyleBasini, G., Ramoni, R., Grolli, S., Bussolati, S., Assogna, L., & Grasselli, F. (2026). Fine Particulate Matter (PM) Effects on Swine Granulosa and Ovarian Endothelial Cells. Animals, 16(1), 81. https://doi.org/10.3390/ani16010081






