Assessing Physiological and Behavioral Stress Parameters in Trained Goats During Repeated Blood Sampling
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
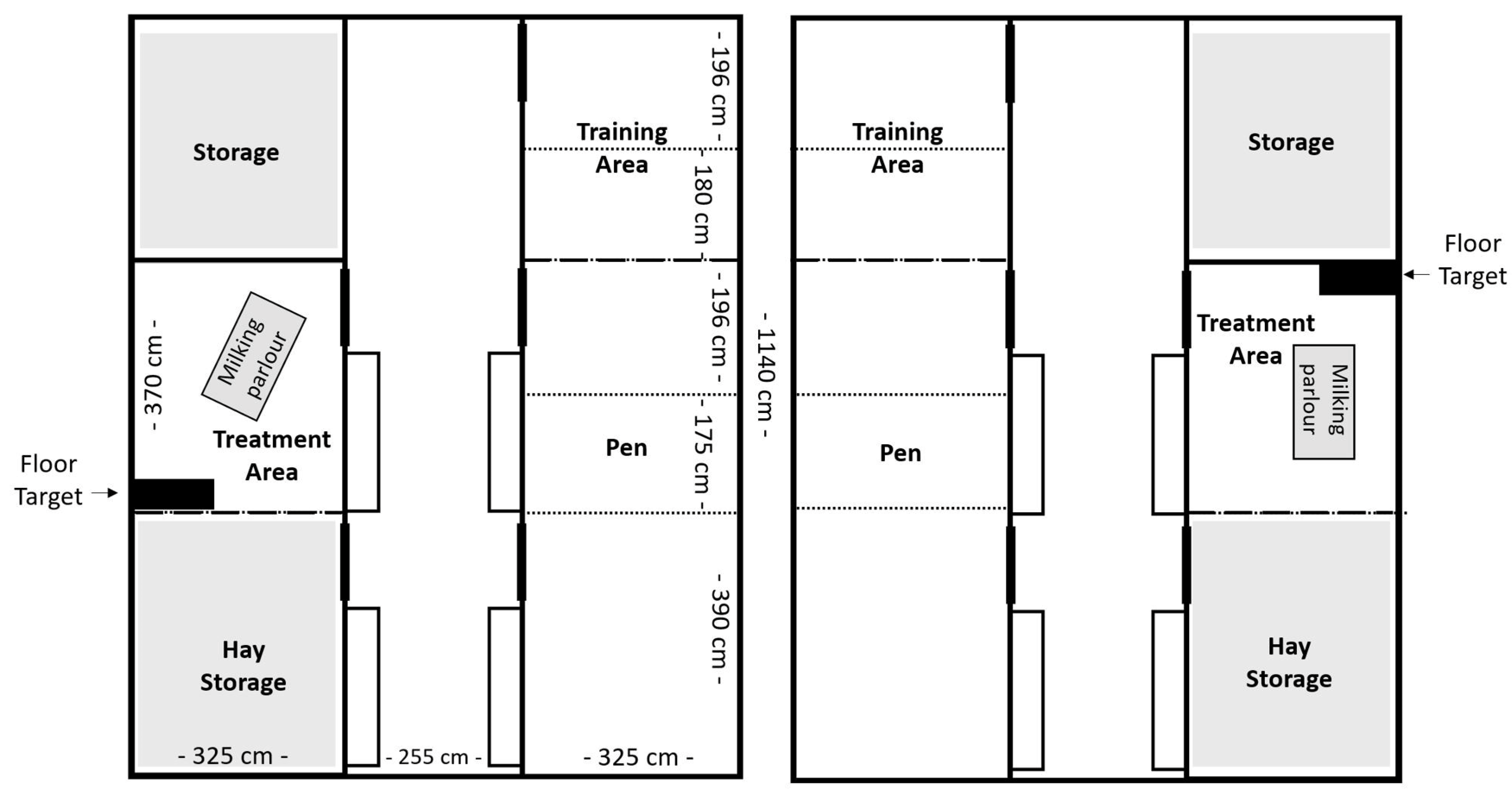
2.1. Animals and Housing
2.2. Training
2.3. Blood Sampling
2.3.1. Blood Serum Cortisol
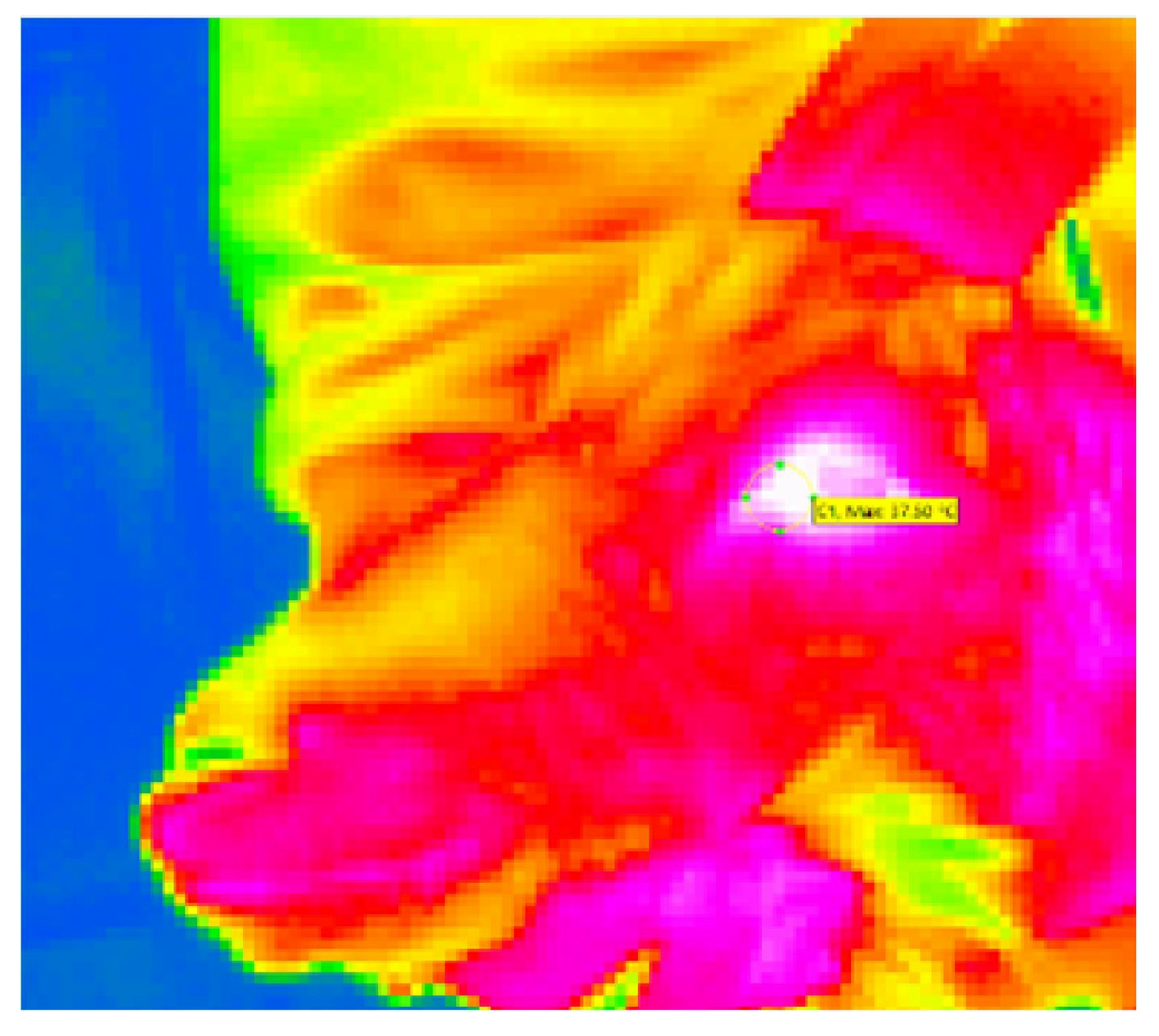
2.3.2. Eye Temperature
2.3.3. Behavior
2.4. Statistical Analysis
3. Results
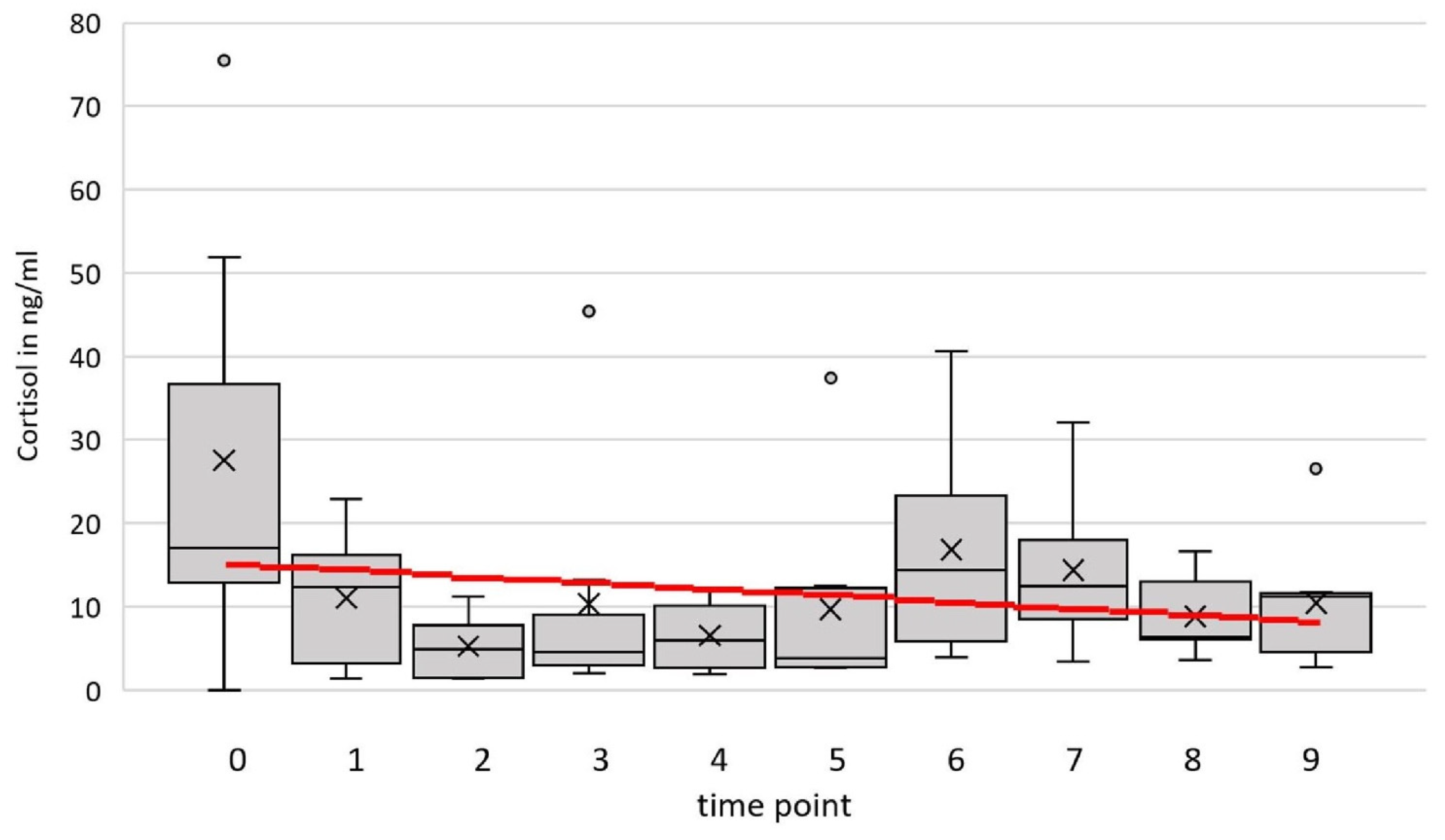
3.1. Blood Serum Cortisol
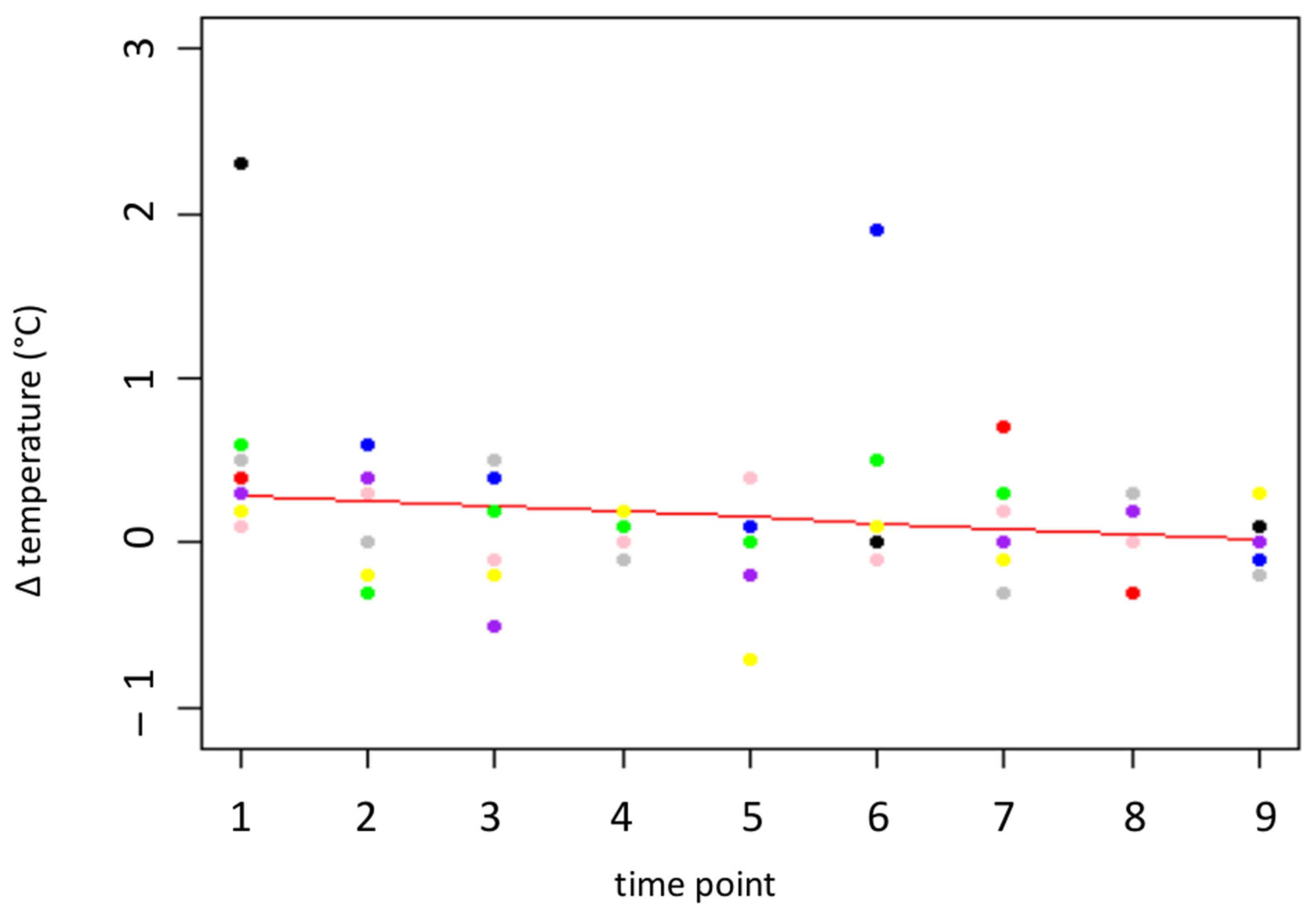
3.2. Eye Temperature
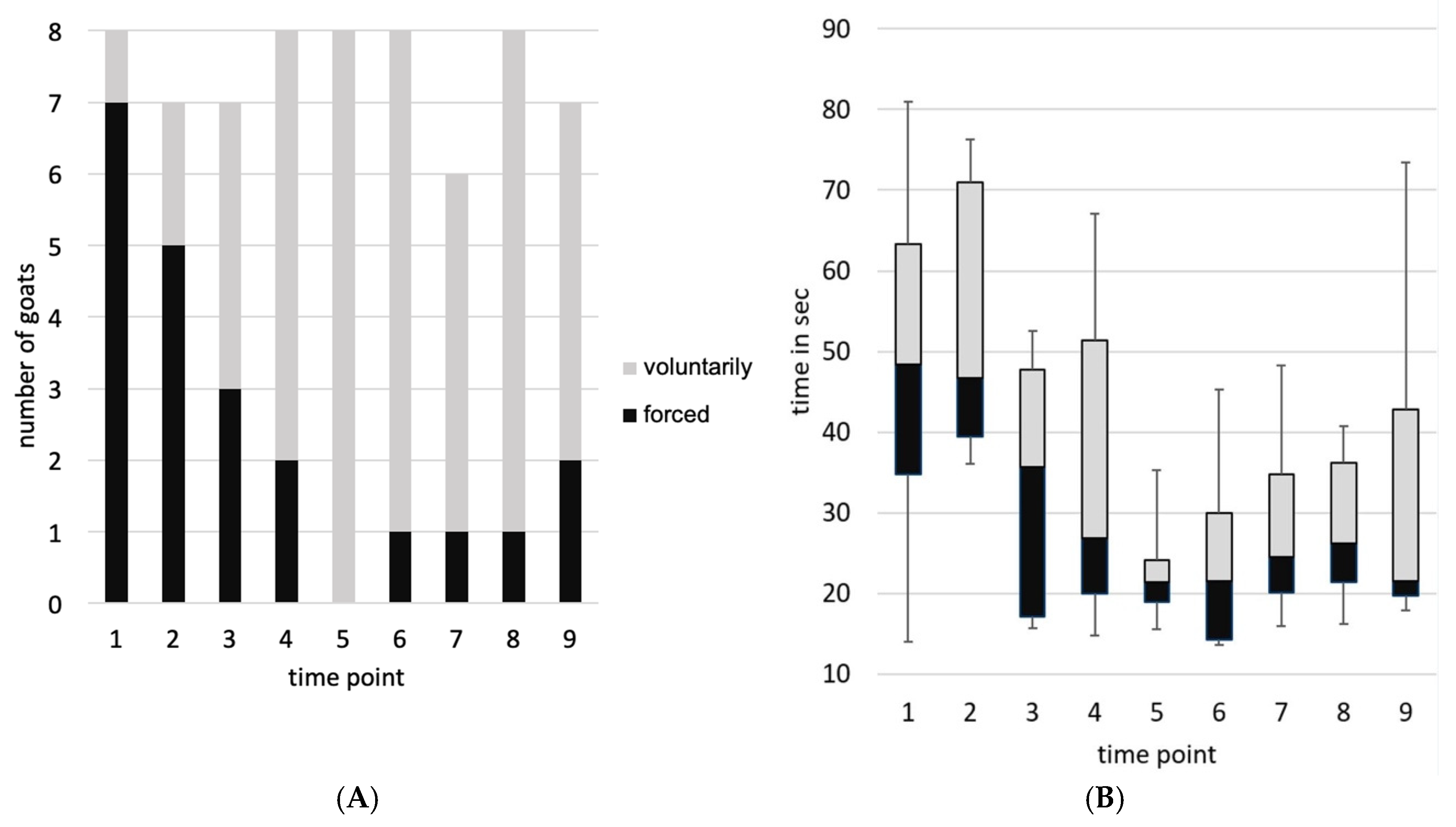
3.3. Behavior
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bailey, J. Does the Stress of Laboratory Life and Experimentation on Animals Adversely Affect Research Data? A Critical Review. Altern. Lab. Anim. 2018, 46, 291–305. [Google Scholar] [CrossRef]
- Baumans, V. Use of Animals in Experimental Research: An Ethical Dilemma? Gene Ther. 2004, 11, S64–S66. [Google Scholar] [CrossRef] [PubMed]
- Selye, H. Stress and Distress. Compr. Ther. 1975, 1, 9–13. [Google Scholar]
- Pluut, H.; Curșeu, P.L.; Fodor, O.C. Development and Validation of a Short Measure of Emotional, Physical, and Behavioral Markers of Eustress and Distress (MEDS). Healthcare 2022, 10, 339. [Google Scholar] [CrossRef]
- Mendl, M.; Burman, O.H.; Paul, E.S. An Integrative and Functional Framework for the Study of Animal Emotion and Mood. Proc. Biol. Sci. 2010, 277, 2895–2904. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Summary Report on the Statistics on the Use of Animals for Scientific Purposes in the Member States of the European Union and Norway in 2022; Commission Staff Working Document; European Commission: Brussels, Belgium, 2024. [Google Scholar]
- Balcombe, J.P.; Barnard, N.D.; Sandusky, C. Laboratory Routines Cause Animal Stress. Contemp. Top. Lab. Anim. Sci. 2004, 43, 42–51. [Google Scholar] [PubMed]
- Tosto, M.S.L.; Santos, S.A.; Filho, R.; Rodrigues, T.; Nicory, I.M.C.; de Carvalho, G.G.P.; Bittencourt, R.F.; Ayres, M.C.C.; Pereira, T.C.J. Metabolic and Behavior Changings during the Transition Period as Predictors of Calving Proximity and Welfare of Dairy Goats. Vet. Anim. Sci. 2021, 11, 100168. [Google Scholar] [CrossRef]
- Li, R.; Wang, L.; Chen, B.; Zhang, Y.; Qi, P. Effects of Transportation on Blood Indices, Oxidative Stress, Rumen Fermentation Parameters and Rumen Microbiota in Goats. Animals 2024, 14, 1616. [Google Scholar] [CrossRef]
- European Parliament and Council of the European Union. Directive 2010/63/EU of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, L276, 33–79. Available online: https://eur-lex.europa.eu (accessed on 3 April 2023).
- Kruger, L.P.; Nedambale, T.L.; Scholtz, M.M.; Webb, E.C. The Effect of Environmental Factors and Husbandry Practices on Stress in Goats. Small Rumin. Res. 2016, 141, 1–4. [Google Scholar] [CrossRef]
- Price, E.O.; Thos, J. Behavioral Responses to Short-Term Social Isolation in Sheep and Goats. Appl. Anim. Ethol. 1980, 6, 331–339. [Google Scholar] [CrossRef]
- Wang, J.Y.; Zhang, H.T.; Chang, J.Y.; Woodward, D.J.; Baccalá, L.A.; Luo, F. Anticipation of Pain Enhances the Nociceptive Transmission and Functional Connectivity within Pain Network in Rats. Mol. Pain. 2008, 4, 34. [Google Scholar] [CrossRef]
- Imfeld-Mueller, S.; Van Wezemael, L.; Stauffacher, M.; Gygax, L.; Hillmann, E. Do Pigs Distinguish between Situations of Different Emotional Valences during Anticipation? Appl. Anim. Behav. Sci. 2011, 131, 86–93. [Google Scholar] [CrossRef]
- Bisogni, S.; Dini, C.; Olivini, N.; Ciofi, D.; Giusti, F.; Caprilli, S.; Gonzalez Lopez, J.R.; Festini, F. Perception of Venipuncture Pain in Children Suffering from Chronic Diseases. BMC Res. Notes 2014, 7, 735. [Google Scholar] [CrossRef]
- Dai, F.; Mazzola, S.; Cannas, S.; Heinzl, E.U.L.; Padalino, B.; Minero, M.; Dalla Costa, E. Habituation to Transport Helps Reducing Stress-Related Behavior in Donkeys During Loading. Front. Vet. Sci. 2020, 7, 593138. [Google Scholar] [CrossRef]
- Directorate-General for Environment (European Commission). Caring for Animals Aiming for Better Science—Directive 2010/63/EU on Protection of Animals Used for Scientific Purposes—Severity Assessment Framework; Publications Office: Luxembourg, 2018. [Google Scholar] [CrossRef]
- Hydbring-Sandberg, E.; von Walter, L.W.; Forkman, B. Cortisol Is Not Enough: A Complex Stress Reaction in Tethered Goats. Anim. Welf. 2022, 31, 91–98. [Google Scholar] [CrossRef]
- Kannan, G.; Terrill, T.H.; Kouakou, B.; Gazal, O.S.; Gelaye, S.; Amoah, E.A.; Samaké, S. Transportation of Goats: Effects on Physiological Stress Responses and Live Weight Loss. J. Anim. Sci. 2000, 78, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Ralph, C.R.; Tilbrook, A.J. Invited Review: The Usefulness of Measuring Glucocorticoids for Assessing Animal Welfare. J. Anim. Sci. 2016, 94, 457–470. [Google Scholar] [CrossRef]
- Jerem, P.; Jenni-Eiermann, S.; McKeegan, D.; McCafferty, D.J.; Nager, R.G. Eye Region Surface Temperature Dynamics during Acute Stress Relate to Baseline Glucocorticoids Independently of Environmental Conditions. Physiol. Behav. 2019, 210, 112627. [Google Scholar] [CrossRef] [PubMed]
- Arfuso, F.; Acri, G.; Piccione, G.; Sansotta, C.; Fazio, F.; Giudice, E.; Giannetto, C. Eye Surface Infrared Thermography Usefulness as a Noninvasive Method of Measuring Stress Response in Sheep during Shearing: Correlations with Serum Cortisol and rectal Temperature Values. Physiol. Behav. 2022, 250, 113781. [Google Scholar] [CrossRef]
- Bartolomé, E.; Azcona, F.; Cañete-Aranda, M.; Perdomo-González, D.I.; Ribes-Pons, J.; Terán, E.M. Testing Eye Temperature Assessed with Infrared Thermography to Evaluate Stress in Meat Goats Raised in a Semi-Intensive Farming System: A Pilot Study. Arch. Anim. Breed. 2019, 62, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Tamioso, P.R.; Rucinque, D.S.; Taconeli, C.A.; da Silva, G.P.; Molento, C.F.M. Behavior and Body Surface Temperature as Welfare Indicators in Selected Sheep Regularly Brushed by a Familiar Observer. J. Vet. Behav. 2017, 19, 27–34. [Google Scholar] [CrossRef]
- Proctor, H.; Carder, G. Can Changes in Nasal Temperature Be Used as an Indicator of Emotional State in Cows? Appl. Anim. Behav. Sci. 2016, 184, 1–6. [Google Scholar] [CrossRef]
- Blessing, W.W. The Lower Brainstem and Bodily Homeostasis; Oxford University Press: New York, NY, USA, 1997. [Google Scholar]
- Stewart, M.; Webster, J.R.; Verkerk, G.A.; Schaefer, A.L.; Colyn, J.J.; Stafford, K.J. Non-Invasive Measurement of Stress in Dairy Cows Using Infrared Thermography. Physiol. Behav. 2007, 92, 520–525. [Google Scholar] [CrossRef]
- Rose, P.E.; Riley, L.M. Conducting Behavioural Research in the Zoo: A Guide to Ten Important Methods, Concepts and Theories. J. Zool. Bot. Gard. 2021, 2, 421–444. [Google Scholar] [CrossRef]
- Forkman, B.; Boissy, A.; Meunier-Salaün, M.C.; Canali, E.; Jones, R.B. A Critical Review of Fear Tests Used on Cattle, Pigs, Sheep, Poultry and Horses. Physiol. Behav. 2007, 92, 340–374. [Google Scholar] [CrossRef]
- Baciadonna, L.; Nawroth, C.; Briefer, E.F.; McElligott, A.G. Perceptual Lateralization of Vocal Stimuli in Goats. Curr. Zool. 2019, 65, 67–74. [Google Scholar] [CrossRef]
- Siebert, K.; Langbein, J.; Schön, P.-C.; Tuchscherer, A.; Puppe, B. Degree of Social Isolation Affects Behavioural and Vocal Response Patterns in Dwarf Goats (Capra hircus). Appl. Anim. Behav. Sci. 2011, 131, 53–62. [Google Scholar] [CrossRef]
- Briefer, E.F.; Tettamanti, F.; McElligott, A.G. Emotions in Goats: Mapping Physiological, Behavioural and Vocal Profiles. Anim. Behav. 2015, 99, 131–143. [Google Scholar] [CrossRef]
- Just, H.; Bode, L.; Wagner, B.; Meier, J.; Kersten, S.; Fischer-Tenhagen, C.; Jahnke, A.; Kürbis, C.; Lüth, A.; Pieper, R.; et al. Linear and Branched Poly- and Perfluoroalkyl Acids: Transfer from Oral Exposure via Hay into the Milk of Dairy Goats (Capra aegagrus hircus). Department Experimental Toxicology and ZEBET, German Federal Institute for Risk Assessment (BfR): Berlin, Germany, 2025; manuscript in preparation. [Google Scholar]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- Skinner, B.F. How to Teach Animals. Sci. Am. 1951, 185, 26–29. [Google Scholar] [CrossRef]
- Sutherland, M.A.; Worth, G.M.; Dowling, S.K.; Lowe, G.L.; Cave, V.M.; Stewart, M. Evaluation of Infrared Thermography as a Non-Invasive Method of Measuring the Autonomic Nervous Response in Sheep. PLoS ONE 2020, 15, e0233558. [Google Scholar] [CrossRef]
- Friard, O.; Gamba, M. BORIS: A Free, Versatile Open-Source Event-Logging Software for Video/Audio Coding and Live Observations. Methods Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Microsoft Corporation. Microsoft Excel. 2018. Available online: https://office.microsoft.com/excel (accessed on 2 August 2021).
- Andanson, S.; Boissy, A.; Veissier, I. Conditions for Assessing Cortisol in Sheep: The Total Form in Blood V. the Free Form in Saliva. Animal 2020, 14, 1916–1922. [Google Scholar] [CrossRef]
- Kannan, G.; Estrada-Reyes, Z.M.; Batchu, P.; Kouakou, B.; Terrill, T.H.; Naldurtiker, A. Social Isolation of Goats: Significance of Visual Contact with Conspecifics on Behavioral and Physiological Responses. J. Anim. Sci. 2021, 99, skab150. [Google Scholar] [CrossRef]
- Joyce-Zuniga, N.M.; Newberry, R.C.; Robbins, C.T.; Ware, J.V.; Jansen, H.T.; Nelson, O.L. Positive Reinforcement Training for Blood Collection in Grizzly Bears (Ursus arctos horribilis) Results in Undetectable Elevations in Serum Cortisol Levels: A Preliminary Investigation. J. Appl. Anim. Welf. Sci. 2016, 19, 210–215. [Google Scholar] [CrossRef]
- Fiderer, D.; Thoene-Reineke, C.; Wiegard, M. Clicker Training in Minipigs to Reduce Stress during Blood Collection-An Example of Applied Refinement. Animals 2024, 14, 2819. [Google Scholar] [CrossRef]
- Marin, N.; Moragon, A.; Gil, D.; Garcia-Garcia, F.; Bisbal, V. Acclimation and Blood Sampling: Effects on Stress Markers in C57Bl/6J Mice. Animals 2023, 13, 2816. [Google Scholar] [CrossRef] [PubMed]
- Aragona, F.; Rizzo, M.; Arfuso, F.; Acri, G.; Fazio, F.; Piccione, G.; Giannetto, C. Eye Temperature Measured with Infrared Thermography to Assess Stress Responses to Road Transport in Horses. Animals 2024, 14, 1877. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, K.; Simmler, M.; Langbein, J.; Nawroth, C.; Keil, N. Responsiveness of Domesticated Goats Towards Various Stressors Following Long-Term Cognitive Test Exposure. PeerJ 2022, 10, e12893. [Google Scholar] [CrossRef]
- Coleman, K.; Pranger, L.; Maier, A.; Lambeth, S.P.; Perlman, J.E.; Thiele, E.; Schapiro, S.J. Training Rhesus Macaques for Venipuncture Using Positive Reinforcement Techniques: A Comparison with Chimpanzees. J. Am. Assoc. Lab. Anim. Sci. 2008, 47, 37–41. [Google Scholar] [PubMed]
- Callealta, I.; Lueders, I.; Luther-Binoir, I.; Ganswindt, A. Positive Reinforcement Conditioning as a Tool for Frequent Minimally Invasive Blood and Vaginal Swab Sampling in African Lions (Panthera leo). J. Appl. Anim. Welf. Sci. 2020, 23, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Hutson, G.D. The Influence of Barley Food Rewards on Sheep Movement through a Handling System. Appl. Anim. Behav. Sci. 1985, 14, 263–273. [Google Scholar] [CrossRef]
- Sankey, C.; Richard-Yris, M.A.; Henry, S.; Fureix, C.; Nassur, F.; Hausberger, M. Reinforcement as a mediator of the perception of humans by horses (Equus caballus). Anim. Cogn. 2010, 13, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Ami, N.; Oshima, H. Assessment of Needle Insertion Pain with Flexor Reflex Responses in Anesthetized Rats. Pain Res. 2012, 27, 215–225. [Google Scholar] [CrossRef]
| Goat | Training Sessions | Duration (min) | Achievement |
|---|---|---|---|
| 1 | 10 | 120.1 | Hand Target (>5 m) Floor Target (without additional signals) + Touch (>5 s) and Chin on Hand (2 s) |
| 2 | 10 | 107.7 | Hand Target (>5 m) Floor Target (with additional signals) + Touch (>5 s) and Chin on Hand (2 s) |
| 3 | 8 | 99.0 | Hand Target (>5 m) Floor Target (with additional signals) + Touch (<5 s) and Chin on Hand (7 s) |
| 4 | 8 | 92.6 | Hand Target (>5 m) Floor Target (with additional signals) + Touch (>5 s) and Chin on Hand (2 s) |
| 5 | 8 | 111.7 | Hand Target (>5 m) Floor Target (without additional signals) + Touch (<5 s) and Chin on Hand (3 s) |
| 6 | 7 | 90.7 | Hand Target (>5 m) Floor Target (with additional signals) + Touch (<5 s) and Chin on Hand (1 s) |
| 7 | 7 | 95.1 | Hand Target (>5 m) Floor Target (with additional signals) + Touch (<5 s) and Chin on Hand (2 s) |
| 8 | 7 | 114.2 | Hand Target (>5 m) Floor Target (with additional signals) + Touch (<5 s) and Chin on Hand (1 s) |
| Designation | Description | Unit/Characteristic |
|---|---|---|
| Predictor Variables | ||
| Time Point | Time of sampling | 1–8/9 |
| Goat | n = 8 | 1–8 |
| Pen | 1 (goats 1–4) or 2 (goats 5–8, PFAS exposed) | 1 or 2 |
| Temperature | Temperature in pen 1 (goats 1–4) and pen 2 (goats 5–8) | In °C |
| Humidity | Relative humidity during daily temperature measurement | In % |
| Person 1 | Restraining person | Individual identifier |
| Person 2 | Person taking blood | Individual identifier |
| Outcome Variables | ||
| Cortisol | Cortisol value from blood serum | ng/mL |
| Thermo | Temperature difference at the medial canthus 5 s after puncturing the skin compared to approx. 3 s before puncturing the skin (T2-T1) | In °C |
| Behavior | ||
| Latency | Duration from entering the sample area to assuming the position on the floor target | In seconds |
| Compliance | Taking the position for blood collection. Differentiation between “voluntary/lured” (voluntary: floor or hand target; lured: presentation of concentrated feed) and “forced” (led on collar/leash, at least temporarily taut) | “voluntary/lured” OR “forced” |
| Escape Behavior | Did the goat show intention or action to leave the ground target by shifting the weight towards the exit or moving at least one leg off the ground target? | Yes/No |
| Defensive Behavior | Did the goat show aversive behavior (either kicking with one leg or hitting the head) during blood collection? | Yes (less than 3 times)/Yes (more than 3 times)/No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Meier, J.; Just, H.; Steinfath, M.; Fischer-Tenhagen, C. Assessing Physiological and Behavioral Stress Parameters in Trained Goats During Repeated Blood Sampling. Animals 2026, 16, 105. https://doi.org/10.3390/ani16010105
Meier J, Just H, Steinfath M, Fischer-Tenhagen C. Assessing Physiological and Behavioral Stress Parameters in Trained Goats During Repeated Blood Sampling. Animals. 2026; 16(1):105. https://doi.org/10.3390/ani16010105
Chicago/Turabian StyleMeier, Jennifer, Hildegard Just, Matthias Steinfath, and Carola Fischer-Tenhagen. 2026. "Assessing Physiological and Behavioral Stress Parameters in Trained Goats During Repeated Blood Sampling" Animals 16, no. 1: 105. https://doi.org/10.3390/ani16010105
APA StyleMeier, J., Just, H., Steinfath, M., & Fischer-Tenhagen, C. (2026). Assessing Physiological and Behavioral Stress Parameters in Trained Goats During Repeated Blood Sampling. Animals, 16(1), 105. https://doi.org/10.3390/ani16010105





