A Pilot Study on Management Practices in Dairy Farms in the Basque Country: Focus on Colostrum Feeding and Vaccination
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
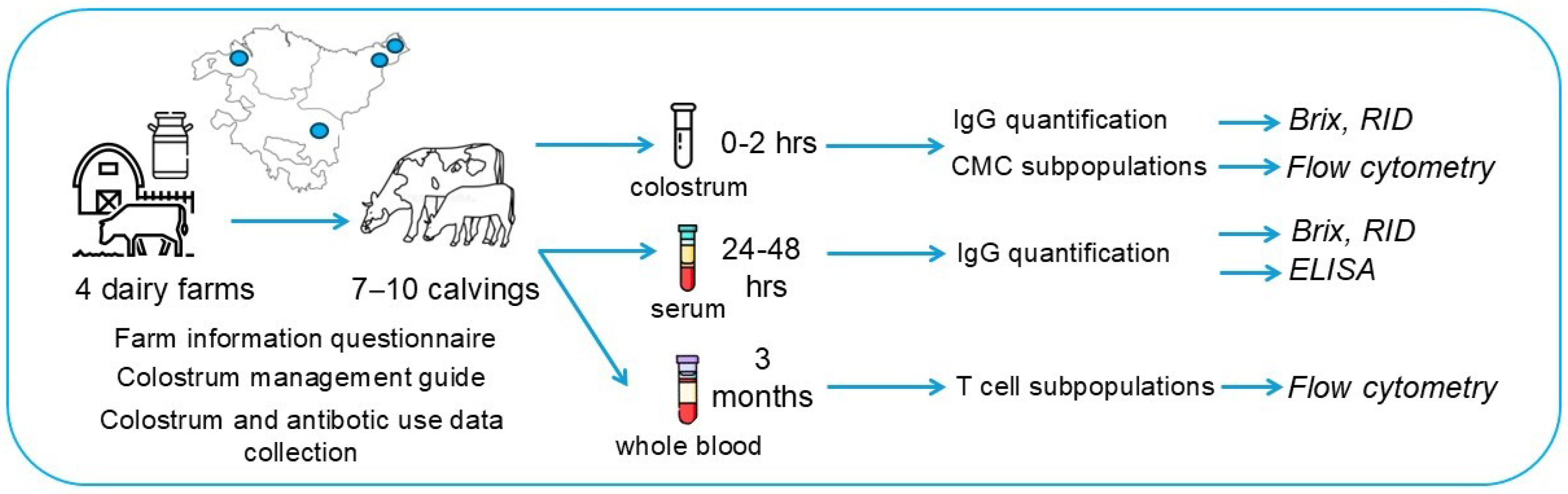
2.1. Study Design
2.2. Colostrum and Serum IgG Measurement
2.2.1. In-Farm
2.2.2. In-Lab
2.3. Analysis of Lymphocyte Subpopulations of Colostrum and Peripheral Blood
2.3.1. Peripheral Blood Mononuclear Cell (PBMC) Isolation
2.3.2. Colostrum Mononuclear Cell (CMC) Isolation
2.4. CMC and PBMC Subpopulation Analysis
2.5. Data Analysis and Statistics
3. Results
3.1. Management Practices and Vaccination Differences Between Farms
3.2. Reported Antibiotic Treatments
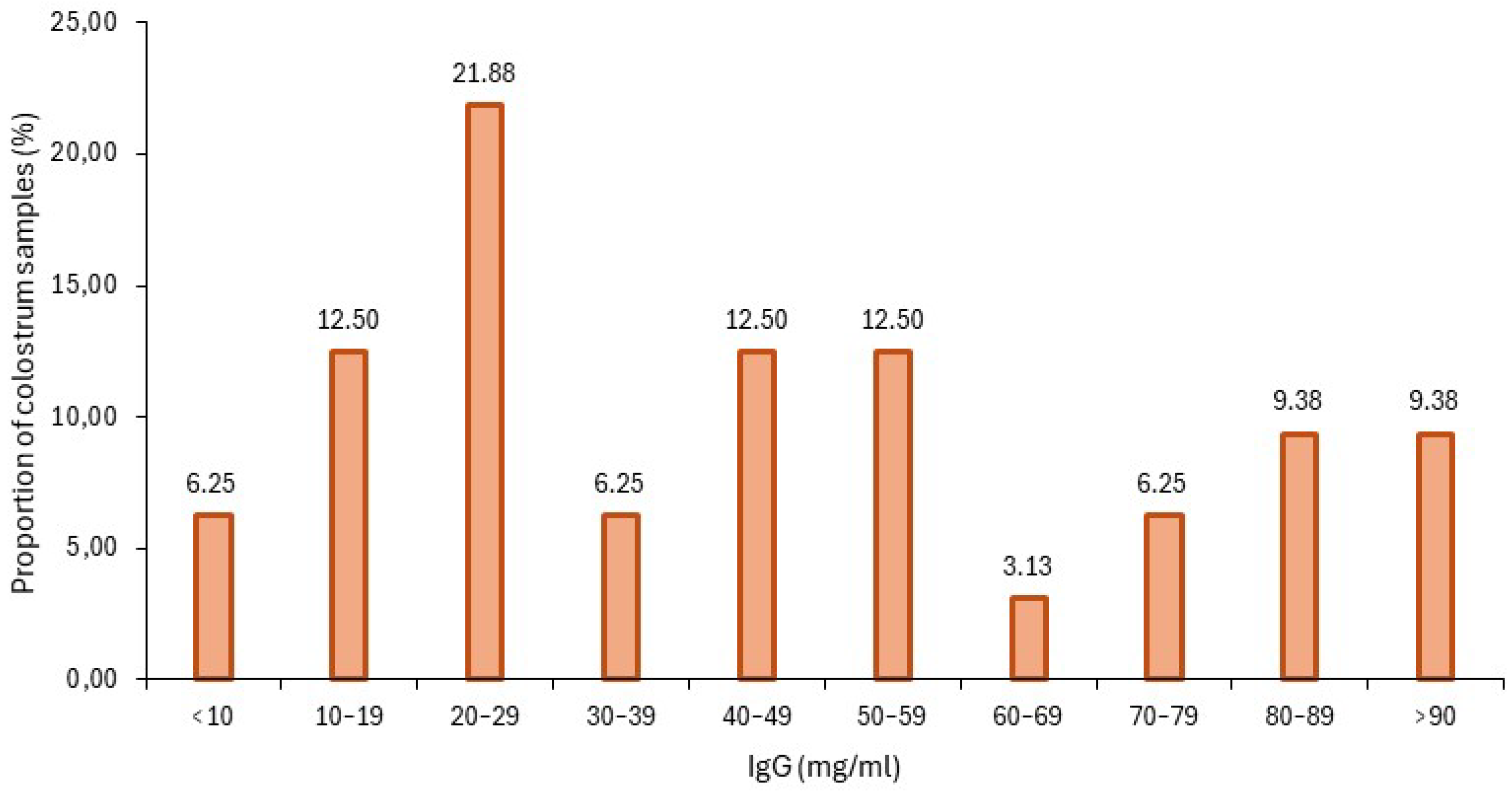
3.3. Colostrum IgG Estimation with Brix Refractometer
3.4. Refractometry and RID Correlation for IgG Estimation in Colostrum
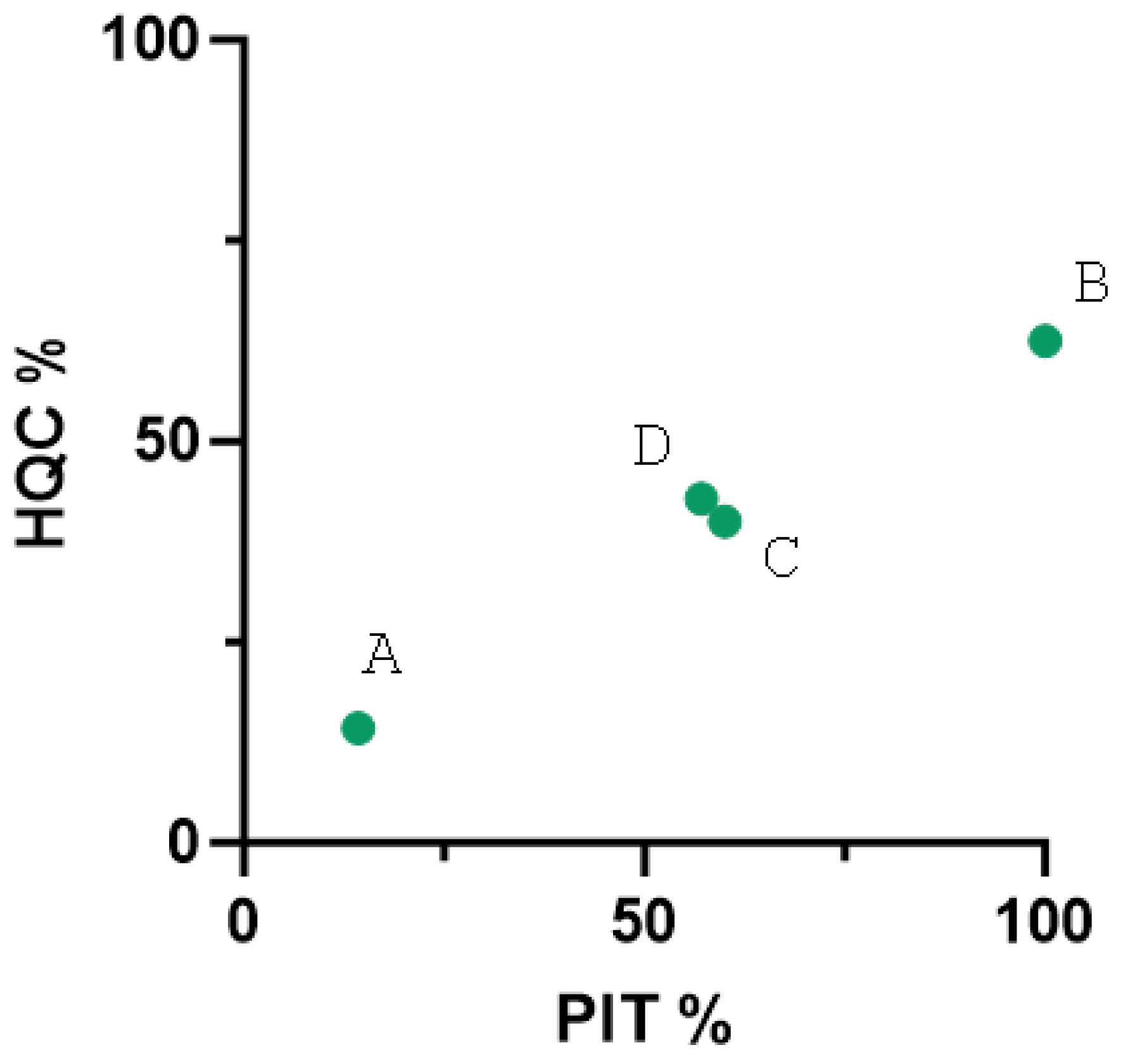
3.5. Colostrum Quality Among Farms
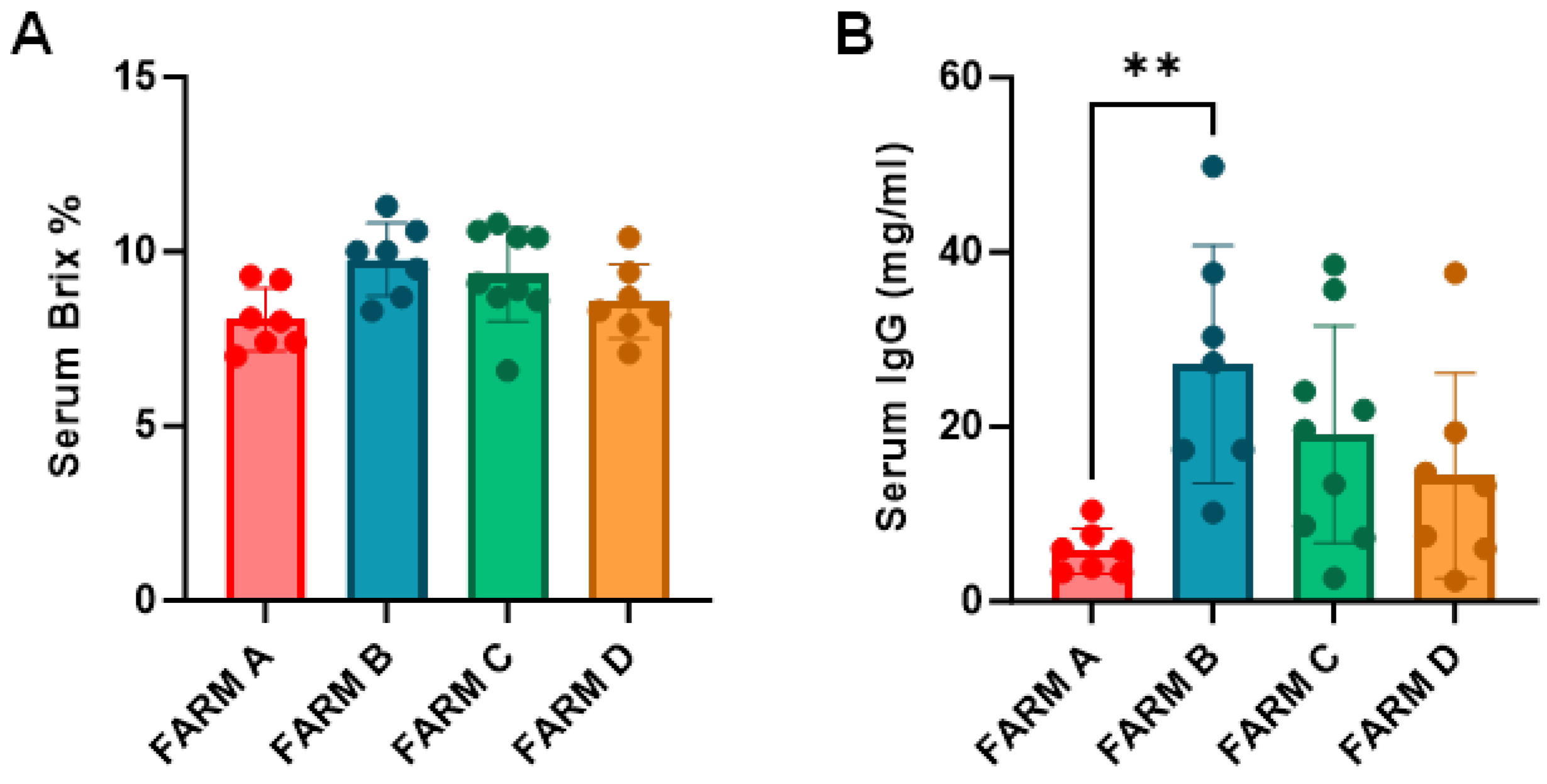
3.6. Passive Immunity Among Farms
3.7. Refractometry and RID Correlation for IgG Estimation in Serum
3.8. CD4, CD8 and γδ T Cell Levels in Colostrum and Blood
3.9. CD4, CD8 and γδ T Cell Levels in Non-Vaccinated Animals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PIT | Passive immunity transfer |
| Ig | Immunoglobulin |
| FPIT | Failure of passive immune transfer |
| RID | radioimmunodiffusion |
| RT | Room temperature |
| PBMC | Peripheral blood mononuclear cell |
| CMC | Colostrum mononuclear cell |
| HQC | High-quality colostrum |
| DCT | Dry cow therapy |
| NON-VAX | Non-vaccinated farm |
| VAX | Vaccinated farms |
| MDR | Multi drug resistance |
| AMR | Antimicrobial resistance |
References
- Peter, A.T. Bovine Placenta: A Review on Morphology, Components, and Defects from Terminology and Clinical Perspectives. Theriogenology 2013, 80, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Barrington, G.M.; Parish, S.M. Overview of Immunologic Development in Utero. Vet. Clin. N. Am. Food Anim. Pract. 2001, 17, 463–476. [Google Scholar] [CrossRef]
- Foley, J.A.; Otterby, D.E. Availability, Storage, Treatment, Composition, and Feeding Value of Surplus Colostrum: A Review. J. Dairy Sci. 1978, 61, 1033–1060. [Google Scholar] [CrossRef]
- DeNise, S.K.; Robison, J.D.; Stott, G.H.; Armstrong, D.V. Effects of Passive Immunity on Subsequent Production in Dairy Heifers. J. Dairy Sci. 1989, 72, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Godden, S.M.; Lombard, J.E.; Woolums, A.R. Colostrum Management for Dairy Calves. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 535–556. [Google Scholar] [CrossRef]
- Lora, I.; Barberio, A.; Contiero, B.; Paparella, P.; Bonfanti, L.; Brscic, M.; Stefani, A.L.; Gottardo, F. Factors Associated with Passive Immunity Transfer in Dairy Calves: Combined Effect of Delivery Time, Amount and Quality of the First Colostrum Meal. Animal 2018, 12, 1041–1049. [Google Scholar] [CrossRef]
- Sutter, F.; Venjakob, P.L.; Heuwieser, W.; Borchardt, S. Association between Transfer of Passive Immunity, Health, and Performance of Female Dairy Calves from Birth to Weaning. J. Dairy Sci. 2023, 106, 7043–7055. [Google Scholar] [CrossRef] [PubMed]
- Dewell, R.D.; Hungerford, L.L.; Keen, J.E.; Laegreid, W.W.; Dee Griffin, D.; Rupp, G.P.; Grotelueschen, D.M. Association of Neonatal Serum Immunoglobulin G1 with Health and performance in Beef Calves. Sci. Rep. Orig. Study JAVMA 2006, 228, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Faber, S.N.; Faber, N.E.; Mccauley, T.C.; Ax, R.L. Case Study: Effects of Colostrum Ingestion on Lactational Performance 1. Prof. Anim. Sci. 2005, 21, 420–425. [Google Scholar] [CrossRef]
- Crouch, C.F.; Oliver, S.; Hearle, D.C.; Buckley, A.; Chapman, A.J.; Francis, M.J. Lactogenic Immunity Following Vaccination of Cattle with Bovine Coronavirus. Vaccine 2001, 19, 189–196. [Google Scholar] [CrossRef]
- Franklin, S.T.; Newman, M.C.; Newman, K.E.; Meek, K.I. Immune Parameters of Dry Cows Fed Mannan Oligosaccharide and Subsequent Transfer of Immunity to Calves. J. Dairy Sci. 2005, 88, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Civra, A.; Altomare, A.; Francese, R.; Donalisio, M.; Aldini, G.; Lembo, D. Colostrum from Cows Immunized with a Veterinary Vaccine against Bovine Rotavirus Displays Enhanced In Vitro Anti-Human Rotavirus Activity. J. Dairy Sci. 2019, 102, 4857–4869. [Google Scholar] [CrossRef]
- Liebler-Tenorio, E.; Riedel-Caspari, G.; Pohlenz, J. Uptake of Colostral Leukocytes in the Intestinal Tract of Newborn Calves. Vet. Immunol. Immunopathol. 2002, 85, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Reber, A.J.; Hippen, A.R.; Hurley, D.J. Effects of the Ingestion of Whole Colostrum or Cell-Free Colostrum on the Capacity of Leukocytes in Newborn Calves to Stimulate or Respond in One-Way Mixed Leukocyte Cultures. Am. J. Vet. Res. 2005, 66, 1854–1860. [Google Scholar] [CrossRef]
- Riedel-Caspari, G. The Influence of Colostral Leukocytes on the Course of an Experimental Escherichia coli Infection and Serum Antibodies in Neonatal Calves. Vet. Immunol. Immunopathol. 1993, 35, 275–288. [Google Scholar] [CrossRef]
- Donovan, D.D.; Reber, A.J.; Gabbard, J.D.; Aceves-Avila, M.; Galland, K.L.; Holbert, K.A.; Ely, L.O.; Hurley, D.J. Effect of Maternal Cells Transferred with Colostrum on Cellular Responses to Pathogen Antigens in Neonatal Calves. Am. J. Vet. Res. 2007, 68, 778–782. [Google Scholar] [CrossRef]
- Robbers, L.; van de Mheen, R.; Benedictus, L.; Jorritsma, R.; Nielen, M.; Bijkerk, H.J.C.; van der Grein, S.G.; Ravesloot, L.; Koets, A.P. Evidence for Transfer of Maternal Antigen Specific Cellular Immunity against Mycobacterium avium subsp. paratuberculosis via Colostrum in a Goat Twin Model. Vet. Immunol. Immunopathol. 2022, 246, 110402. [Google Scholar] [CrossRef] [PubMed]
- Tello, M.; Ocejo, M.; Oporto, B.; Lavín, J.L.; Hurtado, A. Within-Farm Dynamics of ESBL-Producing Escherichia Coli in Dairy Cattle: Resistance Profiles and Molecular Characterization by Long-Read Whole-Genome Sequencing. Front. Microbiol. 2022, 13, 936843. [Google Scholar] [CrossRef]
- Real Decreto 53/2013. Por el que se Establecen las Normas Básicas Aplicables para la Protección de los Animales Utilizados en Experimentación y Otros Fines Científicos, Incluyendo la Docencia; Boletin Oficial del Estado: Madrid, Spain, 2013. [Google Scholar]
- McGuirk, S.M.; Collins, M. Managing the Production, Storage, and Delivery of Colostrum. Vet. Clin. N. Am. Food Anim. Pract. 2004, 20, 593–603. [Google Scholar] [CrossRef]
- Lombard, J.; Urie, N.; Garry, F.; Godden, S.; Quigley, J.; Earleywine, T.; McGuirk, S.; Moore, D.; Branan, M.; Chamorro, M.; et al. Consensus Recommendations on Calf- and Herd-Level Passive Immunity in Dairy Calves in the United States. J. Dairy Sci. 2020, 103, 7611–7624. [Google Scholar] [CrossRef]
- Deelen, S.M.; Ollivett, T.L.; Haines, D.M.; Leslie, K.E. Evaluation of a Brix Refractometer to Estimate Serum Immunoglobulin G Concentration in Neonatal Dairy Calves. J. Dairy Sci. 2014, 97, 3838–3844. [Google Scholar] [CrossRef]
- Bielmann, V.; Gillan, J.; Perkins, N.R.; Skidmore, A.L.; Godden, S.; Leslie, K.E. An Evaluation of Brix Refractometry Instruments for Measurement of Colostrum Quality in Dairy Cattle. J. Dairy Sci. 2010, 93, 3713–3721. [Google Scholar] [CrossRef] [PubMed]
- Quigley, J.D.; Lago, A.; Chapman, C.; Erickson, P.; Polo, J. Evaluation of the Brix Refractometer to Estimate Immunoglobulin G Concentration in Bovine Colostrum. J. Dairy Sci. 2013, 96, 1148–1155. [Google Scholar] [CrossRef] [PubMed]
- Elsohaby, I.; McClure, J.T.; Waite, L.A.; Cameron, M.; Heider, L.C.; Keefe, G.P. Using Serum and Plasma Samples to Assess Failure of Transfer of Passive Immunity in Dairy Calves. J. Dairy Sci. 2019, 102, 567–577. [Google Scholar] [CrossRef]
- Morrill, K.M.; Conrad, E.; Lago, A.; Campbell, J.; Quigley, J.; Tyler, H. Nationwide Evaluation of Quality and Composition of Colostrum on Dairy Farms in the United States. J. Dairy Sci. 2012, 95, 3997–4005. [Google Scholar] [CrossRef] [PubMed]
- Godden, S. Colostrum Management for Dairy Calves. Vet. Clin. N. Am. Food Anim. Pract. 2008, 24, 19–39. [Google Scholar] [CrossRef]
- Moore, M.; Tyler, J.W.; Chigerwe, M.; Dawes, M.E.; Middleton, J.R. Effect of Delayed Colostrum Collection on Colostral IgG Concentration in Dairy Cows. J. Am. Vet. Med. Assoc. 2005, 226, 1316–1319. [Google Scholar] [CrossRef]
- Claudia Casagrande, A.; Carolina Machado, G.P.; Lucas Rebelatto Brunetto, A.; Vedovatto, M.; Miotto Galli, G.; Schafer Da Silva, A. Cow Milk or Milk Replacer in the Diet of Holstein Calves: Effects on Complete Blood Count, Biochemistry Variables, and Performance. Animal Nutrition 2022, 24, 1–13. [Google Scholar] [CrossRef]
- Wellert, S.; Hartschuh, J. Feeding Milk Replacer Versus Whole Milk. Available online: https://dairy.osu.edu/sites/dairy/files/imce/DIBS/DIBS%2039%20Feeding%20Milk%20Replacer%20Versus%20Whole%20Milk%2039-20.pdf (accessed on 9 January 2025).
- Juste, R.A.; Geijo, M.V.; Elguezabal, N.; Sevilla, I.A.; Alonso-Hearn, M.; Garrido, J.M. Paratuberculosis Vaccination Specific and Non-Specific Effects on Cattle Lifespan. Vaccine 2021, 39, 1631–1641. [Google Scholar] [CrossRef]
- Yan, J.; Nielsen, T.B.; Lu, P.; Talyansky, Y.; Slarve, M.; Reza, H.; Novakovic, B.; Netea, M.G.; Keller, A.E.; Warren, T.; et al. A Protein-Free Vaccine Stimulates Innate Immunity and Protects against Nosocomial Pathogens. Sci. Transl. Med. 2023, 15, eadf9556. [Google Scholar] [CrossRef]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef]
- Brandon, M.R.; Watson, D.L.; Lascelles, A.K. The Mechanism of Transfer of Immunoglobulin into Mammary Secretion of Cows. Aust. J. Exp. Biol. Med. Sci. 1971, 6, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.; Ashfield, A.; Earley, B.; Welsh, M.; Gordon, A.; Morrison, S.J. Evaluation of Factors Associated with Immunoglobulin G, Fat, Protein, and Lactose Concentrations in Bovine Colostrum and Colostrum Management Practices in Grassland-Based Dairy Systems in Northern Ireland. J. Dairy Sci. 2017, 100, 2068–2079. [Google Scholar] [CrossRef]
- Hagiwara, K.; Domi, M.; Ando, J. Bovine Colostral CD8-Positive Cells Are Potent IFN-γ-Producing Cells. Vet. Immunol. Immunopathol. 2008, 124, 93–98. [Google Scholar] [CrossRef]
- Myles, I.A.; Datta, S.K. Frontline Science: Breast Milk Confers Passive Cellular Immunity via CD8-Dependent Mechanisms. J. Leukoc. Biol. 2021, 4, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.-H.; Jores, R.; Crowley, M.P. Recognition by g/δ T Cells. Annu. Rev. Immunol. 1996, 14, 511–543. [Google Scholar] [CrossRef] [PubMed]
- Chase, C.C.L.; Hurley, D.J.; Reber, A.J. Neonatal Immune Development in the Calf and Its Impact on Vaccine Response. Vet. Clin. N. Am. Food Anim. Pract. 2008, 24, 87–104. [Google Scholar] [CrossRef]
- Krueger, L.A.; Reinhardt, T.A.; Beitz, D.C.; Stuart, R.L.; Stabel, J.R. Effects of Fractionated Colostrum Replacer and Vitamins A, D, and E on Haptoglobin and Clinical Health in Neonatal Holstein Calves Challenged with Mycobacterium avium subsp. paratuberculosis. J. Dairy Sci. 2016, 99, 2884–2895. [Google Scholar] [CrossRef]
- Ocejo, M.; Oporto, B.; Lavín, J.L.; Hurtado, A. Monitoring Within-Farm Transmission Dynamics of Antimicrobial-Resistant Campylobacter in Dairy Cattle Using Broth Microdilution and Long-Read Whole Genome Sequencing. Sci. Rep. 2023, 13, 12529. [Google Scholar] [CrossRef]
- Ocejo, M.; Mugica, M.; Oporto, B.; Lavín, J.L.; Hurtado, A. Whole-Genome Long-Read Sequencing to Unveil Enterococcus Antimicrobial Resistance in Dairy Cattle Farms Exposed a Widespread Occurrence of Enterococcus lactis. Microbiol. Spectr. 2024, 12, e03672-23. [Google Scholar] [CrossRef]


| Under One Year | |||||
|---|---|---|---|---|---|
| Farm | Number of Non-Milked Heads | Number of Treatments | Number of Treated Heads | % Treated/Non-Milked Heads | |
| A | 110 | 6 | 6 | 5.45 | |
| C | 140 | 20 | 20 | 14.28 | |
| D | 130 | 6 | 6 | 4.61 | |
| In Lactation | |||||
| Farm | Number of Milked Heads | Condition | Number of Treatments | Number of Treated Heads | % Treated/Milked Heads |
| A | 200 | Mastitis | 124 | 49 | 24.5 |
| Lameness | 115 | 50 | 25 | ||
| Respiratory | 1 | 1 | 0.5 | ||
| Total | 240 | 100 | 50 | ||
| C | 100 | Mastitis | 54 | 36 | 36 |
| Lameness | 32 | 32 | 32 | ||
| Respiratory | 3 | 3 | 3 | ||
| Total | 89 | 71 | 71 | ||
| D | 120 | Mastitis | 31 | 20 | 16.66 |
| Lameness | NR | NR | NR | ||
| Respiratory | 2 | 2 | 1.66 | ||
| Total | 33 | 22 | 18.33 | ||
| Farm | Pearson | p | n |
|---|---|---|---|
| A | 0.986 | <0.0001 | 7 |
| B | 0.661 | 0.106 | 7 |
| C | 0.916 | 0.0005 | 9 |
| D | 0.854 | 0.0145 | 7 |
| Total | 0.841 | <0.0001 | 30 |
| Colostrum Quality | ||||
|---|---|---|---|---|
| Farm | Brix% Mean (std.) | IgG (mg/mL) Mean (std.) | HQC% | n |
| A | 20.24 (2.82) | 32.26 (14.89) | 14.29 | 7 |
| B | 21.13 (5.88) | 53.09 (33.40) | 62.50 | 8 |
| C | 18.77 (6.61) | 45.17 (28.84) | 40.00 | 10 |
| D | 20.71 (7.25) | 47.15 (32.35) | 42.86 | 7 |
| Total | 20.21 (5.75) | 44.76 (28.17) | 40.63 | 32 |
| Farm | Excellent (>25 mg/mL) | Good (17.9–24.9 mg/mL) | Fair (10–17.9 mg/mL) | Poor (<10 mg/mL) | PIT | % PIT | % FPIT | n |
|---|---|---|---|---|---|---|---|---|
| A | 0 | 0 | 1 | 6 | 1 | 14.29 | 85.71 | 7 |
| B | 4 | 1 | 3 | 0 | 8 | 100.00 | 0.00 | 8 |
| C | 2 | 3 | 1 | 4 | 6 | 60.00 | 40.00 | 10 |
| D | 1 | 1 | 2 | 3 | 4 | 57.14 | 42.86 | 7 |
| Total | 7 | 3 | 8 | 13 | 19 | 59.38 | 40.62 | 32 |
| Colostrum | Blood | ||||||
|---|---|---|---|---|---|---|---|
| Farm | CD4 Mean (std.) | CD8 Mean (std.) | WC1 Mean (std.) | CD4 Mean (std.) | CD8 Mean (std.) | WC1 Mean (std.) | n |
| A | 16.43 (21.8) | 0.42 (0.46) | 5.35 (6.10) | 10.74 (5.29) | 4.59 (3.59) | 38.35 (21.08) | 5 |
| B | 12.44 (15.66) | 0.11 (0.07) | 10.61 (16.96) | 56.20 (19.40) | 11.75 (2.06) | 22.57 (3.65) | 3 |
| C | 12.31 (16.71) | 4.29 (16.7) | 1.83 (2.67) | 25.36 (7.31) | 18.24 (10.34) | 45.63 (17.55) | 8 |
| D | 17.83 (3.53) | 1.03 (0.73) | 5.35 (2.26) | 28.74 (16.39) | 10.82 (6.33) | 50.67 (17.48) | 4 |
| All | 14.46 (15.39) | 2.04 (3.55) | 4.73 (7.13) | 27.01 (17.71) | 12.37 (8.94) | 41.36 (18.46) | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oyanguren, M.; Molina, E.; Mugica, M.; Badiola, A.; Hurtado, A.; Aduriz, G.; Elguezabal, N. A Pilot Study on Management Practices in Dairy Farms in the Basque Country: Focus on Colostrum Feeding and Vaccination. Animals 2025, 15, 1336. https://doi.org/10.3390/ani15091336
Oyanguren M, Molina E, Mugica M, Badiola A, Hurtado A, Aduriz G, Elguezabal N. A Pilot Study on Management Practices in Dairy Farms in the Basque Country: Focus on Colostrum Feeding and Vaccination. Animals. 2025; 15(9):1336. https://doi.org/10.3390/ani15091336
Chicago/Turabian StyleOyanguren, Maddi, Elena Molina, Maitane Mugica, Ainara Badiola, Ana Hurtado, Gorka Aduriz, and Natalia Elguezabal. 2025. "A Pilot Study on Management Practices in Dairy Farms in the Basque Country: Focus on Colostrum Feeding and Vaccination" Animals 15, no. 9: 1336. https://doi.org/10.3390/ani15091336
APA StyleOyanguren, M., Molina, E., Mugica, M., Badiola, A., Hurtado, A., Aduriz, G., & Elguezabal, N. (2025). A Pilot Study on Management Practices in Dairy Farms in the Basque Country: Focus on Colostrum Feeding and Vaccination. Animals, 15(9), 1336. https://doi.org/10.3390/ani15091336






