Physiological and Immunomodulatory Effects of Purslane Extract in Cirrhinus mrigala Juveniles: Implications for Sustainable Production
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Declaration of Ethics
2.2. Fish Rearing
2.3. Study Design
2.4. Purslane Extract Preparation
2.5. Formulation of Diet Pellets
2.6. Proximate Analysis
2.7. Study of Growth
2.8. Hematological Studies
2.9. Mineral Estimation
2.10. Activity of Antioxidant Enzymes and Immune Response
2.11. Statistical Analyses
3. Results
3.1. Assessment of Growth Performance and Survival
3.2. Whole-Body Nutrient Analysis
3.3. Hematology
3.4. Whole-Body Mineralization
3.5. Antioxidant Status
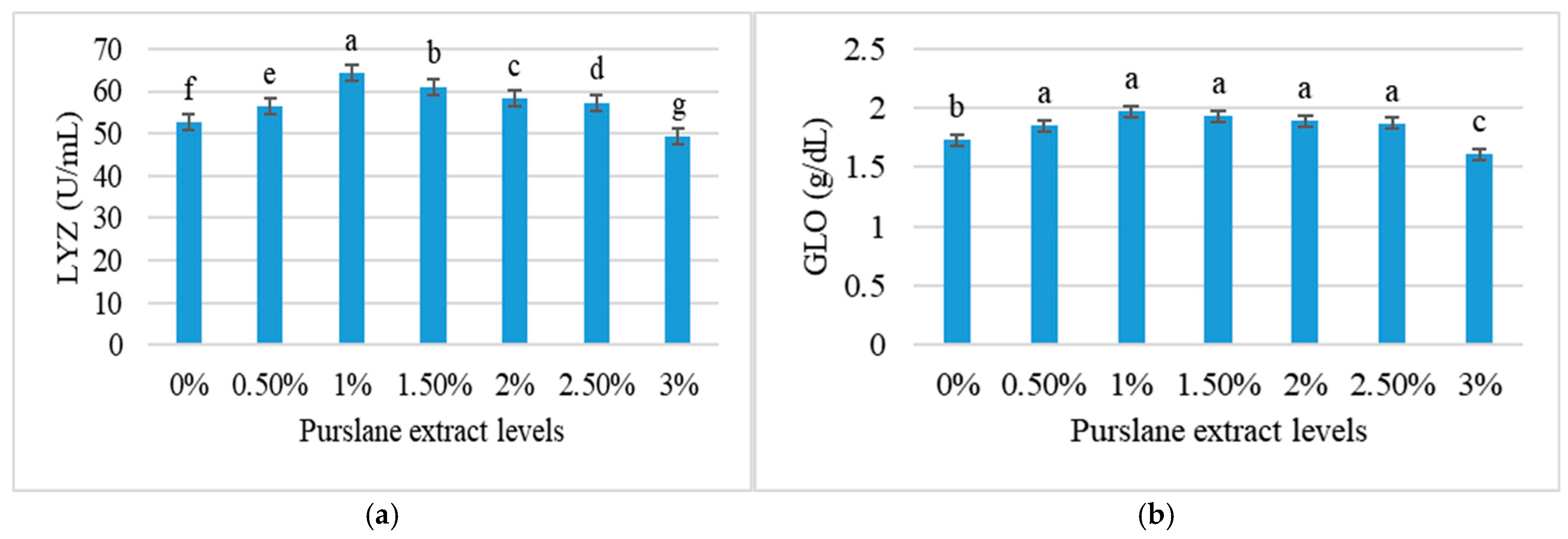
3.6. Immune Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, G.; Bhatnagar, A.; Alok, K.; Ajay, S. Enzymatic profiling and feeding preferences of catla: Catla catla, rohu: Labeo rohita and mrigala: Cirrhinus mrigala in rural polyculture ponds. J. Aquac. Res. Dev. 2018, 9, 553. [Google Scholar] [CrossRef]
- Marković, Z.Z.; Poleksić, V.D. The role and importance of aquaculture for the ecological sustainability of fish resources in the inland water of Serbia. In Ecological Sustainability of Fish Resources of Inland Waters of the Western Balkans: Freshwater Fish Stocks, Sustainable Use and Conservation; Springer International Publishing: Cham, Switzerland, 2024; pp. 575–602. [Google Scholar]
- Austin, B.; Lawrence, A.; Can, E.; Carboni, C.; Crockett, J.; Demirtas, N.; Schleder, D.; Adolfo, J.; Kayis, S.; Karacalar, U.; et al. Selected topics in sustainable aquaculture research: Current and future focus: Sustain. Aquac. Res. 2022, 1, 74–125. [Google Scholar] [CrossRef]
- Hussain, S.M.; Naeem, E.; Ali, S.; Adrees, M.; Riaz, D.; Paray, B.A.; Naeem, A. Evaluation of growth, nutrient absorption, body composition and blood indices under dietary exposure of iron oxide nanoparticles in Common carp (Cyprinus carpio). J. Anim. Physiol. Anim. Nut. 2024, 108, 366–373. [Google Scholar] [CrossRef]
- Gao, S.; Chen, W.; Cao, S.; Sun, P.; Gao, X. Microalgae as fishmeal alternatives in aquaculture: Current status, existing problems, and possible solutions. Environ. Sci. Pollut. Res. 2024, 31, 16113–16130. [Google Scholar] [CrossRef] [PubMed]
- Reverter, M.; Bontemps, N.; Lecchini, D.; Banaigs, B.; Sasal, P. Use of plant extracts in fish aquaculture as an alternative to chemotherapy: Current status and future perspectives. Aquaculture 2014, 433, 50–61. [Google Scholar] [CrossRef]
- Amalia, R.L.R.; Suryaningrum, L.H.; Sumitro, S.; Budiyanti, B.; Rohmy, S.; Nur, B.; Mulyasari, M. Valorization of weed Portulaca oleracea L. as an alternative to fish feed ingredient. In BIO Web of Conferences; EDP Sciences: Les Ulis, France, 2024; Volume 87, p. 03029. [Google Scholar]
- Holkem, A.T.; Silva, M.P.D.; Favaro-Trindade, C.S. Probiotics and plant extracts: A promising synergy and delivery systems. Critic. Rev. Food Sci. Nut. 2023, 63, 9561–9579. [Google Scholar] [CrossRef]
- Syanya, F.J.; Mathia, W.M.; Harikrishnan, M. Current status and trend on the adoption of fish feed additives for sustainable tilapia aquaculture production: A review. Asia J. Fish Aquat. Res. 2023, 22, 10–25. [Google Scholar] [CrossRef]
- Nowosad, J.; Jasiński, S.; Arciuch-Rutkowska, M.; Abdel-Latif, H.M.R.; Wróbel, M.; Mikiewicz, M.; Zielonka, Ł.; Kotsyumbas, I.Y.; Muzyka, V.P.; Brezvvn, O.M.; et al. Effects of Bee Pollen on Growth Performance, Intestinal Microbiota and Histomorphometry in African Catfish. Animals 2023, 13, 132. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; El-Ashram, S.; Yilmaz, S.; Naiel, M.A.E.; Kari, Z.A.; Hamid, N.K.A.; Dawood, M.A.O.; Nowosad, J.; Kucharczyk, D. The effectiveness of Arthrospira platensis and microalgae in relieving stressful conditions affecting finfish and shellfish species: An overview. Aquacult. Rep. 2022, 24, 101135. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Yilmaz, S.; Kucharczyk, D. Editorial: Functionality and applications of phytochemicals in aquaculture nutrition. Front. Vet. Sci. 2023, 10, 1218542. [Google Scholar] [CrossRef]
- Vijayaram, S.; Razafindralambo, H.; Ghafarifarsani, H.; Sun, Y.Z.; Hoseinifar, S.H.; Van Doan, H. Synergetic response on herbal and probiotic applications: A review. Fish Physiol. Biochem. 2024, 50, 1315–1329. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Poyatos, M.D.P.; Llorent-Martínez, E.J.; Ruiz-Medina, A. Phytochemical composition and antioxidant activity of Portulaca oleracea: Influence of the steaming cooking process. Foods 2021, 10, 94. [Google Scholar] [CrossRef]
- Karakas, S.; Bolat, I.; Dikilitas, M. The use of halophytic companion plant (Portulaca oleracea L.) on some growth, fruit, and biochemical parameters of strawberry plants under salt stress. Horticulturae 2021, 7, 63. [Google Scholar] [CrossRef]
- Sangeetha, S.; Kiran, R.S.; Abbulu, K.; Battu, S. A Review on traditional herb Portulaca oleracea. World J. Pharm. Res. 2020, 9, 578–601. [Google Scholar] [CrossRef]
- Montoya-García, C.O.; García-Mateos, R.; Becerra-Martínez, E.; Toledo-Aguilar, R.; Volke-Haller, V.H.; Magdaleno-Villar, J.J. Bioactive compounds of purslane (Portulaca oleracea L.) according to the production system: A review. Sci. Hortic. 2023, 308, 111584. [Google Scholar] [CrossRef]
- Hanan, A.A.; Sobhy, M.H.; Kawkab, A.A.; Azza, K.A.; Zeinab, A.R.; Wedad, A.H. Chemical and remedial effects of portulacaoleracea plant. Life Sci. J. 2014, 11, 31–42. [Google Scholar]
- Uddin, M.K.; Juraimi, A.S.; Hossain, M.S.; Nahar, M.A.U.; Ali, M.E.; Rahman, M.M. Purslane weed (Portulaca oleracea): A prospective plant source of nutrition, omega-3 fatty acid, and antioxidant attributes. Sci. World J. 2014, 2014, 951019. [Google Scholar] [CrossRef]
- Ruiz, M.C. Effects of Purslane (Portulaca oleracea L.) and Shewanella putrefaciens Probiotic Enriched Diet on Gilthead Seabream (Sparus aurata L.). Ph.D. Thesis, Universidade do Algarve, Algarve, Portugal, 2017. [Google Scholar]
- Abdel-Razek, N.; Awad, S.M.; Abdel-Tawwab, M. Effect of dietary purslane (Portulaca oleracea L.) leaves powder on growth, immunostimulation, and protection of Nile tilapia, Oreochromis niloticus against Aeromonas hydrophila infection. Fish. Physiol. Biochem. 2019, 45, 1907–1917. [Google Scholar] [CrossRef]
- Rowland, S.J.; Ingram, B.A. Diseases of Australian native fishes. Fish. Bull. 1991, 4, 21–23. [Google Scholar]
- Allen, G.; Rowland, S. Development of an experimental diet for silver perch (Bidanus bidanus). Austas. Aquac. 1992, 6, 39–40. [Google Scholar]
- Shanker, N.; Maneesh Kumar, M.; Juvvi, P.; Debnath, S. Moisture sorption characteristics of ready-to-eat snack food enriched with purslane leaves. J. Food Sci. Technol. 2019, 56, 1918–1926. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, M.; Rashidi, A.A.; Taherian, A.A.; Vakili, Z.; Mehran, M. The protective effect of hydroalcoholic extract of rosa canina (Dog Rose) fruit on liver function and structure in streptozotocin-induced diabetes in rats. J. Dietary Supplem. 2018, 15, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Lovell, T. Nutrition and Feeding of Fish; Van Nostrand Reinhold: New York, NY, USA, 1989; Volume 260. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 20th ed.; AOAC International: Rockville, MD, USA, 2016. [Google Scholar]
- Yousaf, Z.; Hussain, S.M.; Ali, S.; Sarker, P.K.; Al-Ghanim, K.A. Recuperative effects of cinnamon (Cinnamomum zeylanicum) in Catla catla after sub-lethal exposure to lead. Biol. Trace Elem. Res. 2024, 203, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Hussain, S.M.; Sultana, T.; Rasul, A.; Ali, S. Potential role of purslane (Portulaca oleracea) extract supplemented sunflower meal-based diets on the performance of commercially important fish, Labeo rohita. Pak. J. Zool. 2024, 1–9. [Google Scholar] [CrossRef]
- Javahery, S.; Nekoubin, H.; Moradlu, A.H. Effect of anaesthesia with clove oil in fish. Fish Physiol. Biochem. 2012, 38, 1545–1552. [Google Scholar] [CrossRef]
- Brown, B.A. Routine hematology procedures. In Hematology: Principles and Procedures, 5th ed.; Lea & Febiger: Philadelphia, PA, USA, 1988; pp. 7–122. [Google Scholar]
- Blaxhall, P.C.; Daisley, K.W. Routine hematological methods for use with fish blood. J. Fish Biol. 1973, 5, 771–781. [Google Scholar] [CrossRef]
- Wedemeyer, G.A.; Yasutake, W.T. Clinical Methods for the Assessment of the Effects of Environmental Stress on Fish Health; Department of the Interior, Fish and Wildlife Service: Fairfax, VA, USA, 1977; Volume 89. [Google Scholar]
- Winterbourn, C.C. Superoxide as an intracellular radical sink. Free. Radic. Biol. Med. 1993, 14, 85–90. [Google Scholar] [CrossRef]
- Claiborne, A. Catalase activity. In CRC Handbook of Methods in Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.B.; Hafeman, D.G.; Hoekstra, W. Selenium: Biochemical role as a component of glutathione peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzym. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Ellis, A.E. Lysozyme assays. In Techniques in Fish Immunology; Stolen, J.S., Fletcher, T.C., Anderson, D.P., Roberson, B.S., Muiswinkel, W.B., Eds.; SOS Publications: Fair Haven, NJ, USA, 1990; pp. 101–103. [Google Scholar]
- Steel, R.G.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics, 3rd ed.; McGraw Hill international Book Co., Inc.: New York, NY, USA, 1996; pp. 336–352. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; Iowa State University Press: Ames, IA, USA, 1991; p. 503. [Google Scholar]
- Zaki, M.A.; Alabssawy, A.N.; Nour, A.E.A.M.; El Basuini, M.F.; Dawood, M.A.; Alkahtani, S.; Abdel-Daim, M.M. The impact of stocking density and dietary carbon sources on the growth, oxidative status and stress markers of Nile tilapia (Oreochromis niloticus) reared under biofloc conditions. Aquac. Rep. 2020, 16, 100282. [Google Scholar] [CrossRef]
- Hoseinifar, S.H.; Dadar, M.; Van Doan, H.; Harikrishnan, R. Feed additives impacts on shellfish microbiota, health, and development. In Microbial Communities in Aquaculture Ecosystems; Springer: Cham, Switzerland, 2019; pp. 143–163. [Google Scholar] [CrossRef]
- Abdel-Latif, H.M.R.; Citarasu, T.; Turgay, E.; Yilmaz, E.; Yousefi, M.; Shekarabi, P.H.; Ahmadifar, E.; Nowosad, J.; Kucharczyk, D.; Yilmaz, S. Control of yersiniosis in rainbow trout, Oncorhynchus mykiss: Innovative non-antibiotic feed-based strategies. Ann. Anim. Sci. 2024. [Google Scholar] [CrossRef]
- Güllü, K.; Acar, Ü.; Kesbiç, O.S.; Yılmaz, S.; Ağdamar, S.; Ergün, S.; Türker, A. Beneficial effects of Oral Allspice, Pimenta dioica powder supplementation on the hemato-immunological and serum biochemical responses of Oreochromis mossambicus. Aquac. Res. 2016, 47, 2697–2704. [Google Scholar] [CrossRef]
- Adel, M.; Dawood, M.A.; Shafiei, S.; Sakhaie, F.; Shekarabi, S.P.H. Dietary Polygonum minus extract ameliorated the growth performance, humoral immune parameters, immune-related gene expression and resistance against Yersinia ruckeri in rainbow trout (Oncorhynchus mykiss). Aquaculture 2020, 519, 734738. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Q.; Ye, F.; Tang, H.; Xiong, Y.; Wu, Y.; Wang, L.; Feng, X.; Zhang, S.; Wan, Y.; et al. Dietary purslane (Portulaca oleracea L.) promotes the growth performance of broilers by modulation of gut microbiota. AMB Express 2021, 11, 31. [Google Scholar] [CrossRef]
- Şahin, D.; Meryem, Ö.Z.; Aral, O.; Bahtiyar, M.; Taşçi, S. Growth and pigmentation of goldfish (Carassius auratus L., 1758) fed on a diet supplemented with purslane (Portulaca sp.) Extract. Magnesium 2021, 1727, 1725. [Google Scholar] [CrossRef]
- Mohammadalikahni, M.; Shamsaie Mehrjan, M.; Haghighi, M.; Soltani, M.; Kamali, A. Effects of oral administration of dried purslane (Portulaca oleracea) extract on some growth indices, carcass quality and intestinal microbial flora of rainbow trout (Oncorhynchus mykiss) fry. J. Anim. Environ. 2020, 12, 229–236. [Google Scholar]
- Petropoulos, S.; Karkanis, A.; Martins, N.; Ferreira, I.C. Phytochemical composition and bioactive compounds of common purslane (Portulaca oleracea L.) as affected by crop management practices. Trends Food Sci Technol. 2016, 55, 1–10. [Google Scholar] [CrossRef]
- Díaz-Vázquez, I.E.; Cuevas-Rodríguez, B.L.; Zavala-Leal, O.I.; Cuevas-Rodríguez, E.O.; Arámbul-Muñoz, E.; Sánchez-Magaña, L.M.; Valdez-González, F.J. Effects of adding purslane (Portulaca oleracea) as a source of omega-3 fatty acids on muscle composition, fatty acid profile, and productive performance in Tilapia (Oreochromis niloticus). Israeli J. Aquac-Bamidgeh. 2025, 77, 158–167. [Google Scholar] [CrossRef]
- Ahmadifar, E.; Hoseinifar, S.H.; Adineh, H.; Moghadam, M.S.; Dawood, M.A. Assessing the impact of purslane (L.) on growth performance, anti-oxidative, and immune activities in grass carp. Annal. Anim. Sci. 2020, 20, 1427–1440. [Google Scholar] [CrossRef]
- Srivastava, R.; Srivastava, V.; Singh, A. Multipurpose benefits of an underexplored species purslane (Portulaca oleracea L.): A critical review. Environ. Manag. 2023, 72, 309–320. [Google Scholar] [CrossRef]
- Waititu, S.M.; Sanjayan, N.; Hossain, M.M.; Leterme, P.; Nyachoti, C.M. Improvement of the nutritional value of high-protein sunflower meal for broiler chickens using multi-enzyme mixtures. Poult. Sci. 2018, 97, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.X.; Xin, H.L.; Rahman, K.; Wang, S.J.; Peng, C.; Zhang, H. Portulaca oleracea L.: A review of phytochemistry and pharmacological effects. BioMed Res. Int. 2015, 2015, 925631. [Google Scholar] [CrossRef]
- Habibian, M.; Sadeghi, G.; Karimi, A. Effects of purslane (Portulaca oleracea L.) powder on growth performance, blood indices, and antioxidant status in broiler chickens with triiodothyronine-induced ascites. Arch. Anim. Breed. 2017, 60, 315–325. [Google Scholar] [CrossRef]
- Okafor, I.A.; Ayalokunrin, M.B.; Orachu, L.A. A review on Portulaca oleracea (purslane) plant-its nature and biomedical benefits. Int. J. Biomed. Res. 2014, 5, 75–80. [Google Scholar] [CrossRef]
- Uddin, M.K.; Juraimi, A.S.; Ali, M.E.; Ismail, M.R. Evaluation of Antioxidant Properties and Mineral Composition of Purslane (Portulaca oleracea L.) at Different Growth Stages. Int. J. Mol. Sci. 2012, 13, 10257–10267. [Google Scholar] [CrossRef]
- Shalaei, M.; Hosseini, S.M. Effect of different levels of purslane seed on blood parameters, plasma minerals, liver enzymes and some egg characteristics in laying hens. Anim. Prod. Res. 2014, 3, e45–e54. [Google Scholar]
- National Research Council. Nutrient Requirements of Fish and Shrimp; The National Academies Press: Washington, DC, USA, 2011. [CrossRef]
- Zemheri-Navruz, F.; Acar, Ü.; Yılmaz, S. Dietary supplementation of olive leaf extract increases haematological, serum biochemical parameters and immune related genes expression level in common carp (Cyprinus carpio) juveniles. Fish Shellfish. Immunol. 2019, 89, 672–676. [Google Scholar] [CrossRef]
- Magnadóttir, B. Innate immunity of fish (overview). Fish Shellfish Immunol. 2006, 20, 137–151. [Google Scholar] [CrossRef]

| Feed components | |||||||
| (%) | T0 (Control) | T1 | T2 | T3 | T4 | T5 | T6 |
| Sunflower meal | 54 | 54 | 54 | 54 | 54 | 54 | 54 |
| Rice polish | 8 | 8 | 8 | 8 | 8 | 8 | 8 |
| Vitamin Premix * | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Fish meal | 17 | 17 | 17 | 17 | 17 | 17 | 17 |
| Fish oil | 6 | 6 | 6 | 6 | 6 | 6 | 6 |
| Chromic oxide | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Ascorbic acid | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Mineral mixture ** | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Wheat flour *** | 11 | 10.5 | 10 | 9.5 | 9 | 8.5 | 8 |
| Purslane extract | 0 | 0.5 | 1 | 1.5 | 2 | 2.5 | 3 |
| Proximate Composition of diets | |||||||
| Crude protein (%) | 30.34 | 30.43 | 30.37 | 30.31 | 30.38 | 30.35 | 30.39 |
| Crude lipid (%) | 8.06 | 8.11 | 8.07 | 8.08 | 8.06 | 8.08 | 8.11 |
| Gross energy (Kcal/g) | 3.43 | 3.46 | 3.42 | 3.45 | 3.47 | 3.43 | 3.44 |
| Proximate Composition of ingredients | |||||||
| Gross energy (Kcal/g) | Crude protein (%) | Crude fat (%) | Dry matter (%) | Carbohydrates (%) | Crude fiber (%) | Ash (%) | |
| Sunflower meal | 3.43 | 43.53 | 10.50 | 91.51 | 25.78 | 10.65 | 9.54 |
| Wheat flour | 3.48 | 10.76 | 2.10 | 92.39 | 82.87 | 2.12 | 2.15 |
| Rice, Polish | 3.53 | 11.24 | 13.63 | 92.27 | 50.18 | 13.19 | 11.76 |
| Fish meal | 3.31 | 52.67 | 6.35 | 91.43 | 19.43 | 1.38 | 20.17 |
| Nutrient content (%) | |
| Moisture | 12.34 |
| Crude fat | 4.27 |
| Carbohydrates | 42.51 |
| Ash | 2.13 |
| Crude protein | 18.52 |
| Crude fiber | 20.64 |
| Mineral content (ppm) | |
| Iron | 22 |
| Phosphorus | 330 |
| Potassium | 650 |
| Calcium | 661 |
| Magnesium | 657 |
| Purslane Extract | Treatments | Initial Weight (g) | Final Weight (g) | Weight Gain (WG, g) | FCR | SGR | Feed Intake (g/Fish/Day) | WG (%) | PER | Survival Rate (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 0% | T0 (Control) | 8.37 ± 0.09 a | 18.50 ± 0.30 f | 10.13 ± 0.35 e | 2.11 ± 0.17 ab | 0.88 ± 0.03 e | 0.24 ± 0.01 d | 121.14 ± 5.03 e | 0.32 ± 0.01 f | 96 |
| 0.5% | T1 | 8.34 ± 0.08 a | 19.32 ± 0.16 e | 10.98 ± 0.13 e | 1.88 ± 0.03 bc | 0.93 ± 0.01 e | 0.23 ± 0.00 d | 131.66 ± 2.01 e | 0.36 ± 0.01 e | 100 |
| 1% | T2 | 8.23 ± 0.05 ab | 29.54 ± 0.33 a | 21.31 ± 0.30 a | 1.32 ± 0.05 d | 1.42 ± 0.01 a | 0.31 ± 0.02 a | 258.82 ± 2.80 a | 0.70 ± 0.00 a | 100 |
| 1.5% | T3 | 8.32 ± 0.05 ab | 27.08 ± 0.23 b | 18.77 ± 0.21 b | 1.41 ± 0.06 d | 1.31 ± 0.01 b | 0.29 ± 0.02 ab | 225.65 ± 2.34 b | 0.61 ± 0.01 b | 100 |
| 2% | T4 | 8.21 ± 0.08 ab | 23.79 ± 0.20 c | 15.58 ± 0.28 c | 1.58 ± 0.11 cd | 1.18 ± 0.02 c | 0.27 ± 0.02 bc | 189.68 ± 5.10 c | 0.51 ± 0.01 c | 99 |
| 2.5% | T5 | 8.12 ± 0.09 b | 20.52 ± 0.31 d | 12.39 ± 0.37 d | 1.74 ± 0.08 c | 1.03 ± 0.03 d | 0.24 ± 0.01 cd | 152.61 ± 6.10 d | 0.41 ± 0.01 d | 99 |
| 3% | T6 | 8.29 ± 0.09 ab | 16.69 ± 0.38 g | 8.40 ± 0.45 f | 2.43 ± 0.20 a | 0.78 ± 0.04 f | 0.23 ± 0.02 d | 101.33 ± 6.36 f | 0.27 ± 0.01 g | 95 |
| Purslane Extract | Treatments | Protein (%) | Ash (%) | Fat (%) | Moisture (%) |
|---|---|---|---|---|---|
| 0% | T0 (Control) | 13.28 ± 0.14 d | 1.83 ± 0.09 d | 5.43 ± 0.16 ab | 78.88 ± 0.10 a |
| 0.5% | T1 | 13.52 ± 0.17 d | 2.50 ± 0.07 ab | 5.36 ± 0.13 ab | 78.46 ± 0.13 ab |
| 1% | T2 | 15.37 ± 0.16 a | 2.63 ± 0.14 a | 3.20 ± 0.22 e | 78.34 ± 0.10 ab |
| 1.5% | T3 | 15.06 ± 0.18 ab | 2.62 ± 0.10 a | 3.83 ± 0.11 d | 77.89 ± 0.12 c |
| 2% | T4 | 14.63 ± 0.12 bc | 2.36 ± 0.06 b | 4.27 ± 0.11 c | 78.21 ± 0.20 b |
| 2.5% | T5 | 14.30 ± 0.20 c | 2.28 ± 0.08 bc | 5.09 ± 0.18 b | 78.20 ± 0.15 b |
| 3% | T6 | 13.07 ± 0.21 d | 2.05 ± 0.09 cd | 5.63 ± 0.16 a | 78.84 ± 0.17 a |
| Purslane Extract | Treatments | RBCs (10−6 mm−3) | Hb (g/100mL) | PCV (%) | WBCs (10−6 mm−3) | MCV (fl) | MCH (pg) | PLT | MCHC (%) |
|---|---|---|---|---|---|---|---|---|---|
| 0% | T0 (Control) | 1.63 ± 0.12 e | 6.95 ± 0.09 e | 21.57 ± 0.25 f | 6.63 ± 0.0.09 de | 133.02 ± 8.58 b | 42.89 ± 0.3.81 a | 56.39 ± 0.0.06 f | 32.21 ± 0.76 a |
| 0.5% | T1 | 2.09 ± 0.08 d | 7.42 ± 0.10 d | 22.43 ± 0.17 e | 6.90 ± 0.06 cd | 107.23 ± 3.06 c | 35.48 ± 0.79 b | 58.62. ± 0.13 e | 33.08 ± 0.24 a |
| 1% | T2 | 3.86 ± 0.10 a | 8.75 ± 0.10 a | 29.44 ± 0.34 a | 8.06 ± 0.10 a | 76.34 ± 1.25 e | 22.71 ± 0.80 e | 66.86 ± 0.23 a | 31.81 ± 3.43 a |
| 1.5% | T3 | 3.10 ± 0.14 b | 8.30 ± 0.08 b | 26.82 ± 0.28 b | 7.74 ± 0.15 b | 86.59 ± 3.29 de | 26.79 ± 0.93 de | 64.74 ± 0.06 b | 30.93 ± 0.21 a |
| 2% | T4 | 2.77 ± 0.07 c | 7.89 ± 0.14 c | 24.08 ± 0.18 c | 7.46 ± 0.12 b | 86.95 ± 1.85 de | 28.50 ± 1.20 cd | 62.77 ± 0.11 c | 32.77 ± 0.68 a |
| 2.5% | T5 | 2.33 ± 0.04 d | 7.59 ± 0.06 d | 23.10 ± 0.21 d | 7.09 ± 0.08 c | 99.17 ± 2.57 cd | 32.56 ± 0.33 bc | 59.37 ± 0.07 d | 32.84 ± 0.52 a |
| 3% | T6 | 1.52 ± 0.08 e | 6.75 ± 0.09 e | 22.50 ± 0.15 de | 6.52 ± 0.10 e | 148.60 ± 6.94 a | 44.59 ± 2.75 a | 55.57 ± 0.11 g | 29.99 ± 0.60 a |
| Purslane Extract | Treatments | Ca (%) | Mg (%) | Na (mg/g) | Cu (μg/g) | K (%) | Fe (μg/g) | P (%) | Mn (μg/g) | Zn (μg/g) |
|---|---|---|---|---|---|---|---|---|---|---|
| 0% | T0 (Control) | 0.68 ± 0.15 bc | 2.78 ± 0.14 bc | 4.91 ± 0.13 de | 3.06 ± 0.11 d | 4.79 ± 0.07 de | 43.49 ± 0.15 f | 0.78 ± 0.16 bc | 4.89 ± 0.10 ef | 2.99 ± 0.07 de |
| 0.5% | T1 | 0.74 ± 0.12 abc | 2.89 ± 0.21 abc | 5.07 ± 0.10 cde | 3.21 ± 0.05 cd | 4.98 ± 0.16 de | 45.38 ± 0.13 e | 0.82 ± 0.14 bc | 5.23 ± 0.12 e | 3.13 ± 0.14 cde |
| 1% | T2 | 1.06 ± 0.10 a | 3.35 ± 0.15 a | 5.78 ± 0.15 a | 4.01 ± 0.16 a | 7.85 ± 0.10 a | 61.59 ± 0.15 a | 1.26 ± 0.16 a | 10.71 ± 0.23 a | 3.99 ± 0.14 a |
| 1.5% | T3 | 0.94 ± 0.10 ab | 3.21 ± 0.17 ab | 5.62 ± 0.15 ab | 3.74 ± 0.09 ab | 6.18 ± 0.11b | 56.62 ± 0.17 b | 1.10 ± 0.08 ab | 8.93 ± 0.14 b | 3.70 ± 0.15 ab |
| 2% | T4 | 0.86 ± 0.10 abc | 3.09 ± 0.14 abc | 5.46 ± 0.11 abc | 3.50 ± 0.12 bc | 5.46 ± 0.13 c | 52.01 ± 0.11 c | 0.98 ± 0.15 abc | 6.91 ± 0.18 c | 3.45 ± 0.14 bc |
| 2.5% | T5 | 0.78 ± 0.13 abc | 2.98 ± 0.17 abc | 5.27 ± 0.26 bcd | 3.36 ± 0.09 c | 5.12 ± 0.21 cd | 48.38 ± 0.16 d | 0.88 ± 0.06 abc | 5.76 ± 0.09 d | 3.26 ± 0.11 cd |
| 3% | T6 | 0.60 ± 0.12 c | 2.67 ± 0.16 c | 4.73 ± 0.13 e | 2.75 ± 0.09 e | 4.65 ± 0.12 e | 39.34 ± 0.11 g | 0.62 ± 0.19 c | 4.71 ± 0.12 f | 2.81 ± 0.09 e |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faisal, M.; Hussain, S.M.; Ali, S.; Kucharczyk, D.; Al-Ghanim, K.A. Physiological and Immunomodulatory Effects of Purslane Extract in Cirrhinus mrigala Juveniles: Implications for Sustainable Production. Animals 2025, 15, 1334. https://doi.org/10.3390/ani15091334
Faisal M, Hussain SM, Ali S, Kucharczyk D, Al-Ghanim KA. Physiological and Immunomodulatory Effects of Purslane Extract in Cirrhinus mrigala Juveniles: Implications for Sustainable Production. Animals. 2025; 15(9):1334. https://doi.org/10.3390/ani15091334
Chicago/Turabian StyleFaisal, Muhammad, Syed Makhdoom Hussain, Shafaqat Ali, Dariusz Kucharczyk, and Khalid A. Al-Ghanim. 2025. "Physiological and Immunomodulatory Effects of Purslane Extract in Cirrhinus mrigala Juveniles: Implications for Sustainable Production" Animals 15, no. 9: 1334. https://doi.org/10.3390/ani15091334
APA StyleFaisal, M., Hussain, S. M., Ali, S., Kucharczyk, D., & Al-Ghanim, K. A. (2025). Physiological and Immunomodulatory Effects of Purslane Extract in Cirrhinus mrigala Juveniles: Implications for Sustainable Production. Animals, 15(9), 1334. https://doi.org/10.3390/ani15091334






