Gas Endeavour: An Innovative Equipment for Estimating Methane Kinetics During In Vitro Rumen Fermentation
Simple Summary
Abstract
1. Introduction
2. In Vitro Gas Production Techniques (IVGPTs)
2.1. Syringe Method (Hohenheim Gas Test)
2.2. In Vitro Methane Measuring Techniques
2.3. Limitations of IVGPTs
| Reference | Device | Water-Bath/Air Incubator | Device Volume, mL | Inoculum/Medium Ratio, mL/mL | Buffer Reference | Duration of Incubation, h | Dietary Substrate, Buffered Medium Ratio mg/mL | Gas Venting and Collection | Pressure Control | Gas Measurement and Analysis |
|---|---|---|---|---|---|---|---|---|---|---|
| Menke et al. [19] | Syringes | Water rotor bath | 30 | 1:2 | [19] | 24 | 6.67 | Manual, endpoint sampling | Yes, movable glass piston | Manual, no analysis |
| Theodorou et al. [24] | Bottles | Air incubator | 60 | 1:9 | [24] | 24–72 | 2–20 | Manual, endpoint sampling | No, pressure increase | Manual, no analysis |
| Mauricio et al. [36] | Bottles | Air incubator | 100 | 1:9 | [24] | n.s. | 10.0 | Manual, endpoint sampling | No, pressure increase | Manual, no analysis |
| Pell and Schofield [23] | Bottles, stirred | Air incubator | 10 | 1:4 | [41] | n.s. | 10.0 | Manual endpoint sampling | No, pressure increase | Manual, CH4 by GC |
| Cone et al. [27] | Bottle, shook | Water bath | 100 | 1:2 | [42] | 48 | 6.67 | Automated, fixed pressure | Yes | Manual, |
| Davies et al. [43] | Bottles | Air incubator | 100 | 1:9 | [24] | n.s. | 10.0 | Automated, fixed pressure | Yes | n.s., n.s. |
| Cornou et al. [33] | Bottles | Air incubator | 60 | 1:2 | [42] | 72 | 8.33 | Automated, fixed pressure | Yes | Manual, no analysis |
| Muetzel et al. [34] | Bottles | Air incubator | 60 | 1:4 | [44,45] | 48 | 10.0 | Automated, Automated | Yes | Automated, CH4 by GC |
| Pellikaan et al. [18] | Bottles, shook | Water bath | 60 | 1:2 | [42] | 72 | 8.33 | Automated, vented pressure | Yes | Manual, CH4 by GC |
| Tagliapietra et al. [20] | Bottles | Air incubator | 75 | 1:2 | [42] | 144 | 6.67 | Automated, Automated | Yes | Manual, CH4 by GC |
| Braidot et al. [35] | Bottles, stirred | Water bath | 500 | 1:2 | [42] | 48 | 6.67 | Automated, Automated | No, volume base | Automated, CH4—infrared |
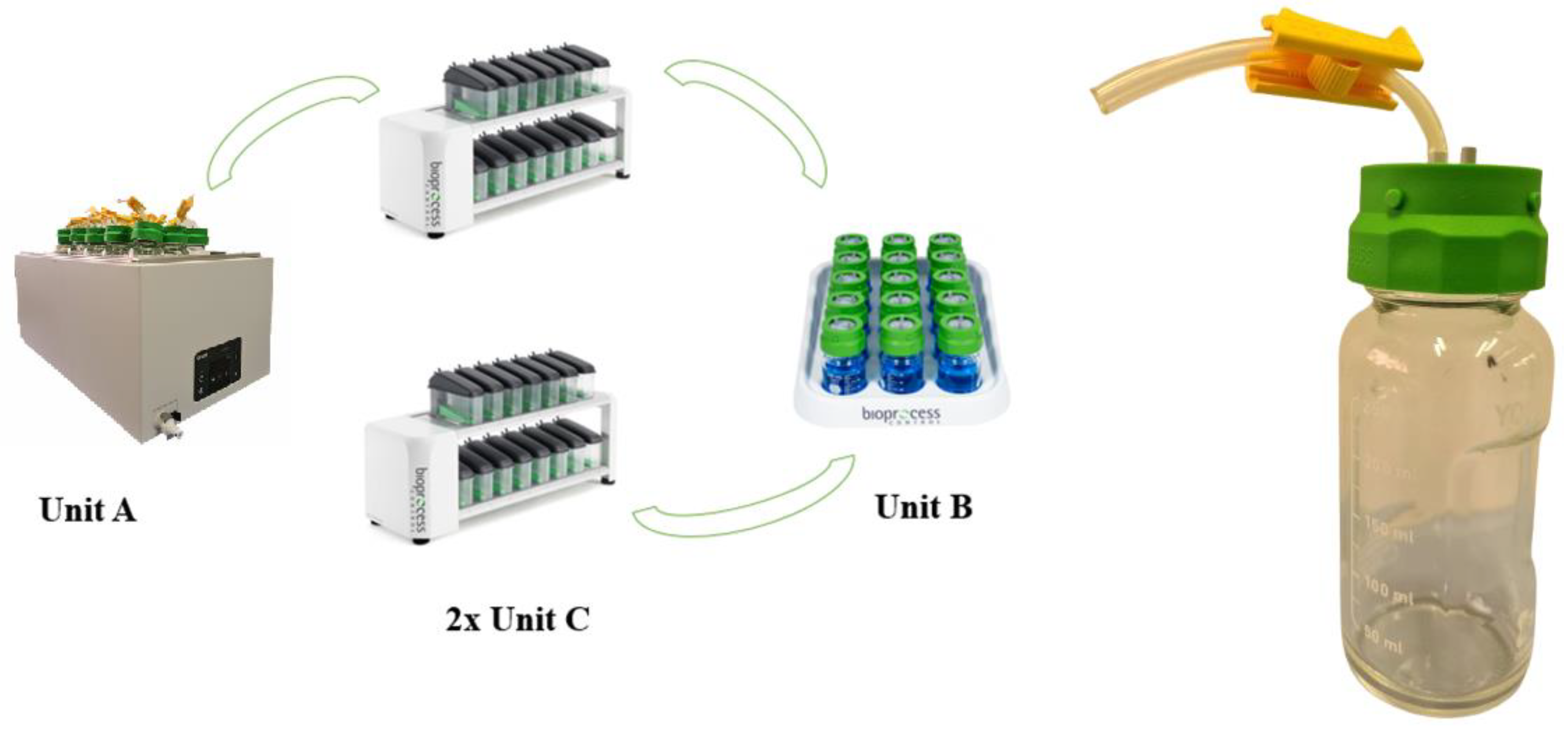
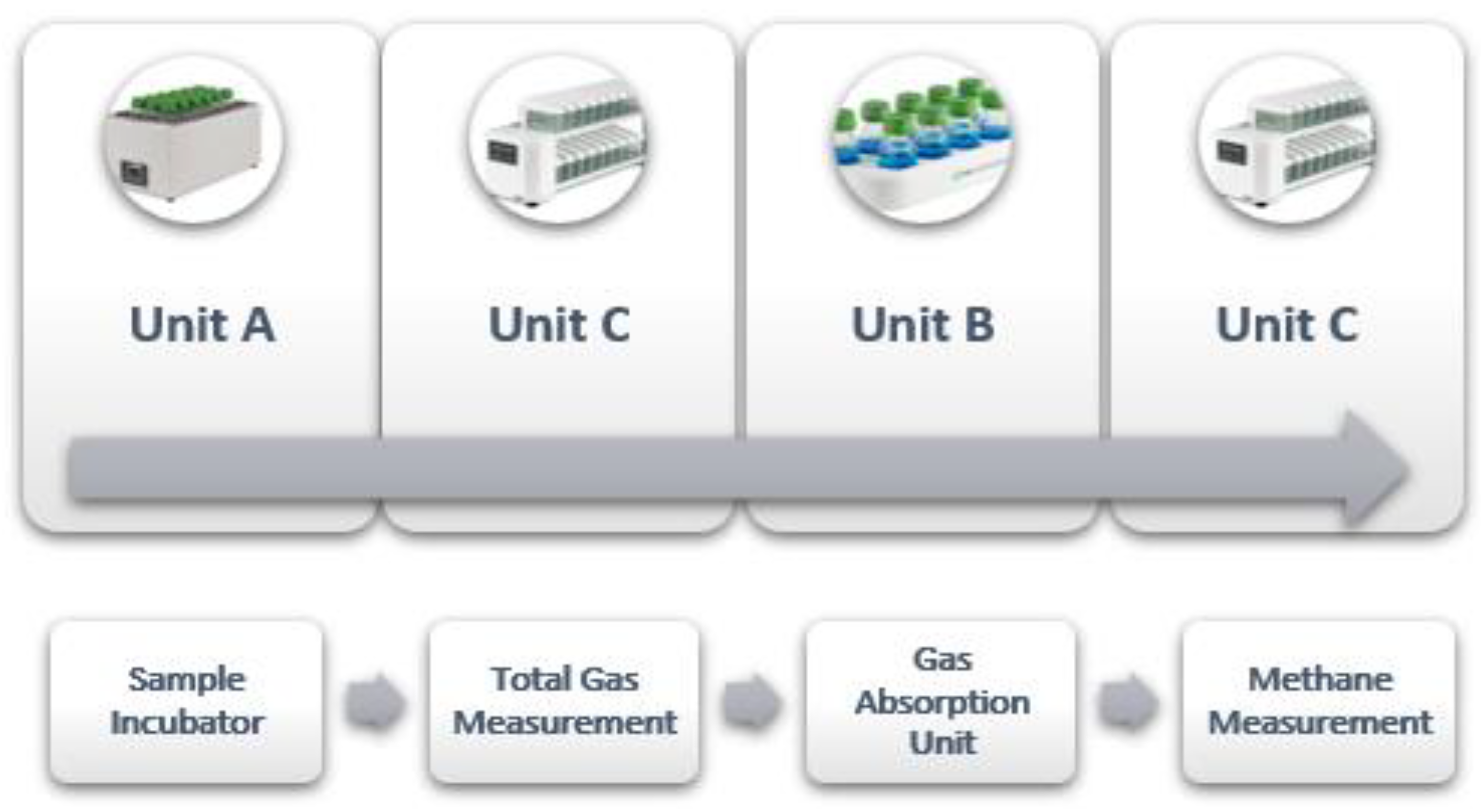
3. The Gas Endeavour System
3.1. Parts and Functionality
3.2. Area of Application of the Gas Endeavour System
3.2.1. Biomethane Potential
3.2.2. Animal Nutrition
3.3. Challenges and Considerations in the Practical Use of GES
3.3.1. Buffer, Rumen Inoculum, and Feed Substrate Ratio
3.3.2. Bubbling of CO2
3.3.3. Effect of Shaking on Gas Production
3.3.4. Effect of Headspace Pressure on Gas Production
3.3.5. Effect of Headspace Pressure on Methane Production
3.3.6. Normalization of Gas and Methane Measurement for Temperature, Pressure, and Vapour Interference
3.4. Gas Endeavour Properties and Characteristics: Strengths and Weaknesses
3.4.1. Gas and Methane Measurements
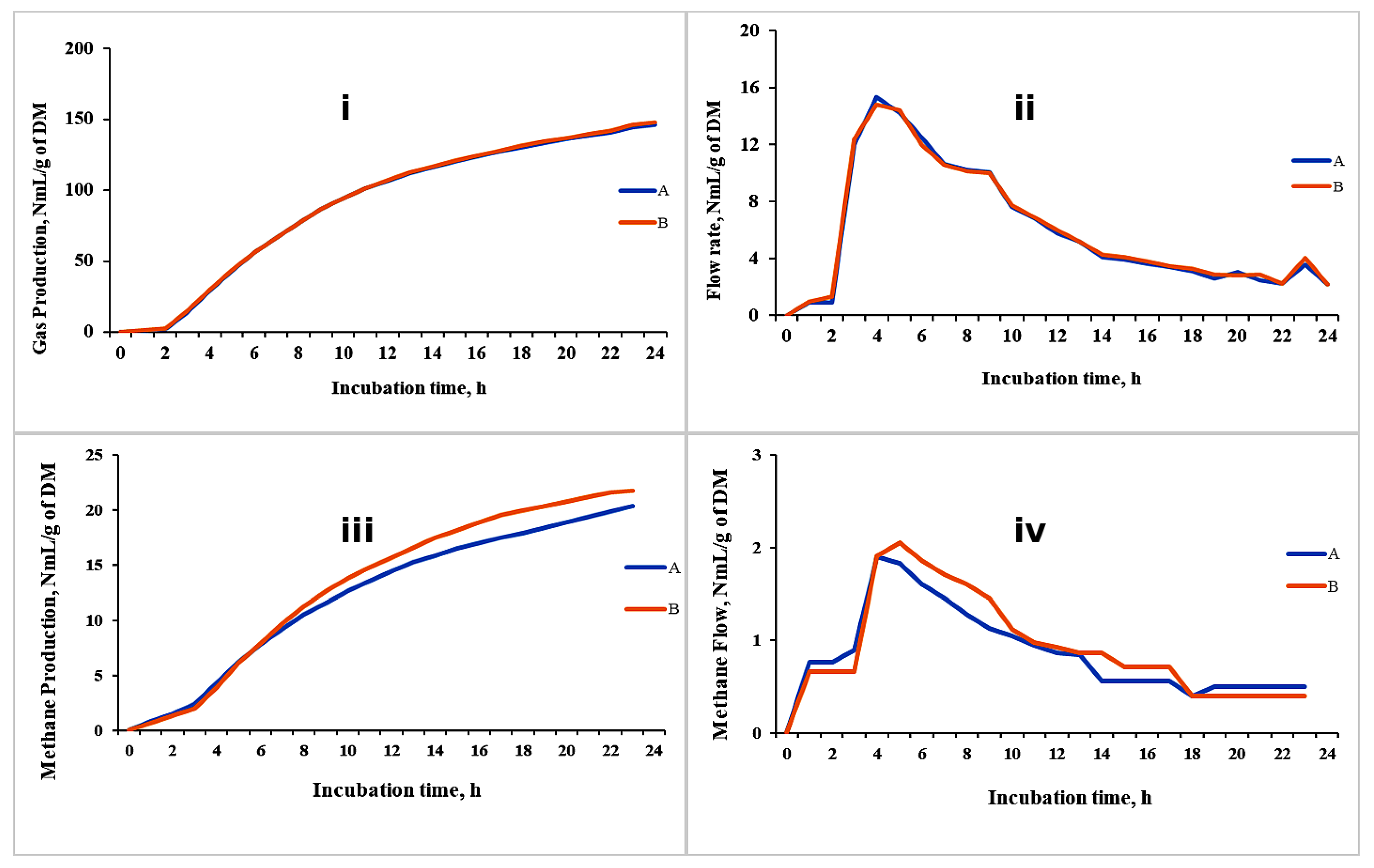
3.4.2. Gas and CH4 Flow Rate and Kinetics
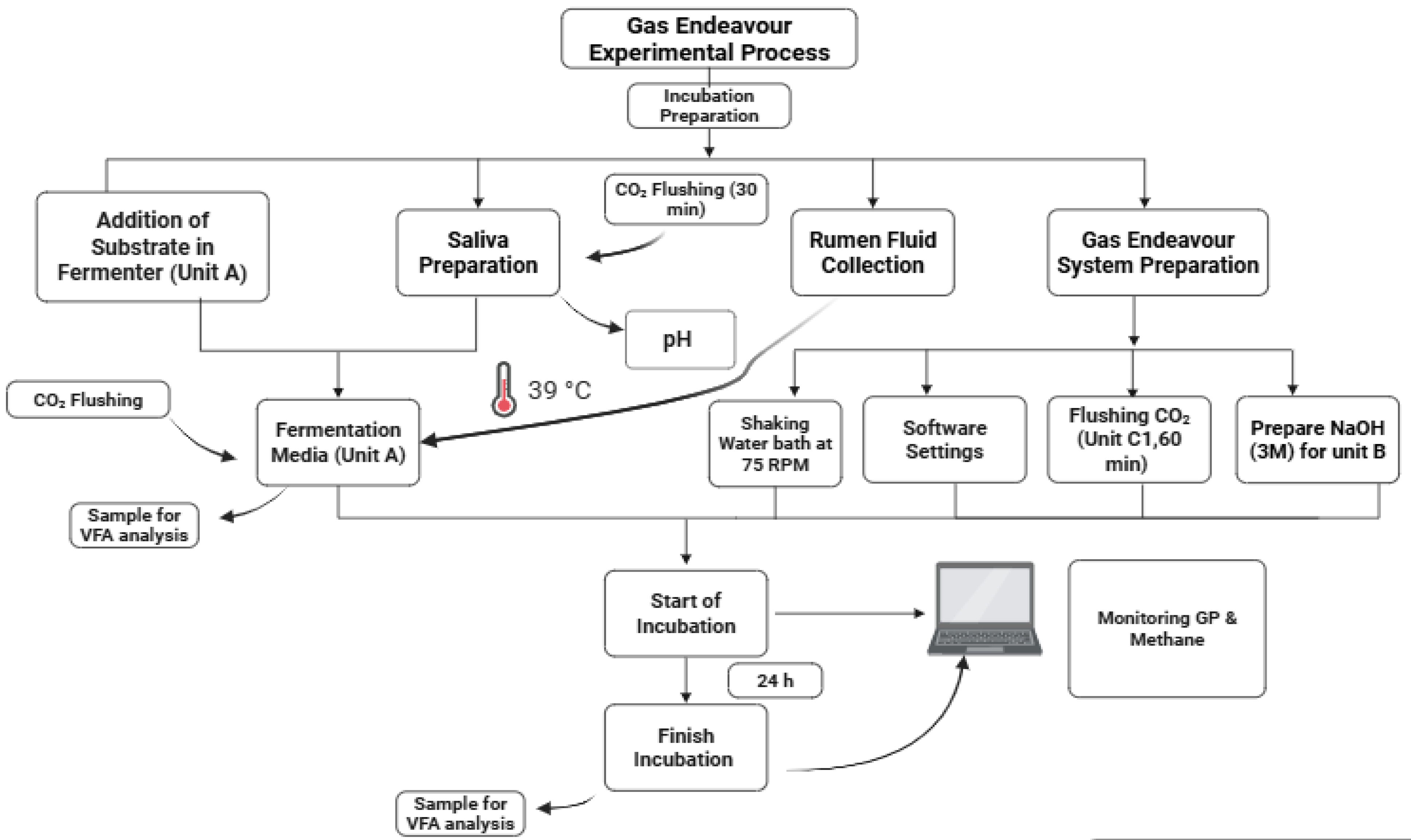
3.4.3. Experimental Design and Results of the Demonstrative Trial
3.4.4. Real-Time Monitoring
3.4.5. Limitations
4. Potential Future Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization (FAO). FAOSTAT Statistical Database; FAO: Rome, Italy, 2024. [Google Scholar]
- Tubiello, F.N.; Salvatore, M.; Rossi, S.; Ferrara, A.; Fitton, N.; Smith, P. The FAOSTAT database of greenhouse gas emissions from agriculture. Environ. Res. Lett. 2013, 8, 015009. [Google Scholar] [CrossRef]
- Ramin, M.; Huhtanen, P. Development of an in vitro method for determination of methane production kinetics using a fully automated in vitro gas system—A modelling approach. Anim. Feed Sci. Technol. 2012, 174, 190–200. [Google Scholar] [CrossRef]
- Garnsworthy, P.C.; Craigon, J.; Hernandez-Medrano, J.H.; Saunders, N. On-farm methane measurements during milking correlate with total methane production by individual dairy cows. J. Dairy Sci. 2012, 95, 3166–3180. [Google Scholar] [CrossRef] [PubMed]
- Pinares, C.; Waghorn, G. Technical Manual on Respiration Chamber Designs; Ministry of Agriculture and Forestry: Wellington, New Zealand, 2014. [Google Scholar]
- Storm, I.M.; Hellwing, A.L.F.; Nielsen, N.I.; Madsen, J. Methods for measuring and estimating methane emission from ruminants. Animals 2012, 2, 160–183. [Google Scholar] [CrossRef]
- Sejian, V.; Lal, R.; Lakritz, J.; Ezeji, T. Measurement and prediction of enteric methane emission. Int. J. Biometeorol. 2011, 55, 1–16. [Google Scholar] [CrossRef]
- Johnson, K.; Huyler, M.; Westberg, H.; Lamb, B.; Zimmerman, P. Measurement of methane emissions from ruminant livestock using a sulfur hexafluoride tracer technique. Environ. Sci. Technol. 1994, 28, 359–362. [Google Scholar] [CrossRef]
- Lassey, K.R. On the importance of background sampling in applications of the SF6 tracer technique to determine ruminant methane emissions. Anim. Feed Sci. Technol. 2013, 180, 115–120. [Google Scholar] [CrossRef]
- Zhao, Y.; Nan, X.; Yang, L.; Zheng, S.; Jiang, L.; Xiong, B. A review of enteric methane emission measurement techniques in ruminants. Animals 2020, 10, 1004. [Google Scholar] [CrossRef]
- Zimmerman, P.R.Z.S. Method and system for monitoring and reducing ruminant methane production. US8307785B2, 13 November 2012. [Google Scholar]
- Place, S.E.; Pan, Y.; Zhao, Y.; Mitloehner, F.M. Construction and Operation of a Ventilated Hood System for Measuring Greenhouse Gas and Volatile Organic Compound Emissions from Cattle. Animals 2011, 1, 433–446. [Google Scholar] [CrossRef]
- Silveira, S.R.; Terry, S.A.; Biffin, T.E.; Maurício, R.M.; Pereira, L.G.R.; Ferreira, A.L.; Ribeiro, R.S.; Sacramento, J.P.; Tomich, T.R.; Machado, F.S. Replacement of soybean meal with soybean cake reduces methane emissions in dairy cows and an assessment of a face-mask technique for methane measurement. Front. Vet. Sci. 2019, 6, 295. [Google Scholar] [CrossRef]
- Almeida, A.K.; Hegarty, R.S.; Cowie, A. Meta-analysis quantifying the potential of dietary additives and rumen modifiers for methane mitigation in ruminant production systems. Anim. Nutr. 2021, 7, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Tilley, J.; Terry, d.R. A two-stage technique for the in vitro digestion of forage crops. Grass Forage Sci. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Rymer, C.; Huntington, J.; Williams, B.; Givens, D. In vitro cumulative gas production techniques: History, methodological considerations and challenges. Anim. Feed Sci. Technol. 2005, 123, 9–30. [Google Scholar] [CrossRef]
- Navarro-Villa, A.; O’brien, M.; López, S.; Boland, T.; O’kiely, P. Modifications of a gas production technique for assessing in vitro rumen methane production from feedstuffs. Anim. Feed Sci. Technol. 2011, 166, 163–174. [Google Scholar] [CrossRef]
- Pellikaan, W.; Hendriks, W.; Uwimana, G.; Bongers, L.; Becker, P.; Cone, J. A novel method to determine simultaneously methane production during in vitro gas production using fully automated equipment. Anim. Feed Sci. Technol. 2011, 168, 196–205. [Google Scholar] [CrossRef]
- Menke, K.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The estimation of the digestibility and metabolizable energy content of ruminant feedingstuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- Tagliapietra, F.; Cattani, M.; Bailoni, L.; Schiavon, S. In vitro rumen fermentation: Effect of headspace pressure on the gas production kinetics of corn meal and meadow hay. Anim. Feed Sci. Technol. 2010, 158, 197–201. [Google Scholar] [CrossRef]
- Blümmel, M.; Orskov, E. Comparison of in vitro gas production and nylon bag degradability of roughages in predicting of food intake in cattle. Anim. Feed. Sci. Technol. 1993, 40, 109–119. [Google Scholar] [CrossRef]
- Makkar, H.; Blümmel, M.; Becker, K. Formation of complexes between polyvinyl pyrrolidones or polyethylene glycols and tannins, and their implication in gas production and true digestibility in in vitro techniques. Br. J. Nutr. 1995, 73, 897–913. [Google Scholar] [CrossRef]
- Pell, A.; Schofield, P. Computerized monitoring of gas production to measure forage digestion in vitro. J. Dairy Sci. 1993, 76, 1063–1073. [Google Scholar] [CrossRef]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Mauricio, R.M.; Mould, F.L.; Dhanoa, M.S.; Owen, E.; Channa, K.S.; Theodorou, M.K. A semi-automated in vitro gas production technique for ruminant feedstuff evaluation. Anim. Feed Sci. Technol. 1999, 79, 321–330. [Google Scholar] [CrossRef]
- Massaro, S.; Jantzen, B.; Axel, A.M.D.; Tagliapietra, F.; Hansen, H.H. Effect of Iodoform in Maize and Clover Grass Silages: An In Vitro Study. Ruminants 2024, 4, 418–432. [Google Scholar] [CrossRef]
- Cone, J.W.; van Gelder, A.H.; Visscher, G.J.; Oudshoorn, L. Influence of rumen fluid and substrate concentration on fermentation kinetics measured with a fully automated time related gas production apparatus. Anim. Feed Sci. Technol. 1996, 61, 113–128. [Google Scholar] [CrossRef]
- Ankom. ANKOM RF Gas Production System. Available online: https://www.ankom.com/product-catalog/ankom-rf-gas-production-system (accessed on 18 March 2024).
- Jouany, J.P.; Lassalas, B. Gas pressure inside a rumen in vitro system stimulates the use of hydrogen. In Proceedings of the 3rd Joint RRI-INRA Gastrointestinal Tract Microbiology Symposium Beyond Antimicrobials: The Future of Gut Microbiology, Aberdeen, UK, 12–15 June 2002. [Google Scholar]
- Hess, P.A.; Giraldo, P.; Williams, R.; Moate, P.; Beauchemin, K.; Eckard, R. A novel method for collecting gas produced from the in vitro ankom gas production system. J. Anim. Sci. 2016, 94, 570. [Google Scholar] [CrossRef]
- Tunkala, B.Z.; DiGiacomo, K.; Hess, P.S.A.; Dunshea, F.R.; Leury, B.J. Rumen fluid preservation for in vitro gas production systems. Anim. Feed Sci. Technol. 2022, 292, 115405. [Google Scholar] [CrossRef]
- Regadas Filho, J.G.L.; Tedeschi, L.O.; Fonseca, M.A.; Cavalcanti, L.F.L. Comparison of fermentation kinetics of four feedstuffs using an in vitro gas production system and the ANKOM Gas Production System. In Proceedings of the 2014 ADSA-ASAS-CSAS Joint Annual Meeting, Kansas City, MO, USA, 20–24 July 2014; Available online: https://www.researchgate.net/publication/268087640 (accessed on 4 May 2025).
- Cornou, C.; Storm, I.M.D.; Hindrichsen, I.K.; Worgan, H.; Bakewell, E.; Ruiz, D.R.Y.; Abecia, L.; Tagliapietra, F.; Cattani, M.; Ritz, C. A ring test of a wireless in vitro gas production system. Anim. Prod. Sci. 2013, 53, 585–592. [Google Scholar] [CrossRef]
- Muetzel, S.; Hunt, C.; Tavendale, M.H. A fully automated incubation system for the measurement of gas production and gas composition. Anim. Feed Sci. Technol. 2014, 196, 1–11. [Google Scholar] [CrossRef]
- Braidot, M.; Sarnataro, C.; Romanzin, A.; Spanghero, M. A new equipment for continuous measurement of methane production in a batch in vitro rumen system. J. Anim. Physiol. Anim. Nutr. 2023, 107, 747–753. [Google Scholar] [CrossRef]
- Mauricio, R.M.; Owen, E.; Mould, F.L.; Givens, I.; Theodorou, M.K.; France, J.; Davies, D.R.; Dhanoa, M.S. Comparison of bovine rumen liquor and bovine faeces as inoculum for an in vitro gas production technique for evaluating forages. Animal Feed Science and Technology 2001, 89, 33–48. [Google Scholar] [CrossRef]
- Mutimura, M.; Myambi, C.; Gahunga, P.; Mgheni, D.; Laswai, G.; Mtenga, L.; Gahakwa, D.; Kimambo, A.; Ebong, C. Rumen liquor from slaughtered cattle as a source of inoculum for in vitro gas production technique in forage evaluation. Agric. J 2013, 8, 173–180. [Google Scholar]
- Stocker, T. Climate Change 2013: The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Spanghero, M.; Chiaravalli, M.; Colombini, S.; Fabro, C.; Froldi, F.; Mason, F.; Moschini, M.; Sarnataro, C.; Schiavon, S.; Tagliapietra, F. Rumen Inoculum Collected from Cows at Slaughter or from a Continuous Fermenter and Preserved in Warm, Refrigerated, Chilled or Freeze-Dried Environments for In Vitro Tests. Animals 2019, 9, 815. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Ruiz, D.R.; Bannink, A.; Dijkstra, J.; Kebreab, E.; Morgavi, D.P.; O’Kiely, P.; Reynolds, C.K.; Schwarm, A.; Shingfield, K.J.; Yu, Z.; et al. Design, implementation and interpretation of in vitro batch culture experiments to assess enteric methane mitigation in ruminants—A review. Anim. Feed. Sci. Technol. 2016, 216, 1–18. [Google Scholar] [CrossRef]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analyses (Apparatus, Reagents, Procedures, and Some Applications); US Agricultural Research Service: Washington, DC, USA, 1970. [Google Scholar]
- Menke, K.H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Davies, Z.; Mason, D.; Brooks, A.; Griffith, G.; Merry, R.; Theodorou, M. An automated system for measuring gas production from forages inoculated with rumen fluid and its use in determining the effect of enzymes on grass silage. Anim. Feed Sci. Technol. 2000, 83, 205–221. [Google Scholar] [CrossRef]
- Mould, F.; Kliem, K.; Morgan, R.; Mauricio, R. In vitro microbial inoculum: A review of its function and properties. Anim. Feed. Sci. Technol. 2005, 123, 31–50. [Google Scholar] [CrossRef]
- Mould, F.; Morgan, R.; Kliem, K.; Krystallidou, E. A review and simplification of the in vitro incubation medium. Anim. Feed Sci. Technol. 2005, 123, 155–172. [Google Scholar] [CrossRef]
- BPC Instruments. Gas Endeavour Anaerobic Testing Handbook, Version 1.0; BPC Instruments AB: Lund, Sweden, 2024. [Google Scholar]
- Liu, J.; Strömberg, S.; van Gorp, J.; Nistor, M. Importance of Accurate and Correct Quantitative Measurements in a New Volumetric Gas Measuring Technique for In Vitro Assessment of Ruminant Feeds. In Proceedings of the 7th Nordic Feed Science Conference, Uppsala, Sweden, 14–15 June 2016. [Google Scholar]
- Uveges, Z.; Damak, M.; Klátyik, S.; Ramay, M.W.; Fekete, G.; Varga, Z.; Gyuricza, C.; Székács, A.; Aleksza, L. Biomethane Potential in Anaerobic Biodegradation of Commercial Bioplastic Materials. Fermentation 2023, 9, 261. [Google Scholar] [CrossRef]
- Swiechowski, K.; Rasaq, W.A.; Syguła, E. Anaerobic digestion of brewer’s spent grain with biochars—biomethane production and digestate quality effects. Front. Energy Res. 2023, 11. [Google Scholar] [CrossRef]
- Wu, H.; Li, J.; Liao, Q.; Fu, Q.; Liu, Z. Enhanced biohydrogen and biomethane production from Chlorella sp. with hydrothermal treatment. Energy Convers. Manag. 2020, 205, 112373. [Google Scholar] [CrossRef]
- Carrillo-Reyes, J.; Buitrón, G.; Moreno-Andrade, I.; Tapia-Rodríguez, A.C.; Palomo-Briones, R.; Razo-Flores, E.; Aguilar-Juárez, O.; Arreola-Vargas, J.; Bernet, N.; Braga, A.F.M.; et al. Standardized protocol for determination of biohydrogen potential. MethodsX 2020, 7, 100754. [Google Scholar] [CrossRef] [PubMed]
- Dolci, G.; Venturelli, V.; Catenacci, A.; Ciapponi, R.; Malpei, F.; Romano Turri, S.E.; Grosso, M. Evaluation of the anaerobic degradation of food waste collection bags made of paper or bioplastic. J. Environ. Manag. 2022, 305, 114331. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.M.D.; de Almeida, J.F.M.; Escócio, V.A.; da Silva, A.L.N.; de Sousa, A.M.F.; Visconte, L.L.Y.; Furtado, C.R.G.; Pacheco, E.B.A.V.; Leite, M.C.A.M. Evaluation of rheological behavior, anaerobic and thermal degradation, and lifetime prediction of polylactide/poly(butylene adipate-co-terephthalate)/powdered nitrile rubber blends. Polym. Bull. 2019, 76, 2899–2913. [Google Scholar] [CrossRef]
- Elgemark, E. Intensively Processed Silage Using Bio-Extruder: Effects on Gas Production and Forage Digestibility. Master’s Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2019. [Google Scholar]
- Psenovschi, G.; Vintila, A.C.N.; Neamtu, C.; Vlaicu, A.; Capra, L.; Dumitru, M.; Enascuta, C.-E. Biogas Production Using “Gas Endeavour” Automatic Gas Flow System. Chem. Proc. 2023, 13, 17. [Google Scholar] [CrossRef]
- Dębowski, M.; Kisielewska, M.; Kazimierowicz, J.; Zieliński, M. Methane Production from Confectionery Wastewater Treated in the Anaerobic Labyrinth-Flow Bioreactor. Energies 2023, 16, 571. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Kazimierowicz, J.; Walery, M. Aquatic Macrophyte Biomass Periodically Harvested Form Shipping Routes and Drainage Systems in a Selected Region of Poland as a Substrate for Biogas Production. Appl. Sci. 2023, 13, 4184. [Google Scholar] [CrossRef]
- Chan, S.; Nishi, K.; Koyama, M.; Toda, T.; Matsuyama, T.; Ida, J. Combined effects of various conductive materials and substrates on enhancing methane production performance. Biomass Bioenergy 2023, 178, 106977. [Google Scholar] [CrossRef]
- Syguła, E.; Rasaq, W.A.; Świechowski, K. Effects of Iron, Lime, and Porous Ceramic Powder Additives on Methane Production from Brewer’s Spent Grain in the Anaerobic Digestion Process. Materials 2023, 16, 5245. [Google Scholar] [CrossRef]
- Szwarc, D.; Nowicka, A.; Zieliński, M. Comparison of the Effects of Pulsed Electric Field Disintegration and Ultrasound Treatment on the Efficiency of Biogas Production from Chicken Manure. Appl. Sci. 2023, 13, 8154. [Google Scholar] [CrossRef]
- Kleinheinz, G.; Hernandez, J. Comparison of two laboratory methods for the determination of biomethane potential of organic feedstocks. J. Microbiol. Methods. 2016, 130, 54–60. [Google Scholar] [CrossRef]
- Nolan, P.; Luostarinen, S.; Doyle, E.; O’kiely, P. Anaerobic digestion of perennial ryegrass prepared by cryogenic freezing versus thermal drying methods, using contrasting in vitro batch digestion systems. Renew. Energy. 2016, 87, 273–278. [Google Scholar] [CrossRef]
- Yang, H.; Rustas, B.-O.; Eriksson, T. Rumen In Vitro Total Gas Production of Timothy, Red Clover and the Mixed Silage After Extrusion. In Proceedings of the 9th Nordic Feed Science Conference, Uppsala, Sweden, 12–13 June 2018; SLU/HUV Report 298. pp. 181–183. [Google Scholar]
- Quarantelli, A.; Renzi, M.; Simoni, M.; Martuzzi, F.; Rosato, M.; Zambini, E.M.; Righi, F. Evaluation of an additive capable to improve ruminal fermentations through the use of an automated gas production system. Ital. J. Anim. Sci. 2019, 18, 30–31. [Google Scholar]
- Bertignon, M. Analisi di additivi in grado di migliorare le fermentazioni ruminali attraverso l’uso di un sistema automatizzato di gas production. Bachelor’s Thesis, Università di Parma, Dipartimento di Scienze Medico-Veterinarie, Parma, Italy.
- Vigh, A.; Criste, A.; Gragnic, K.; Moquet, L.; Gerard, C. Ruminal solubility and bioavailability of inorganic trace mineral sources and effects on fermentation activity measured in vitro. Agriculture 2023, 13, 879. [Google Scholar] [CrossRef]
- Muragijeyezu, P.C. Investigating the potential of different dietary fibers to stimulate butyrate production in vitro. Master’s Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2020. [Google Scholar]
- Diamond, L.W.; Akinfiev, N.N. Solubility of CO2 in water from− 1.5 to 100 C and from 0.1 to 100 MPa: Evaluation of literature data and thermodynamic modelling. Fluid Phase Equilibria 2003, 208, 265–290. [Google Scholar] [CrossRef]
- Sun, Q.; Tian, H.; Li, Z.; Guo, X.; Liu, A.; Yang, L. Solubility of CO2 in water and NaCl solution in equilibrium with hydrate. Part I: Experimental measurement. Fluid Phase Equilibria 2016, 409, 131–135. [Google Scholar] [CrossRef]
- Jantzen, B.; Hansen, H.H. Differences in Donor Animal Production Stage Affect Repeatability of In Vitro Rumen Fermentation Kinetics. Animals 2023, 13, 2993. [Google Scholar] [CrossRef]
- Wang, M.; Wang, R.; Tang, S.; Tan, Z.; Zhou, C.; Han, X.; Kang, J. Comparisons of manual and automated incubation systems: Effects of venting procedures on in vitro ruminal fermentation. Livest. Sci. 2016, 184, 41–45. [Google Scholar] [CrossRef]
- Lucile, F.; Cézac, P.; Contamine, F.; Serin, J.-P.; Houssin, D.; Arpentinier, P. Solubility of Carbon Dioxide in Water and Aqueous Solution Containing Sodium Hydroxide at Temperatures from (293.15 to 393.15) K and Pressure up to 5 MPa: Experimental Measurements. J. Chem. Eng. Data 2012, 57, 784–789. [Google Scholar] [CrossRef]
- Carroll, J.J.; Slupsky, J.D.; Mather, A.E. The solubility of carbon dioxide in water at low pressure. J. Phys. Chem. Ref. Data. 1991, 20, 1201–1209. [Google Scholar] [CrossRef]
- Patra, A.K.; Yu, Z. Effects of gas composition in headspace and bicarbonate concentrations in media on gas and methane production, degradability, and rumen fermentation using in vitro gas production techniques. J. Dairy Sci. 2013, 96, 4592–4600. [Google Scholar] [CrossRef]
- Rosato, M.; Quarantelli, A. Comparing ruminal activity measures performed with Gas Endeavour with literature data. In Proceedings of the 22nd Congress of the Animal Science and Production Association (ASPA), Perugia, Italy, 13–16 June 2017. [Google Scholar]
- Baffa, D.F.; Oliveira, T.S.; Fernandes, A.M.; Camilo, M.G.; Silva, I.N.; Meirelles Júnior, J.R.; Aniceto, E.S. Evaluation of Associative Effects of In Vitro Gas Production and Fermentation Profile Caused by Variation in Ruminant Diet Constituents. Methane 2023, 2, 344–360. [Google Scholar] [CrossRef]
- Boland, T.M.; Pierce, K.M.; Kelly, A.K.; Kenny, D.A.; Lynch, M.B.; Waters, S.M.; Whelan, S.J.; McKay, Z.C. Feed Intake, Methane Emissions, Milk Production and Rumen Methanogen Populations of Grazing Dairy Cows Supplemented with Various C 18 Fatty Acid Sources. Animals 2020, 10, 2380. [Google Scholar] [CrossRef] [PubMed]
- Maccarana, L.; Cattani, M.; Tagliapietra, F.; Bailoni, L.; Schiavon, S. Influence of main dietary chemical constituents on the in vitro gas and methane production in diets for dairy cows. J. Anim. Sci. Biotechnol. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Rota Graziosi, A.; Colombini, S.; Crovetto, G.M.; Galassi, G.; Chiaravalli, M.; Battelli, M.; Reginelli, D.; Petrera, F.; Rapetti, L. Partial replacement of soybean meal with soybean silage in lactating dairy cows diet: Part 1, milk production, digestibility, and N balance. Ital. J. Anim. Sci. 2022, 21, 634–644. [Google Scholar] [CrossRef]
- Xu, H.; Li, Y.; Hua, D.; Mu, H.; Zhao, Y.; Chen, G. Methane production from the anaerobic digestion of substrates from corn stover: Differences between the stem bark, stem pith, and leaves. Sci. Total Environ. 2019, 694, 133641. [Google Scholar] [CrossRef]
- Council, N.R. Nutrient Requirements of Dairy Cattle: Seventh Revised Edition, 2001; The National Academies Press: Washington, DC, USA, 2001; p. 405. [Google Scholar]
- Blümmel, M.; Lebzien, P. Predicting ruminal microbial efficiencies of dairy rations by in vitro techniques. Livest. Prod. Sci. 2001, 68, 107–117. [Google Scholar] [CrossRef]
- Amodeo, C.; Hafner, S.D.; Teixeira Franco, R.; Benbelkacem, H.; Moretti, P.; Bayard, R.; Buffière, P. How different are manometric, gravimetric, and automated volumetric BMP results. Water 2020, 12, 1839. [Google Scholar] [CrossRef]
- Blümmel, M.; Steingass, H.; Becker, K. The relationship between in vitro gas production, in vitro microbial biomass yield and 15N incorporation and its implications for the prediction of voluntary feed intake of roughages. Br. J. Nutr. 1997, 77, 911–921. [Google Scholar] [CrossRef]
- Massaro, S.; Amalfitano, N.; Andersen, J.B.H.; Dallavalle, G.; Angeli, A.; Nikolić, N.; Bailoni, L.; Currò, S.; Vrhovsek, U.; Franciosi, E. Alpine pasture herbs redirected hydrogen towards alternative sinks, inhibiting methane production: in vitro study. Ital. J. Anim. Sci. 2025, 24, 711–727. [Google Scholar] [CrossRef]
- Dijkstra, J.; Kebreab, E.; Bannink, A.; France, J.; Lopez, S. Application of the gas production technique to feed evaluation systems for ruminants. Anim. Feed Sci. Technol. 2005, 123, 561–578. [Google Scholar] [CrossRef]
- Rossi, G.; Massaro, S.; Arango, S.; Currò, S.; Spanghero, M.; Giannuzzi, D.; Schiavon, S.; Bailoni, L.; Tagliapietra, F. Rumen fluid from donor cows fed different additives can affect the in vitro fermentation parameters. Ital. J. Anim. Sci. 2025, 24, 53–60. [Google Scholar] [CrossRef]
- Getachew, G.; DePeters, E.J.; Robinson, P.H.; Fadel, J.G. Use of an in vitro rumen gas production technique to evaluate microbial fermentation of ruminant feeds and its impact on fermentation products. Anim. Feed Sci. Technol. 2005, 123-124, 547–559. [Google Scholar] [CrossRef]

| Diet | |
|---|---|
| Dry matter 1 (% DM) | 58.00 |
| CP 3 (% DM) | 14.68 |
| EE 4 (% DM) | 3.41 |
| CF 5 (% DM) | 5.54 |
| NDF 6 (% DM) | 41.44 |
| ADF 7 (% DM) | 21.12 |
| Gross ADL 8 (% DM) | 4.11 |
| AIA 9 (% DM) | 0.55 |
| Net ADL (% DM) | 3.95 |
| Ash (% DM) | 7.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, R.; Arango, S.; Tagliapietra, F.; Bailoni, L. Gas Endeavour: An Innovative Equipment for Estimating Methane Kinetics During In Vitro Rumen Fermentation. Animals 2025, 15, 1331. https://doi.org/10.3390/ani15091331
Iqbal R, Arango S, Tagliapietra F, Bailoni L. Gas Endeavour: An Innovative Equipment for Estimating Methane Kinetics During In Vitro Rumen Fermentation. Animals. 2025; 15(9):1331. https://doi.org/10.3390/ani15091331
Chicago/Turabian StyleIqbal, Rashid, Sheyla Arango, Franco Tagliapietra, and Lucia Bailoni. 2025. "Gas Endeavour: An Innovative Equipment for Estimating Methane Kinetics During In Vitro Rumen Fermentation" Animals 15, no. 9: 1331. https://doi.org/10.3390/ani15091331
APA StyleIqbal, R., Arango, S., Tagliapietra, F., & Bailoni, L. (2025). Gas Endeavour: An Innovative Equipment for Estimating Methane Kinetics During In Vitro Rumen Fermentation. Animals, 15(9), 1331. https://doi.org/10.3390/ani15091331







