Comparative Analysis of Anti-Müllerian Hormone Concentration in Two Indigenous Slovenian Sheep Breeds
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sampling
2.2. Enzyme Linked Immunoassay for Anti-Müllerian Hormone
2.3. Statistical Analysis
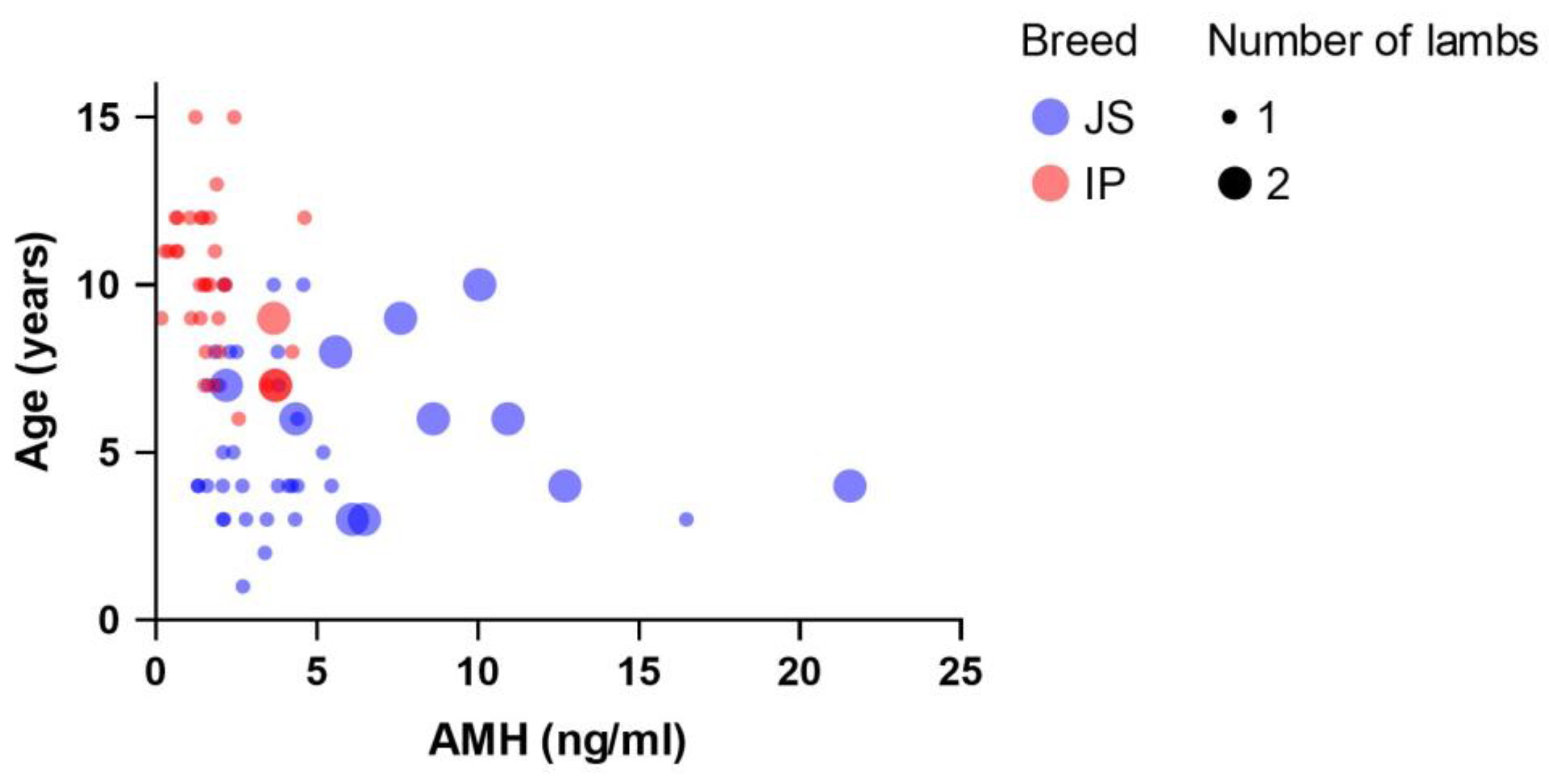
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMH | Anti-Müllerian hormone |
| CV | Coefficient of variation |
| FSH | Follicle stimulating hormone |
| IP | Istrska pramenka |
| JS | Jezersko–Solčava |
References
- Umer, S.; Zhao, S.J.; Sammad, A.; Weldegebriall Sahlu, B.; Yunwei, P.; Zhu, H. AMH: Could It Be Used as A Biomarker for Fertility and Superovulation in Domestic Animals? Genes 2019, 10, 1009. [Google Scholar] [CrossRef] [PubMed]
- Grieve, K.M.; McLaughlin, M.; Dunlop, C.E.; Telfer, E.E.; Anderson, R.A. The controversial existence and functional potential of oogonial stem cells. Maturitas 2015, 82, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Torres Rovira, C.; Gómez-Fernández, J.; Dolz, C. Evaluation of ovarian reserve markers and reproductive outcomes in sheep. Anim. Reprod. Sci. 2014, 152, 1–9. [Google Scholar]
- Mossa, F.; Jimenez-Krassel, F.; Scheetz, D.; Weber-Nielsen, M.; Evans, A.C.O.; Ireland, J.J. Anti-Mullerian Hormone (AMH) and fertility management in agricultural species. Reproduction 2017, 154, R1–R11. [Google Scholar] [CrossRef]
- Mossa, F.; Evans, A.C.O. Review: The ovarian follicular reserve—Implications for fertility in ruminants. Animal 2023, 17 (Suppl. S1), 100744. [Google Scholar] [CrossRef]
- Vigier, B.; Picard, J.Y.; Tran, D.; Legeai, L.; Josso, N. Production of Anti-Mullerian Hormone—Another Homology between Sertoli and Granulosa-Cells. Endocrinology 1984, 114, 1315–1320. [Google Scholar] [CrossRef]
- Bezard, J.; Vigier, B.; Tran, D.; Mauleon, P.; Josso, N. Immunocytochemical study of anti-Mullerian hormone in sheep ovarian follicles during fetal and post-natal development. J. Reprod. Fertil. 1987, 80, 509–516. [Google Scholar] [CrossRef]
- La Marca, A.; Stabile, G.; Artenisio, A.C.; Volpe, A. Serum anti-Mullerian hormone throughout the human menstrual cycle. Hum. Reprod. 2006, 21, 3103–3107. [Google Scholar] [CrossRef] [PubMed]
- Monniaux, D.; Drouilhet, L.; Rico, C.; Estienne, A.; Jarrier, P.; Touze, J.L.; Sapa, J.; Phocas, F.; Dupont, J.; Dalbies-Tran, R.; et al. Regulation of anti-Mullerian hormone production in domestic animals. Reprod. Fertil. Dev. 2012, 25, 1–16. [Google Scholar] [CrossRef]
- Peluso, C.; Fonseca, F.L.A.; Rodart, I.F.; Cavalcanti, V.; Gastaldo, G.; Christofolini, D.M.; Barbosa, C.P.; Bianco, B. AMH: An ovarian reserve biomarker in assisted reproduction. Clin. Chim. Acta 2014, 437, 175–182. [Google Scholar] [CrossRef]
- Ireland, J.L.; Scheetz, D.; Jimenez-Krassel, F.; Themmen, A.P.; Ward, F.; Lonergan, P.; Smith, G.W.; Perez, G.I.; Evans, A.C.; Ireland, J.J. Antral follicle count reliably predicts number of morphologically healthy oocytes and follicles in ovaries of young adult cattle. Biol. Reprod. 2008, 79, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Rico, C.; Fabre, S.; Medigue, C.; di Clemente, N.; Clement, F.; Bontoux, M.; Touze, J.L.; Dupont, M.; Briant, E.; Remy, B.; et al. Anti-mullerian hormone is an endocrine marker of ovarian gonadotropin-responsive follicles and can help to predict superovulatory responses in the cow. Biol. Reprod. 2009, 80, 50–59. [Google Scholar] [CrossRef]
- Baldrighi, J.M.; Sa Filho, M.F.; Batista, E.O.; Lopes, R.N.; Visintin, J.A.; Baruselli, P.S.; Assumpcao, M.E. Anti-Mullerian hormone concentration and antral ovarian follicle population in Murrah heifers compared to Holstein and Gyr kept under the same management. Reprod. Domest. Anim. 2014, 49, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Monniaux, D.; Barbey, S.; Rico, C.; Fabre, S.; Gallard, Y.; Larroque, H. Anti-Mullerian hormone: A predictive marker of embryo production in cattle? Reprod. Fertil. Dev. 2010, 22, 1083–1091. [Google Scholar] [CrossRef]
- Veiga-Lopez, A.; Ye, W.; Padmanabhan, V. Developmental programming: Prenatal testosterone excess disrupts anti-Mullerian hormone expression in preantral and antral follicles. Fertil. Steril. 2012, 97, 748–756. [Google Scholar] [CrossRef]
- Ireland, J.J.; Ward, F.; Jimenez-Krassel, F.; Ireland, J.L.; Smith, G.W.; Lonergan, P.; Evans, A.C. Follicle numbers are highly repeatable within individual animals but are inversely correlated with FSH concentrations and the proportion of good-quality embryos after ovarian stimulation in cattle. Hum. Reprod. 2007, 22, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 31 October 2023).
- Lenth, R. emmeans: Estimated Marginal Means, aka Least-Squares Means. R Package Version 1.10.6-090003. Available online: https://rvlenth.github.io/emmeans/ (accessed on 1 January 2025).
- Suarez-Henriques, P.; Miranda, E.S.-C.C.; Cardoso-Leite, R.; Guilermo-Ferreira, R.; Katiki, L.M.; Louvandini, H. Exploring AMH levels, homeostasis parameters, and ovarian primordial follicle activation in pubertal infected sheep on a high-protein diet. Res. Vet. Sci. 2024, 169, 105158. [Google Scholar] [CrossRef]
- Turgut, A.O.; Koca, D. Anti-Mullerian hormone as a promising novel biomarker for litter size in Romanov sheep. Reprod. Domest. Anim. 2024, 59, e14692. [Google Scholar] [CrossRef]
- Batista, E.O.; Guerreiro, B.M.; Freitas, B.G.; Silva, J.C.; Vieira, L.M.; Ferreira, R.M.; Rezende, R.G.; Basso, A.C.; Lopes, R.N.; Renno, F.P.; et al. Plasma anti-Mullerian hormone as a predictive endocrine marker to select Bos taurus (Holstein) and Bos indicus (Nelore) calves for in vitro embryo production. Domest. Anim. Endocrinol. 2016, 54, 1–9. [Google Scholar] [CrossRef]
- Cushman, R.A.; Yake, H.K.; Snider, A.P.; Lents, C.A.; Murphy, T.W.; Freking, B.A. An extreme model of fertility in sheep demonstrates the basis of controversies surrounding antral follicle count and circulating concentrations of anti-Mullerian hormone as predictors of fertility in ruminants. Anim. Reprod. Sci. 2023, 259, 107364. [Google Scholar] [CrossRef]
- Campbell, B.K.; Clinton, M.; Webb, R. The role of anti-Mullerian hormone (AMH) during follicle development in a monovulatory species (sheep). Endocrinology 2012, 153, 4533–4543. [Google Scholar] [CrossRef] [PubMed]
- Ireland, J.J.; Smith, G.W.; Scheetz, D.; Jimenez-Krassel, F.; Folger, J.K.; Ireland, J.L.; Mossa, F.; Lonergan, P.; Evans, A.C. Does size matter in females? An overview of the impact of the high variation in the ovarian reserve on ovarian function and fertility, utility of anti-Mullerian hormone as a diagnostic marker for fertility and causes of variation in the ovarian reserve in cattle. Reprod. Fertil. Dev. 2011, 23, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Monniaux, D.; Baril, G.; Laine, A.L.; Jarrier, P.; Poulin, N.; Cognie, J.; Fabre, S. Anti-Mullerian hormone as a predictive endocrine marker for embryo production in the goat. Reproduction 2011, 142, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Lahoz, B.; Alabart, J.L.; Monniaux, D.; Mermillod, P.; Folch, J. Anti-Mullerian hormone plasma concentration in prepubertal ewe lambs as a predictor of their fertility at a young age. BMC Vet. Res. 2012, 8, 118. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, C.; Wang, L.; Feng, R.; Guo, Y.; Feng, S.; Zhang, L.; Zheng, Z.; Su, G.; Fan, L.; et al. Correlation analysis of serum reproductive hormones and metabolites during multiple ovulation in sheep. BMC Vet. Res. 2022, 18, 290. [Google Scholar] [CrossRef]
- Scaramuzzi, R.J.; Campbell, B.K.; Downing, J.A.; Kendall, N.R.; Khalid, M.; Muñoz-Gutiérrez, M.; Somchit, A. A review of the effects of supplementary nutrition in the ewe on the concentrations of reproductive and metabolic hormones and the mechanisms that regulate folliculogenesis and ovulation rate. Reprod. Nutr. Dev. 2006, 46, 339–354. [Google Scholar] [CrossRef]
- Werner, L.; van der Schouw, Y.T.; de Kat, A.C. A systematic review of the association between modifiable lifestyle factors and circulating anti-Mullerian hormone. Hum. Reprod. Update 2024, 30, 262–308. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šterbenc, N.; Mrkun, J.; Petročnik, Š.; Sterniša, M.; Pipan, M.Z. Comparative Analysis of Anti-Müllerian Hormone Concentration in Two Indigenous Slovenian Sheep Breeds. Animals 2025, 15, 1332. https://doi.org/10.3390/ani15091332
Šterbenc N, Mrkun J, Petročnik Š, Sterniša M, Pipan MZ. Comparative Analysis of Anti-Müllerian Hormone Concentration in Two Indigenous Slovenian Sheep Breeds. Animals. 2025; 15(9):1332. https://doi.org/10.3390/ani15091332
Chicago/Turabian StyleŠterbenc, Nataša, Janko Mrkun, Špela Petročnik, Meta Sterniša, and Maja Zakošek Pipan. 2025. "Comparative Analysis of Anti-Müllerian Hormone Concentration in Two Indigenous Slovenian Sheep Breeds" Animals 15, no. 9: 1332. https://doi.org/10.3390/ani15091332
APA StyleŠterbenc, N., Mrkun, J., Petročnik, Š., Sterniša, M., & Pipan, M. Z. (2025). Comparative Analysis of Anti-Müllerian Hormone Concentration in Two Indigenous Slovenian Sheep Breeds. Animals, 15(9), 1332. https://doi.org/10.3390/ani15091332






