Insights into the Genetic Connectivity and Climate-Driven Northward Range Expansion of Turbo sazae (Gastropoda: Turbinidae) Along the Eastern Coast of Korea
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
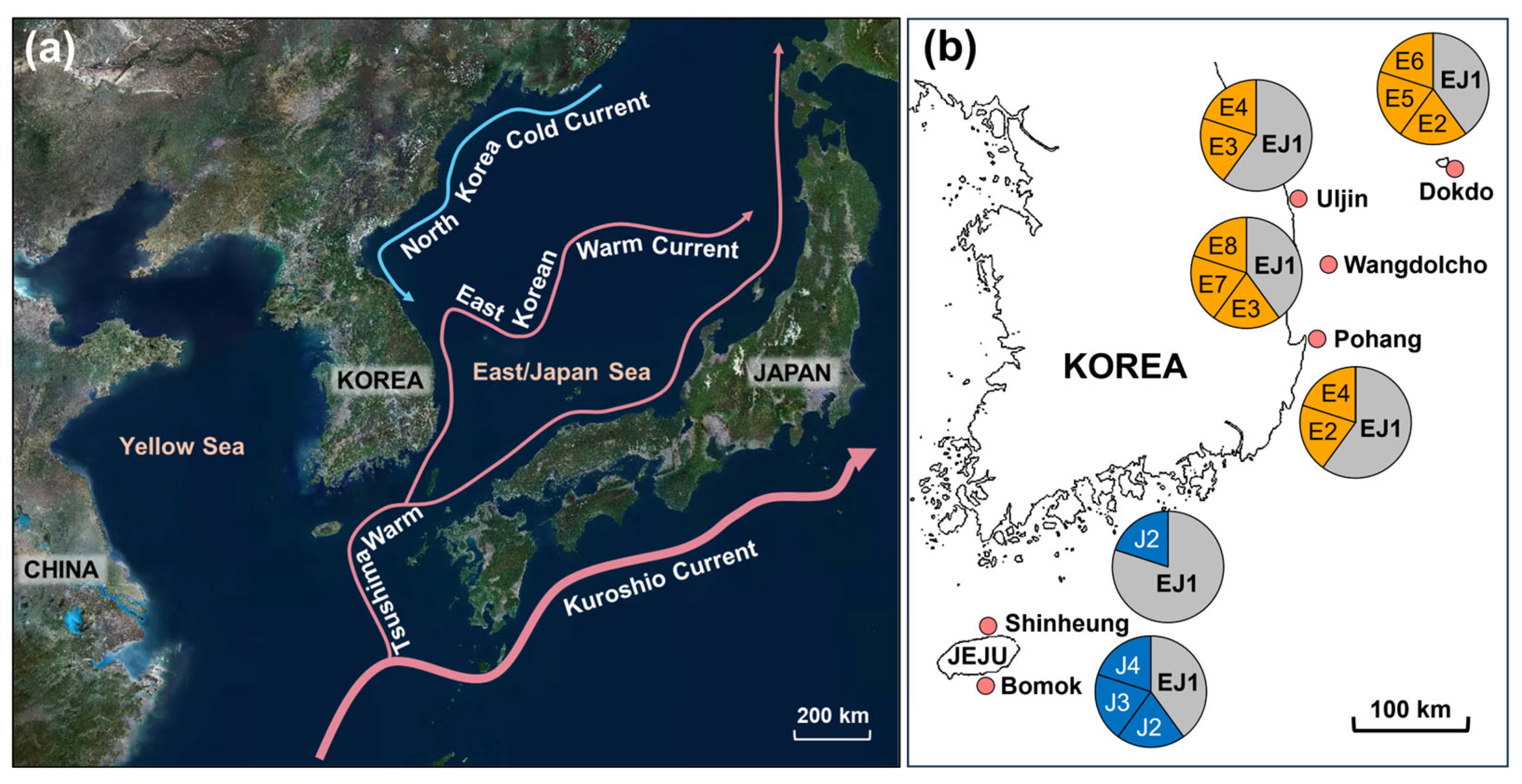
2.1. Study Sites and Sample Collection
2.2. DNA Extraction, PCR, and Sequencing
2.3. Genetic Data Processing and Analysis
2.4. Ethics Statement
3. Results
3.1. Haplotype Frequency and Distribution
3.2. Genetic Diversity Analysis
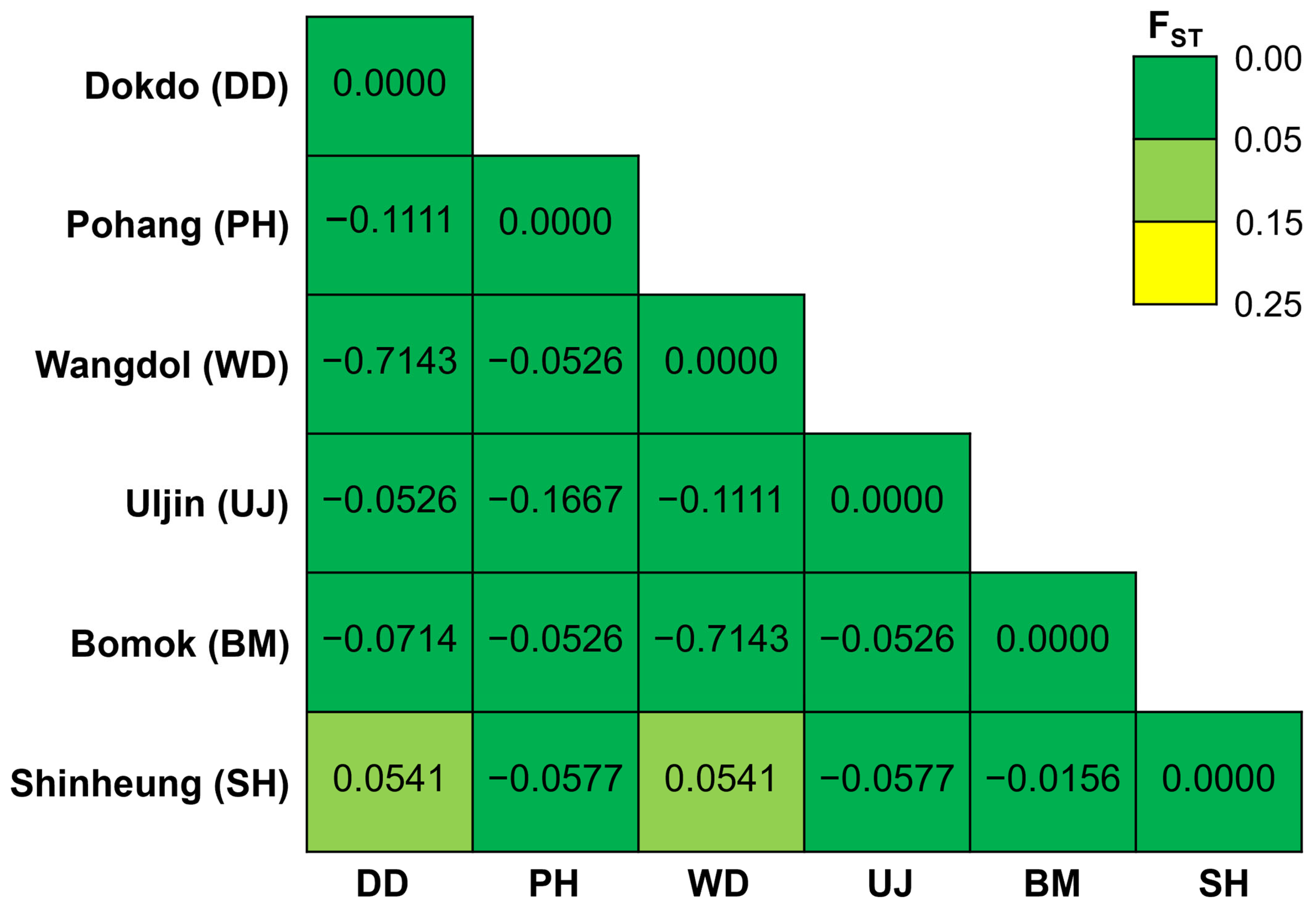
3.3. Genetic Differentiation and Population Structure
3.4. Time to the Most Recent Common Ancestor (MRCA)
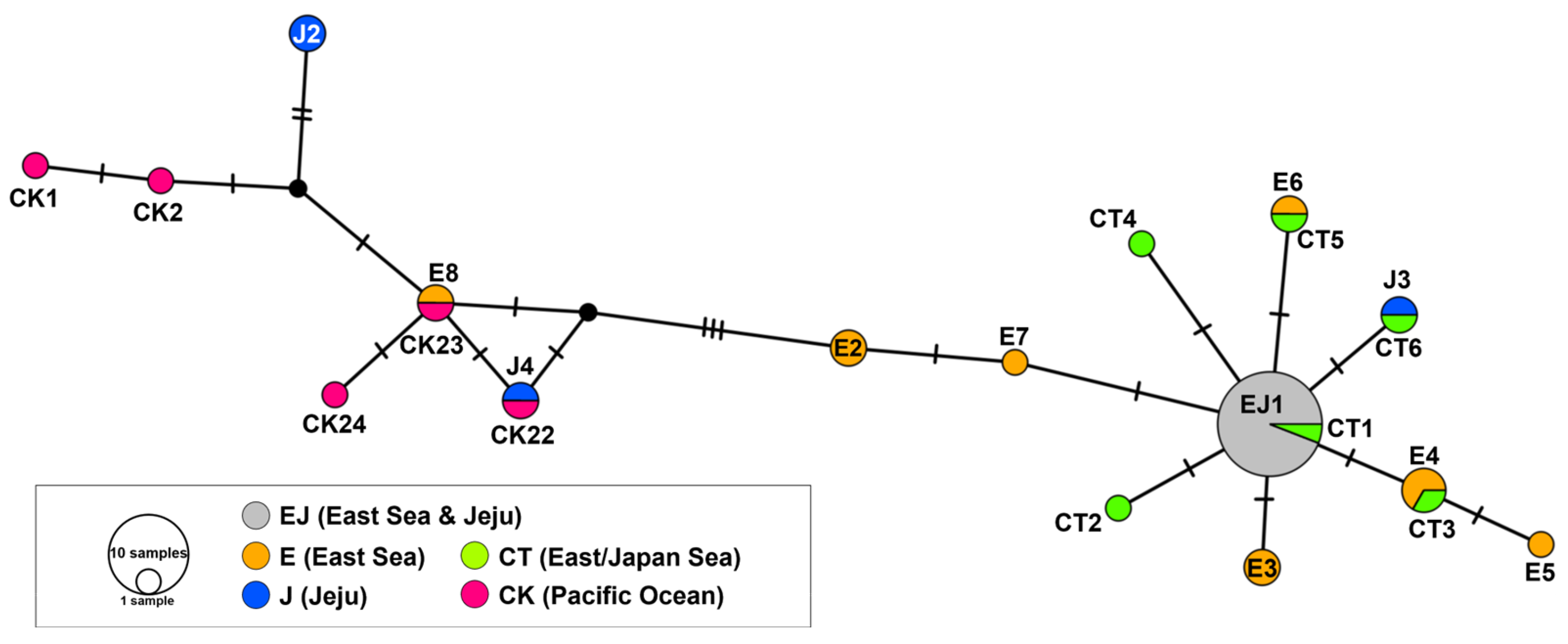
3.5. Haplotype Network Analysis
4. Discussion
4.1. Validation of Species Identification and Sequencing Approaches
4.2. Genetic Connectivity
4.3. Signals of Potential Localized Genetic Structure
4.4. Climate Change and Habitat Expansion
4.5. Implications for Range Expansion and Fisheries Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, I.C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Dahms, C.; Killen, S.S. Temperature change effects on marine fish range shifts: A meta-analysis of ecological and methodological predictors. Glob. Change Biol. 2023, 29, 4459–4479. [Google Scholar] [CrossRef]
- Jung, S. Spatial variability in long-term changes of climate and oceanographic conditions in Korea. J. Environ. Biol. 2008, 29, 519–529. [Google Scholar]
- KMA. Report of Global Atmosphere Watch 2018; National Institute of Meteorological Sciences: Seogwipo, Republic of Korea, 2019; p. 268. [Google Scholar]
- Lee, E.Y.; Park, K.A. Change in the recent warming trend of sea surface temperature in the East Sea (Sea of Japan) over decades (1982–2018). Remote Sens. 2019, 11, 2613. [Google Scholar] [CrossRef]
- Calvet, N.; Bluhm, B.A.; Yoccoz, N.G.; Altenburger, A. Shifting invertebrate distributions in the Barents Sea since pre-1900. Front. Mar. Sci. 2014, 11, 1421475. [Google Scholar] [CrossRef]
- Reddin, C.J.; Aberhan, M.; Raja, N.B.; Kocsis, Á.T. Global warming generates predictable extinctions of warm-and cold-water marine benthic invertebrates via thermal habitat loss. Glob. Change Biol. 2022, 28, 5793–5807. [Google Scholar] [CrossRef]
- Fukuda, H. Nomenclature of the horned turbans previously known as Turbo cornutus [Lightfoot], 1786 and Turbo chinensis Ozawa & Tomida, 1995 (Vetigastropoda: Trochoidea: Turbinidae) from China, Japan and Korea. Molluscan Res. 2017, 37, 268–281. [Google Scholar]
- Yang, H.S.; Park, A.; Park, H.S.; Kang, D.H.; Hong, H.K. Effects of elevated seawater temperatures on cellular immune function in the top shell, Turbo sazae. J. Mar. Sci. Eng. 2024, 12, 1904. [Google Scholar] [CrossRef]
- Ryu, Y.K.; Hong, H.K.; Park, A.; Lee, W.K.; Kim, T.; Heo, S.J.; Park, H.S.; Kim, D.; Oh, C.; Yang, H.S. Effect of diet changes in benthic ecosystems owing to climate change on the physiological responses of Turbo sazae in waters around Jeju Island, Korea. Mar. Environ. Res. 2025, 205, 107001. [Google Scholar] [CrossRef]
- Son, M.H.; Lee, C.I.; Park, J.M.; Kim, H.J.; Riedel, R.; Hwang, I.; Jung, H.K. The northward habitat expansion of the Korean top shell Turbo sazae (Gastropoda: Vetigastropoda: Turbinidae) in the Korean Peninsula: Effects of increasing water temperature. J. Mar. Sci. Eng. 2020, 8, 782. [Google Scholar] [CrossRef]
- Hebert, P.D.; Ratnasingham, S.; De Waard, J.R. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, S96–S99. [Google Scholar] [CrossRef]
- Chiu, Y.W.; Bor, H.; Tan, M.S.; Lin, H.D.; Jean, C.T. Phylogeography and genetic differentiation among populations of the moon turban snail Lunella granulata Gmelin, 1791 (Gastropoda: Turbinidae). Int. J. Mol. Sci. 2013, 14, 9062–9079. [Google Scholar] [CrossRef]
- Pardo-Gandarillas, M.C.; Ibáñez, C.M.; Torres, F.I.; Sanhueza, V.; Fabres, A.; Escobar-Dodero, J.; Méndez, M.A. Phylogeography and species distribution modelling reveal the effects of the Pleistocene ice ages on an intertidal limpet from the South-Eastern Pacific. J. Biogeogr. 2018, 45, 1751–1767. [Google Scholar] [CrossRef]
- Williams, S.; Apte, D.; Ozawa, T.; Kaligis, F.; Nakano, T. Speciation and dispersal along continental coastlines and island arcs in the Indo-West Pacific turbinid gastropod genus Lunella. Evolution 2011, 65, 1752–1771. [Google Scholar] [CrossRef]
- Kojima, S.; Segawa, R.; Hayashi, I. Genetic differentiation among populations of the Japanese turban shell Turbo (Batillus) cornutus corresponding to warm currents. Mar. Ecol. Prog. Ser. 1997, 150, 149–155. [Google Scholar] [CrossRef]
- Yanagimoto, T.; Chow, S.; Sakai, T.; Sawayama, S.; Hayashi, Y.; Saito, K.; Wakabayashi, T.; Yamamoto, J. Genetic and morphological differentiation among local populations of Japanese top shell Turbo sazae. Jpn. Soc. Fish. Sci. 2002, 88, 396–406. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Davis, M.W.; Jorgensen, E.M. ApE, a plasmid editor: A freely available DNA manipulation and visualization program. Front. Bioinform. 2022, 2, 818619. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D.; Nakagawa, S. POPART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Drummond, A.J.; Suchard, M.A.; Xie, D.; Rambaut, A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef]
- Meyer, C.P.; Geller, J.B.; Paulay, G. Fine scale endemism on coral reefs: Archipelagic differentiation in turbinid gastropods. Evolution 2005, 59, 113–125. [Google Scholar]
- Hellberg, M.E.; Vacquier, V.D. Rapid evolution of fertilization selectivity and lysin cDNA sequences in teguline gastropods. Mol. Biol. Evol. 1999, 16, 839–848. [Google Scholar] [CrossRef]
- Rodríguez-Pena, E.; Verísimo, P.; Fernández, L.; González-Tizón, A.; Bárcena, C.; Martínez-Lage, A. High incidence of heteroplasmy in the mtDNA of a natural population of the spider crab Maja brachydactyla. PLoS ONE 2020, 15, e0230243. [Google Scholar] [CrossRef]
- Martínez, M.; Harms, L.; Abele, D.; Held, C. Mitochondrial heteroplasmy and PCR amplification bias lead to wrong species delimitation with high confidence in the South American and Antarctic marine bivalve Aequiyoldia eightsii species complex. Genes 2023, 14, 935. [Google Scholar] [CrossRef]
- Holbourn, A.E.; Kuhnt, W.; Clemens, S.C.; Kochhann, K.G.; Jöhnck, J.; Lübbers, J.; Andersen, N. Late Miocene climate cooling and intensification of southeast Asian winter monsoon. Nat. Commun. 2018, 9, 1584. [Google Scholar] [CrossRef]
- Sohn, Y.K. Geology of Tok Island, Korea: Eruptive and depositional processes of a shoaling to emergent island volcano. Bull. Volcanol. 1995, 56, 660–674. [Google Scholar] [CrossRef]
- Cho, J.S.R.; Kim, Y.M.; Sagong, J.; Lee, J.H.; Yeo, M.Y.; Bahn, S.Y.; Kim, H.M.; Lee, G.S.; Lee, D.H.; Choo, Y.S.; et al. Biodiversity of marine invertebrates on rocky shores of Dokdo, Korea. Zool. Stud. 2012, 51, 710–726. [Google Scholar]
- Teague, W.J.; Tracey, K.L.; Watts, D.R.; Book, J.W.; Chang, K.I.; Hogan, P.J.; Mitchell, D.A.; Suk, M.S.; Wimbush, M.; Yoon, J.H. Observed deep circulation in the Ulleung Basin. Deep-Sea Res. Part II Top. Stud. Oceanogr. 2005, 52, 1802–1826. [Google Scholar] [CrossRef]
- Kim, C.H.; Park, C.H. Detailed bathymetry and seabed characteristics of Wangdol-cho, hupo bank in the East Sea. Econ. Environ. Geol. 2014, 47, 533–540. [Google Scholar] [CrossRef]
- Gove, J.M.; McManus, M.A.; Neuheimer, A.B.; Polovina, J.J.; Drazen, J.C.; Smith, C.R.; Merrifield, M.A.; Friedlander, A.M.; Ehses, J.S.; Young, C.W.; et al. Near-island biological hotspots in barren ocean basins. Nat. Commun. 2016, 7, 10581. [Google Scholar] [CrossRef] [PubMed]
- Slatkin, M.; Hudson, R.R. Pairwise comparisons of mitochondrial DNA sequences in stable and exponentially growing populations. Genetics 1991, 129, 555–562. [Google Scholar] [CrossRef] [PubMed]
| Site | Haplotype Frequency (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EJ1 | E2 | E3 | E4 | E5 | E6 | E7 | E8 | J2 | J3 | J4 | Site-specific Haplotypes | ||
| East/Japan Sea | 50.0 | 10.0 | 10.0 | 10.0 | 5.0 | 5.0 | 5.0 | 5.0 | - | - | - | ||
| Dokdo (DD) | 40.0 | 20.0 | 0.0 | 0.0 | 20.0 | 20.0 | 0.0 | 0.0 | - | - | - | E5, E6 | |
| Pohang (PH) | 60.0 | 20.0 | 0.0 | 20.0 | 0.0 | 0.0 | 0.0 | 0.0 | - | - | - | - | |
| Wangdolcho (WD) | 40.0 | 0.0 | 20.0 | 0.0 | 0.0 | 0.0 | 20.0 | 20.0 | - | - | - | E7, E8 | |
| Uljin (UJ) | 60.0 | 0.0 | 20.0 | 20.0 | 0.0 | 0.0 | 0.0 | 0.0 | - | - | - | - | |
| Jeju Island | 60.0 | - | - | - | - | - | - | - | 20.0 | 10.0 | 10.0 | ||
| Bomok (BM) | 40.0 | - | - | - | - | - | - | - | 20.0 | 20.0 | 20.0 | J3, J4 | |
| Shinheung (SH) | 80.0 | - | - | - | - | - | - | - | 20.0 | - | - | - | |
| Site | N | Hd (±SD) | π | K |
|---|---|---|---|---|
| East/Japan Sea | 20 | 0.747 ± 0.097 | 0.003 | 1.663 |
| Dokdo (DD) | 5 | 0.900 ± 0.161 | 0.003 | 2.000 |
| Pohang (PH) | 5 | 0.700 ± 0.218 | 0.002 | 1.200 |
| Wangdolcho (WD) | 5 | 0.900 ± 0.161 | 0.005 | 3.000 |
| Uljin (UJ) | 5 | 0.700 ± 0.218 | 0.001 | 0.800 |
| Jeju Island | 10 | 0.644 ± 0.152 | 0.006 | 4.111 |
| Bomok (BM) | 5 | 0.900 ± 0.161 | 0.008 | 5.300 |
| Shinheung (SH) | 5 | 0.400 ± 0.237 | 0.006 | 3.600 |
| Group | Variance Components | Variation (%) | Fixation Indices |
|---|---|---|---|
| Among Regions (East × Jeju) | 0.0088 | 2.44 | 0.0244 |
| Among populations within regions | −0.0250 | −6.97 | −0.0714 |
| Within populations | 0.3750 | 104.53 | −0.0453 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, Y.-G.; Kwon, K.; Rho, H.S.; Min, W.-G.; Jeung, H.-D.; Hwang, U.-K.; Ryu, Y.-K.; Park, A.; Hong, H.-K.; Shin, J.-S.; et al. Insights into the Genetic Connectivity and Climate-Driven Northward Range Expansion of Turbo sazae (Gastropoda: Turbinidae) Along the Eastern Coast of Korea. Animals 2025, 15, 1321. https://doi.org/10.3390/ani15091321
Cho Y-G, Kwon K, Rho HS, Min W-G, Jeung H-D, Hwang U-K, Ryu Y-K, Park A, Hong H-K, Shin J-S, et al. Insights into the Genetic Connectivity and Climate-Driven Northward Range Expansion of Turbo sazae (Gastropoda: Turbinidae) Along the Eastern Coast of Korea. Animals. 2025; 15(9):1321. https://doi.org/10.3390/ani15091321
Chicago/Turabian StyleCho, Young-Ghan, Kyungman Kwon, Hyun Soo Rho, Won-Gi Min, Hee-Do Jeung, Un-Ki Hwang, Yong-Kyun Ryu, Areumi Park, Hyun-Ki Hong, Jong-Seop Shin, and et al. 2025. "Insights into the Genetic Connectivity and Climate-Driven Northward Range Expansion of Turbo sazae (Gastropoda: Turbinidae) Along the Eastern Coast of Korea" Animals 15, no. 9: 1321. https://doi.org/10.3390/ani15091321
APA StyleCho, Y.-G., Kwon, K., Rho, H. S., Min, W.-G., Jeung, H.-D., Hwang, U.-K., Ryu, Y.-K., Park, A., Hong, H.-K., Shin, J.-S., & Yang, H.-S. (2025). Insights into the Genetic Connectivity and Climate-Driven Northward Range Expansion of Turbo sazae (Gastropoda: Turbinidae) Along the Eastern Coast of Korea. Animals, 15(9), 1321. https://doi.org/10.3390/ani15091321






