Simple Summary
Genomic selection and genome-wide association studies (GWASs) are powerful tools for improving dairy cattle’s milk production, fertility, and health traits. While these methods have been successfully applied to purebred populations in the United States and Europe, their effectiveness in crossbred dairy cattle, particularly in tropical regions such as Thailand, remains less well explored. Identifying the pleiotropic genes influencing multiple economically important traits can enhance genetic selection strategies. This study evaluated the potential of weighted single-step genomic best linear unbiased prediction (wssGBLUP) to improve GEBV accuracy compared to the standard single-step GBLUP (ssGBLUP) and applied a wssGWAS to identify key genetic markers for production (305-day milk yield), fertility (days open), and health traits (fat-to-protein ratio) in Thai-Holstein crossbred dairy cattle. Further research is needed to validate these findings and optimize breeding programs for tropical dairy systems.
Abstract
Understanding the genetic basis of economically important traits is essential for enhancing the productivity, fertility, and health of dairy cattle. This study aimed to identify the pleiotropic genes associated with the 305-day milk yield (MY305), days open (DO), and milk fat-to-protein ratio (FPR) in Thai-Holstein crossbred dairy cattle using a genome-wide association study (GWAS) approach. The dataset included 18,843 records of MY305 and milk FPR, as well as 48,274 records of DO, collected from first-lactation Thai-Holstein crossbred dairy cattle. A total of 868 genotyped animals and 43,284 informative SNPs out of 50,905 were used for the analysis. The single-nucleotide polymorphism (SNP) effects were evaluated using a weighted single-step GWAS (wssGWAS), which estimated these effects based on genomic breeding values (GEBVs) through a multi-trait animal model with single-step genomic BLUP (ssGBLUP). Genomic regions explaining at least 5% of the total genetic variance were selected for candidate gene analysis. Single-step genomic REML (ssGREML) with a multi-trait animal model was used to estimate components of (co)variance. The heritability estimates from additive genetic variance were 0.262 for MY305, 0.029 for DO, and 0.102 for milk FPR, indicating a moderate genetic influence on milk yield and a lower genetic impact on fertility and milk FPR. The genetic correlations were 0.559 (MY305 and DO), −0.306 (MY305 and milk FPR), and −0.501 (DO and milk FPR), indicating potential compromises in genetic selection. wssGBLUP showed a higher accuracy than ssGBLUP, although the improvement was modest. A total of 24, 46, and 33 candidate genes were identified for MY305, DO, and milk FPR, respectively. Pleiotropic effects, identified by SNPs showing significant influence with more than trait, were observed in 14 genes shared among all three traits, 17 genes common between MY305 and DO, 14 genes common between MY305 and milk FPR, and 26 genes common between DO and milk FPR. Overall, wssGBLUP is a promising approach for improving the genomic prediction of economic traits in multi-trait analyses, outperforming ssGBLUP. This presents a viable alternative for genetic evaluation in dairy cattle breeding programs in Thailand. However, further studies are needed to validate these candidate genes and refine marker selection for production, fertility, and health traits in dairy cattle.
1. Introduction
Sustainable dairy production in tropical regions necessitates cattle breeds with both high milk yield and heat tolerance. Thai-Holstein crossbred cattle, a product of a breeding program initiated in 1956 [1], represent a crucial resource for Thailand’s dairy industry, combining indigenous breeds’ resilience with Holsteins’ superior milk production. Notably, over 95% of these crossbreds result from crosses between Holstein and either the Sahiwal or Thai Native breeds. The crossing of these breeds was intended to increase milk production from taurine cattle, while incorporating tolerance of heat stress, ticks, and tropical diseases. However, a historical focus on maximizing milk yield has inadvertently compromised fertility and overall animal health [2,3,4], highlighting the need for a more integrated breeding approach.
Negative energy balance (NEB), a key factor in the compromises between milk yield, fertility, and health, arises when energy intake fails to meet the high metabolic demands of early lactation [5,6]. During early lactation, if the energy intake of a cow is insufficient to meet metabolic demands, it enters a state of NEB. In severe cases, NEB leads to metabolic stress, reduced feed intake, higher disease susceptibility, metabolic disorders, and impaired reproductive performance [5,7]. These conditions are associated with metabolic diseases such as ketosis and fatty liver syndrome [5,7], where cows mobilize body fat to compensate for energy deficits, resulting in increased ketone production and impaired liver function [8,9]. Additionally, a deficiency in fermentable carbohydrates reduces protein synthesis by rumen bacteria, leading to lower amino acid flow to the udder, decreased milk protein, and an elevated milk fat-to-protein ratio [10,11].
The reproductive consequences of NEB include delayed ovulation, reduced conception rates, and extended calving intervals [12,13], often driven by hormonal imbalances that disrupt the estrous cycle and follicular development [14]. Similarly, an excessively low milk FPR can indicate subclinical rumen acidosis, a metabolic disorder characterized by a sharp drop in rumen pH. This condition can lead to severe health complications [15], including laminitis and immune suppression, increasing susceptibility to infections [16]. Therefore, the optimal milk FPR in tropical Holsteins ranges from 0.9 to 1.9 [8], emphasizing its value as an indicator of overall cow health and productivity.
Milk FPR serves as a potential indicator of NEB and exhibits significant associations with milk yield and fertility traits, demonstrating heritability ranging from 0.19 to 0.54 across various dairy breeds [11,17,18]. Genetic correlations between milk yield and milk FPR vary across multiple breeds, showing positive correlations in some (e.g., Nordic Red and Canadian Holstein: 0.05–0.48 in early lactation [18,19]) but negative correlations in others (e.g., Thai dairy cattle: −0.44 to −0.29 in late lactation [18]). Similarly, correlations between milk FPR and DO range from 0.37 to 0.67 to 0.98 in Thai dairy cattle and from 0.14 to 0.28 in Nordic Red cattle [19], suggesting a compromise between energy balance, milk production, and reproductive performance. Previous studies have primarily focused on phenotypic traits or common genetic variations [20,21,22,23,24,25,26]. Given the importance of genomics selection for these traits [27,28], this study aimed to identify pleiotropic genes associated with MY305, DO, and milk FPR using a GWAS and to analyze their relationships using multi-trait ssGBLUP models.
2. Materials and Methods
2.1. Data Collection
This study examined 18,843 records of MY305 and FPR from first lactation and 48,274 records of DO after first calving in Thai-Holstein crossbred dairy cows collected between 1993 and 2017. The data were provided by the Bureau of Biotechnology in Livestock Production, Department of Livestock Development, Thailand. AI data were recorded on farms that used the DLD’s bulls, while test-day milk and milk composition data were collected only from selected farms. MY305 denotes the total milk production (kg) during the first 305 days of lactation, calculated from test-day milk data. After merging the data, 897 farms, with an average herd size of 54.0 cows/farm, were used in the study. DO was defined as the number of days between calving and conception during the lactation period. A conception record was discarded if the consecutive calving date was unknown. Cows with a DO value less than 20 or greater than 400 were also removed. The calving age was restricted to between 24 and 48 months. Milk FPR was defined as the ratio of milk fat to protein in test-day milk samples, with the average value over the lactation period being used for analysis. Cows were grouped by percentage of Holstein genetics (breeding group, BG) as follows: BG1 > 93.7%, BG2 from 87.5 to 93.6%, and BG3 < 87.5%.
Genotype data were obtained from 882 Thai-Holstein crossbred cows using the Illumina BovineSNP50 Bead Chip (Illumina Inc., San Diego, CA, USA). Genotyped animals were randomly selected based on high, medium, and low breeding values of bulls and dams. Quality control (QC) was applied to both animals and markers based on the following criteria: minor allele frequency (MAF) > 5% and minimum call rate per marker ≥ 90%. The SNPs and animals that did not meet these criteria were excluded. Animals with duplicate genotypes and SNPs with Mendelian conflicts were also discarded. Quality control and SNP analyses were performed using the BLUPF90+ Ver 2.56 software. After QC, 868 genotyped animals and 43,284 informative SNPs for MY305, DO, and milk FPR were retained for analysis. Cows were classified into three breed groups (BGs) based on their proportion of Holstein genetics: BG1 for cows with >93.7% Holstein genetics, BG2 for cows with 87.5–93.6% Holstein genetics, and BG for cows with <87.5% Holstein genetics. The age at calving in this population ranged from 18 to 50 months. The summary statistics of the analyzed traits are presented in Table 1.

Table 1.
Data structure of MY305, DO, and milk FPR for Thai-Holstein crossbred cattle.
2.2. Estimating the Genetic Parameters
The variance components for each trait were estimated by single-step genomic REML (ssGREML) with a multi-trait animal model. The AIREMLF90 and BLUPF90+ programs were used to estimate these components and solve the model equations [29]. The model included fixed effects, the animal’s additive genetic effects, and residual effects, as shown below:
where , , and represent the traits MY305, DO, and milk FPR; , and represent the fixed effects of the breed group (3 levels), age at first calving (32 levels), and herd-month-year of calving (30,299 levels); , and represent the random additive genetic effects vectors; and , , and represent the random residual effects vectors. and and and are the incidence matrices of fixed effects and additive genetic effects, respectively. The assumed covariance structures were as follows:
where matrix denotes the additive relationship among the animals; matrix denotes the identity; , , , , , and denote the variances and covariances for additive genetic effects; and , , , , , and denote the variances and covariances for residual effects.
The estimated heritability () and genetic correlations () between MY305, DO, and milk FPR were calculated using the formula provided by Ravagnolo and Misztal [30], as follows:
where represents the additive genetic variance for trait i, represents the residual variance, and defines the genetic covariance between traits i and j.
2.3. Estimating the Genomic Breeding Values
A weighted single-step genomic approach with multiple-trait genetic analysis was employed for genomic analysis. Instead of using the inverse of the numerator relationship matrix (), the inverse of matrix () was applied in the mixed-model equation [27] described below.
Here, denotes the inverse of a pedigree-based relationship matrix for all the animals involved in the analysis; denotes the inverse of a genomic relationship matrix (); and denotes the inverse of the pedigree-based relationship matrix specific to genotyped animals [31]. The weighting factors τ = 1.00 and ω = 0.50 were selected based on their ability to minimize bias during the preliminary validation study. Due to potential pedigree gaps, inbreeding was excluded from the matrix, although its omission might have influenced the results. The genomic relationship matrix () and weights were constructed using the method described by VanRaden [31], as follows:
In this context, ZDZ′ represents the matrix of gene content adjusted for allele frequencies, is a diagonal matrix containing weights for SNP variances, denotes the total number of SNP markers, represents the frequency of the reference allele for the th SNP, and represents the weight for the th SNP.
The estimated breeding values (EBVs) and genomic breeding values (GEBVs) were calculated using the wssGBLUP option in the BLUPF90+ program. The accuracy of GEBVs was estimated from prediction error variance (PEV) functions available within the BLUPF90+ package.
2.4. Genome-Wide Association Study (GWAS)
The analyses were conducted using the wssGWAS approach. A similar model was applied in the wssGWAS to estimate the association between quantitative traits and individual single-nucleotide polymorphisms (SNPs). SNP effects were determined through an iterative process, identical to the method described by Wang et al. [32], and were implemented in POSTGSF90 Version 1.83 software [33]. The additive genomic breeding value () was converted back to SNP effects (), considering their shared genomic variance ), using the following formula:
The variance for each SNP was estimated as the proportion of variance explained by five adjacent SNPs relative to the additive genetic variance, using the following formula:
Here, represents the estimate of segment variance for the th SNP, comprising 5 adjacent SNPs’ explained variance; denotes the total additive genetic variance; is the vector of gene content for the th SNP across all individuals; and is the estimated effect of the th SNP within the ith region [34]. The threshold for significant SNP variance was determined by selecting SNPs with high explained variance that accounted for at least 5% of the total genomic variance. This threshold has been examined in cattle studies related to complex traits [35,36,37,38]. Manhattan plots for these windows were created using SAS Studio online v.3.81.
2.5. Identification of Candidate and Pleiotropic Genes
Potential candidate genes associated with MY305, DO, and milk FPR were identified by searching for genes near significant SNPs that exceeded the threshold. Genes located in the same genomic region across two or three traits were classified as pleiotropic genes. The distance between candidate genes and SNP locations was set to less than 50 kb (kilobase pairs), in accordance with the previous studies [39,40]. Gene identification was performed using the National Center for Biotechnology Information (NCBI) Map Viewer tool for the bovine genome, with UMD 3.1 assembly as the reference map (https://www.ncbi.nlm.nih.gov/gdv/browser/genome/?id=GCF_000003055.6 (accessed on 24 February 2025)) [35,41]. Further literature and database searches for all identified genes were conducted using the NCBI (https://www.ncbi.nlm.nih.gov/ (accessed on 24 February 2025)) and GeneCards (https://www.genecards.org/ (accessed on 24 February 2025)) platforms.
3. Results
3.1. Estimation of Heritability and Genetic Correlations
Table 2 presents the estimated variance components and heritability values for MY305, DO, and milk FPR. The additive genetic variances () were 176,980 for MY305, 176.45 for DO, and 0.31 for milk FPR, reflecting the genetic contribution to the variation in these traits. The residual variances () were 676,710 for MY305, 6099.85 for DO, and 2.99 for milk FPR, indicating the influence of environmental and non-genetic factors on their variation. The heritability estimates ( ± SE) were 0.262 ± 0.005 for MY305, 0.029 ± 0.0004 for DO, and 0.102 ± 0.002 for milk FPR, indicating that a moderate proportion of variation in MY305 is due to genetic factors, whereas the genetic influence on DO and milk FPR is relatively low. The standard errors (SEs) for all three traits were lower than their respective heritability estimates, indicating statistically reliable estimates.

Table 2.
Variance components and heritability values for MY305, DO, and milk FPR.
Table 3 presents the genetic , phenotypic , and environmental correlations among the MY305, DO, and milk FPR traits. A strong positive genetic correlation was observed between MY305 and DO (0.559). In contrast, strong negative genetic correlations were identified between MY305 and milk FPR (−0.306) and between DO and milk FPR (−0.501), indicating an antagonistic genetic relationship among these traits. Phenotypic and environmental correlations among the three traits were generally low, with values below 0.1 indicating that shared environmental and phenotypic influences have a minimal impact on the relationships between MY305, DO, and milk FPR.

Table 3.
Genetic, phenotypic, and environmental correlations between MY305, DO, and milk FPR.
3.2. Estimation of GEBVs Using ssGBLUP and wssGBLUP
Table 4 presents the average genomic estimated breeding values (GEBVs) obtained using ssGBLUP and wssGBLUP for MY305, DO, and milk FPR, categorized by dataset and breed group. wssGBLUP yielded higher GEBV estimates than ssGBLUP for all three traits. Generally, the GEBVs for MY305 and DO decreased as the percentage of Holstein genetics decreased, whereas the milk FPR increased in both the ssGBLUP and wssGBLUP analyses across all animal datasets. For MY305, both methods produced negative GEBVs, but wssGBLUP (−8.983 to −8.259) resulted in less negative values than ssGBLUP (−12.492 to −11.792), suggesting a potential positive impact on milk yield. A similar trend was observed in the bull dataset, where wssGBLUP produced fewer negative GEBVs for MY305 and better GEBVs for DO and milk FPR. Among dams, wssGBLUP generally improved GEBVs across all traits, although differences in DO and milk FPR were less pronounced than those observed for MY305.

Table 4.
Comparison of breed group effects and breed group averages of EBVs and GEBVs for MY305, DO, and milk using ssGBLUP and wssGBLUP.
Figure 1 presents the averaged accuracy of GEBVs from the two genomic prediction methods, ssGBLUP and wssGBLUP, across all animal groups: all animals, bulls, and dams. wssGBLUP consistently outperformed ssGBLUP, demonstrating higher accuracy across all traits and dataset groups. For instance, in the all-animals group, the accuracy for MY305 increased from 0.138 (ssGBLUP) to 0.176 (wssGBLUP). Similarly, the accuracy for DO improved from 0.112 to 0.124, and for milk FPR, it improved from 0.094 to 0.111. These improvements remained stable across all groups, indicating the robust and reliable advantage of wssGBLUP over ssGBLUP. Across the breed groups, bulls and dams exhibited variation in trait performance under both the ssGBLUP and wssGBLUP models. Bulls generally demonstrated higher genetic potential for MY305, while dams showed slightly greater variability in DO and milk FPR.
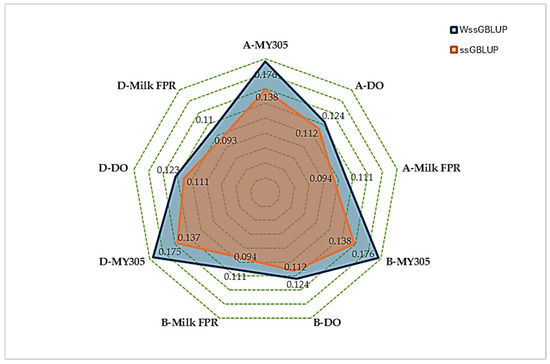
Figure 1.
Comparison of GEBV accuracy between ssGBLUP and wssGBLUP in all animal (A), bull (B), and dam (D) datasets.
3.3. Weighted Single-Step Genome-Wide Association Study
The wssGWAS analysis was successfully conducted using genotypic data from 43,284 SNPs obtained from 868 cows for the MY305, DO, and milk FPR traits. The results are illustrated in the Manhattan plots in Figure 2 and Figure 3, where each color represents a specific chromosome. This study identified 14 SNPs associated with MY305 across seven chromosomes, 19 SNPs linked to DO across five chromosomes, and 16 SNPs associated with milk FPR across five chromosomes, adhering to a distance constraint of less than 50 kb (Table 5, Table 6 and Table 7, respectively).
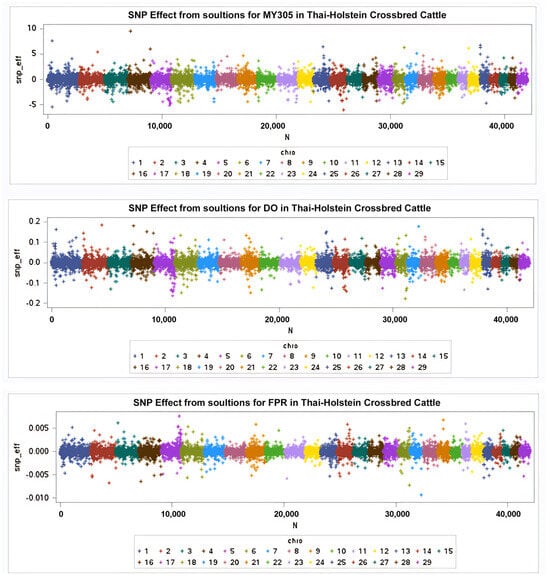
Figure 2.
SNP effects derived from GEBVs for MY305, DO, and milk FPR, respectively.
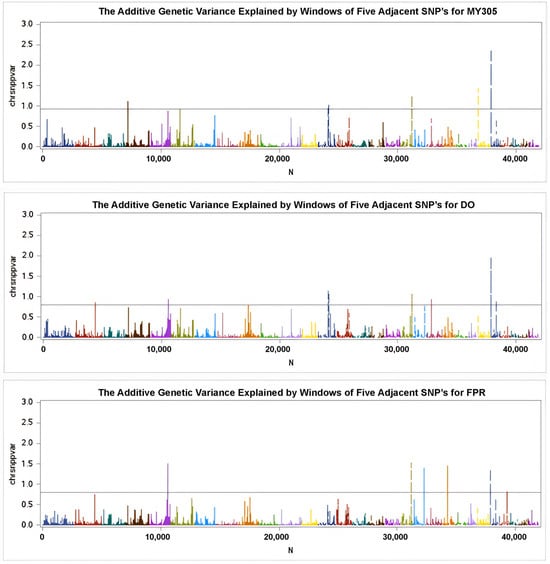
Figure 3.
Manhattan plots of the additive genetic variance explained by windows of five adjacent SNPs for MY305, DO, and milk FPR, respectively. The y-axis shows the SNP variance across the genome, while the x-axis indicates SNPs’ positions on individual chromosomes. The horizontal line marks the threshold level, suggesting significance at a minimum of 5% of the total genetic variance.

Table 5.
Significant SNPs associated with MY305 in Thai-Holstein crossbred cattle.

Table 6.
Significant SNPs associated with DO in Thai-Holstein crossbred cattle.

Table 7.
Significant SNPs associated with milk FPR in Thai-Holstein crossbred cattle.
3.4. Identification of Pleiotropic Genes for MY305, DO, and Milk FPR in Thai-Holstein Crossbred Cattle
Following the candidate gene analysis, we identified genes located in the same genomic region across all three traits, suggesting potential pleiotropic effects (Figure 4). Fourteen genes were identified within the same region for MY305, DO, and milk FPR. Additionally, 17 genes were shared between MY305 and DO, whereas 14 genes were common between DO and milk FPR. Furthermore, we conducted a relationship analysis between SNP variance and SNP effects for MY305, DO, and milk FPR (Figure 5, Figure 6 and Figure 7). A positive linear relationship was observed between the SNP variance in MY305 and DO. In contrast, a negative linear relationship was found between the SNP variance in DO and milk FPR, as well as between that in MY305 and milk FPR. Similar patterns were observed in SNP effect relationships among the three traits.
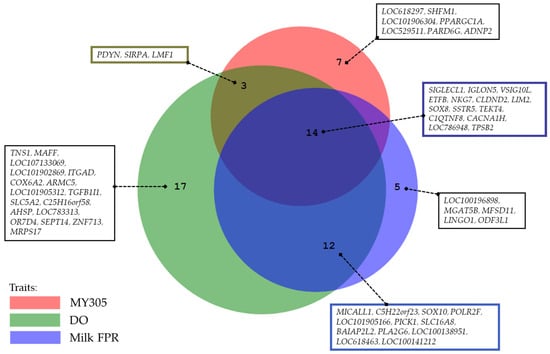
Figure 4.
Pleiotropic genes identified for MY305, DO, and milk FPR.
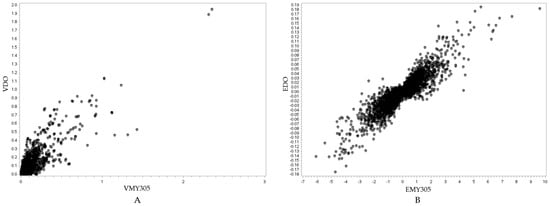
Figure 5.
Visualization of the relationship between the SNP variance of the MY305 and DO traits (r = 0.8728, p < 0.0001) (A), and the SNP effects of the MY305 and DO traits (r = 0.8915, p < 0.0001) (B). In (A), the y-axis represents the SNP variance for the DO trait (VDO), while the x-axis represents the SNP variance for the MY305 trait (VMY305). In (B), the y-axis represents the SNP effect for the DO trait (EDO), while the x-axis represents the SNP effect for the MY305 trait (EMY305).
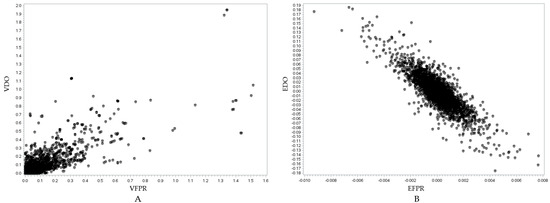
Figure 6.
Visualization of the relationship between the SNP variance of the DO and milk FPR traits (r = 0.8219, p < 0.0001) (A) and the SNP effects of the DO and milk FPR traits (r = −0.8394, p < 0.0001) (B). In (A), the y-axis represents the SNP variance for the DO trait (VDO), while the x-axis represents the SNP variance for the milk FPR trait (VFPR). In (B), the y-axis represents the SNP effect for the DO trait (EDO), while the x-axis represents the SNP effect for the milk FPR trait (EFPR).
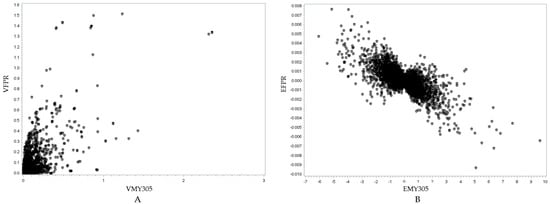
Figure 7.
Visualization of the relationship between the SNP variance of the milk FPR and MY305 traits (r = 0.6993, p < 0.0001) (A), and the SNP effects of the milk FPR and MY305 traits (r = −0.7018, p < 0.0001) (B). In (A), the y-axis represents the SNP variance for the milk FPR trait (VFPR), while the x-axis represents the SNP variance for the MY305 trait (VMY305). In (B), the y-axis represents the SNP effect for the milk FPR trait (EFPR), while the x-axis represents the SNP effect for the MY305 trait (EMY305).
4. Discussion
4.1. Heritabilities
Heritability estimates (h2) are crucial for understanding the genetic influence on these traits and their potential for improvement through selective breeding. The heritability of MY305 (0.262 ± 0.005) suggests that a moderate proportion of variation in this trait is attributable to genetic factors. While genetic selection can enhance MY305, effective management strategies are necessary, especially in challenging environments, to achieve substantial progress (Table 2). The high additive genetic variance () further supports the potential for genetic improvement in MY305. The heritability estimate for MY305 observed in this study aligns with values previously reported in the literature, ranging from 0.19 to 0.27 for Holstein cows [38,39,40,41] and from 0.24 to 0.27 for Guzerat cows [42,43]. Beyond breed differences, the lactation stage also influences heritability variations. Pangmao et al. [44] reported that the heritability for MY305 was generally higher during the first lactation compared to subsequent lactations. Additionally, differences in management practices, climate conditions, and methodological approaches across populations can significantly affect MY305 estimates and heritability [45].
However, the low heritability of DO (0.029 ± 0.0004) suggests that most of the variation in this trait within the population was due to management practices, with genetic factors playing a minor role. As a result, selective breeding may have a limited impact on improving DO, and alternative strategies, such as enhancing environmental conditions and reproductive management, may be more effective. The heritability of DO observed in this study was lower than the values reported for Holstein cows, which range from 0.04 to 0.07 [46,47,48,49], and for Russian Black-and-White cattle (0.07) [50]. Additionally, it was lower than the values previously reported for the same population in 2015 (0.04 to 0.05) [19], but comparable to values reported in a more recent study [22]. The low heritability value may be due to the strong influence of fixed factors on DO and the high residual variance observed in this study (). A previous study identified factors affecting DO, including Holstein genetic proportions and heat stress in hot climates [51,52]. Studies from other subtropical regions have suggested that environmental stress can increase genetic variability in dairy cattle [49,53]. In Thailand’s tropical climate, heat stress poses a significant challenge for livestock, potentially compromising reproductive performance. Additionally, variations in the month and year of calving across different populations may have contributed to the low heritability estimates of DO. To improve this trait, strategies such as better reproductive management, heat stress control, skilled artificial insemination, modern milking facilities, and optimized nutrition should be implemented [54,55].
Low heritability (0.102 ± 0.002) was observed for milk FPR, indicating that fixed factors and residual variance were the primary contributors to its variation within the population. Similar heritability values have been reported in Thai-Holstein crossbreeds (0.17) [17] and Nordic cattle (0.14) [19]. In contrast, higher heritability values have been observed in Holstein cows, ranging from 0.25 to 0.54 [11,56,57,58,59]. The variation in milk FPR heritability estimates across studies may be due to differences in breed, estimation methods, and model effects. Genetic differences between cows and environmental factors such as heat stress have also been reported to influence milk FPR [56]. Additionally, a study utilizing random regression test-day models reported higher milk FPR heritability values [59]. Genetic improvement of milk FPR through selection requires careful management strategies to achieve meaningful progress, particularly in preventing metabolic disorders, such as acidosis and ketosis.
4.2. Genetic Correlations Between MY305, DO, and Milk FPR
Table 3 shows the positive genetic correlation between MY305 and DO (0.559), suggesting that cows with a higher milk yield over 305 days tended to have extended DO. Consequently, selection for increased milk production may compromise fertility. This finding aligns with previous research on Holstein cows [60,61,62], showing that nutrient allocation prioritizes milk production over reproductive functions [63]. Additionally, management strategies that delay the first insemination in high-yielding cows may further contribute to prolonged DO [64]. Moreover, high-producing dairy cows often experience a postpartum negative energy balance (NEB), which can significantly impair fertility by delaying ovulation [13,65]. These results emphasize the need for balanced breeding strategies to improve milk production while maintaining reproductive efficiency.
A negative genetic correlation (−0.306) was identified between MY305 and milk FPR, indicating that cows with higher MY305 often have lower milk FPR. Similar genetic correlations between MY305 and milk FPR have been reported in Thai-Holstein crossbreeds [17,56] and Nordic Red cattle [19]. Negussie et al. [19] found that genetic correlations between test-day FPR and MY during early lactation at 15, 30, and 60 days in milk (DIM) were small but positive (0.01 to 0.13), and after 60 DIM, these correlations became predominantly negative (0.01 to −0.22). These positive genetic correlations in early lactation suggest that high-producing cows mobilize body reserves to meet the energy demands of peak milk production, leading to a higher FPR. However, as lactation progresses, the genetic correlation between FPR and MY declines or becomes negative, indicating recovery from NEB. This trend is consistent with the findings in Canadian Holsteins [18] and German Holsteins [66]. In Thai dairy cattle, this condition may arise because of less intensive management during late lactation compared to early lactation, particularly among dairy farmers who continue to use traditional practices. As a result, these animals often experience inadequate nutrition management [67].
One notable finding from this study is the negative genetic correlation between DO and milk FPR, which indicates that cows with higher DO tend to have a lower milk FPR. These results contradict previous studies that reported low-to-moderate positive genetic correlations between DO and milk FPR [17,19]. This may be attributed to a lack of awareness regarding the nutritional needs of dairy cows during lactation, particularly during the late lactation phase. An extremely low milk FPR indicates acidosis, a condition often caused by excessive concentrate feeding without sufficient high-quality forage. Overfeeding with concentrates leads to excessive starch fermentation, which increases lactic acid production and lowers rumen pH. This can negatively impact reproductive performance, resulting in infertility, prolonged calving-to-conception intervals, and increased incidence of lameness [12,15,68]. Alternatively, the negative genetic correlation between DO and milk FPR may be influenced by differences in the time from calving to first service between trained officers and traditional farmers, which affects DO variation among cows [67].
In our study, the phenotypic and environmental correlations among MY305, DO, and milk FPR were very low, with both positive and negative values close to zero, indicating that these traits share minimal common influences at the observable and environmental levels. This suggests that changes in MY305 are unlikely to have a direct impact on DO and milk FPR, and shared environmental factors do not significantly affect these traits together. As a result, improvements in milk yield management or genetic selection may not necessarily affect fertility and animal health.
4.3. GEBVs for MY305, DO, and Milk FPR
The breed group (BG) effects indicated that a higher proportion of Holstein genetics was associated with increased MY305, longer DO, and lower milk FPR across both methods (Table 4). Holsteins have superior genetic potential for milk production compared to many other breeds, which explains why animals in BG1 exhibited the highest MY305. Studies have consistently shown that Holsteins outperform other breeds in terms of milk production [69,70,71]. However, animals with higher Holstein genetics also tend to have a longer DO, possibly because of their reduced adaptability to tropical environments. This finding aligns with the study by Pongpiachan et al. [69], who reported that purebred Holsteins exhibited lower reproductive efficiency, even when specialized management strategies were employed to mitigate the effects of tropical climates and enhance their diet. Consequently, crossbreeding has been proposed as a strategy to counteract reproductive decline associated with “Holsteinization” [72]. A previous study found that crossbred cows exhibited improved reproductive performance, including shorter DO and calving intervals (CIs), higher conception rates at 28 days, and reduced incidences of mastitis, ketosis, and endometritis [73]. Additionally, high-production Holsteins often experience greater energy deficits, leading to increased mobilization of body fat. This metabolic shift can alter the fat-to-protein ratio (FPR) in milk, often resulting in a lower FPR [67].
The GEBV results presented in Table 4 demonstrate that wssGBLUP consistently produced higher GEBVs than ssGBLUP, particularly for MY305 and DO, indicating that wssGBLUP captured genetic effects in the dataset more effectively. In the all-animal dataset, both methods yielded negative GEBVs for MY305, but the wssGBLUP values (−8.983 to −8.259) were closer to zero, suggesting enhanced GEBV accuracy. A similar trend of improvement was observed in the bull dataset, whereas in the dam dataset, the improvements were less pronounced for DO and milk FPR than for MY305. The GEBV for MY305 in this population was generally negative, in contrast to that of Russian Black-and-White cattle, which was reported to be 0.88 [50]. A negative average GEBV for MY305 suggests that the population’s genetic potential for milk yield was below the baseline or the genetic mean defined by the reference population. This may have resulted from factors such as population structure or the genetic merit of the reference group used for comparison. Research has shown that the composition of the reference population plays a crucial role, as it directly affects the estimated breed composition and subsequent GEBVs [74]. In this study, the reference population consisted of high-performing breeds, which might have led to lower GEBVs for animals from breeds with a lower genetic potential for MY305.
The positive GEBVs for DO indicate that animals have a higher genetic potential for longer reproductive intervals, which may not be desirable based on breeding goals favoring shorter calving intervals. However, the mean GEBVs for DO in the all-animal dataset were lower than those reported in Russian Black-and-White cattle (3.25 to 4.14) [50] but comparable to recent findings of 0.266 to 0.274 [22]. This suggests that the Thai-Holstein population has better genetic values for the DO trait than the Russian Black-and-White cattle population. This improvement may be attributed to the genetic advantages of crossbred cattle, which exhibit superior reproductive efficiency and overall performance compared with purebred Holsteins. For milk FPR, the GEBVs remained consistently negative or near zero across both the ssGBLUP and wssGBLUP methods, indicating that both models provided similar GEBV accuracy for this trait.
The wssGBLUP method consistently demonstrated a higher accuracy than ssGBLUP across all traits and groups (Figure 1). For MY305, the accuracy increased by 27.54%, improving from 0.138 with ssGBLUP to approximately 0.176–0.177 with wssGBLUP. This highlights both the higher heritability of MY305 and the enhanced ability of wssGBLUP to capture key genetic markers compared to other traits. In contrast, DO and milk FPR exhibited smaller accuracy gains, with the averaged GEBV accuracy increasing from 0.112–0.113 to 0.124–0.125 (10.71%), and from 0.094 to approximately 0.110–0.112 (17.02%), respectively. These findings suggest that wssGBLUP improved accuracy by emphasizing key genetic regions, with notable gains for highly heritable traits, such as MY305. These results align with the findings of previous studies, where wssGBLUP provided greater accuracy for production traits, while yielding smaller gains for traits with lower genetic influence. Zhang et al. [75] reported that wssGBLUP enhances accuracy by assigning different weights to SNPs, making it particularly effective for highly heritable traits in dairy cattle. The wssGBLUP method has been shown to effectively identify SNPs linked to traits such as protein content and to enhance the accuracy of GEBVs in Canadian Holstein cattle [76]. Additionally, a study on Hanwoo beef cattle found that wssGBLUP improved the prediction accuracy for carcass traits such as carcass weight, eye muscle area, and yearling weight [77]. In pig breeding, a study revealed that wssGBLUP offered improved estimation reliability compared to ssGBLUP for meat, fattening, and reproductive traits [78].
wssGBLUP is superior to ssGBLUP because it can assign weights to single-nucleotide polymorphisms (SNPs), optimize predictions for specific traits, and iteratively refine accuracy. According to Teissier et al. [79], wssGBLUP assigns different weights to SNPs based on their estimated effects, enabling the more precise identification of quantitative trait loci (QTLs) with significant impacts on traits. By emphasizing SNPs associated with major genes, wssGBLUP enhanced the accuracy of GEBVs for traits strongly influenced by these genes. Additionally, the iterative weighting process of wssGBLUP allows it to adapt to the genetic architecture of different traits, making it highly flexible in modeling both polygenic traits and those controlled by a few major genes [77].
4.4. Identification of Genomic Regions and Candidate Genes
Manhattan plots of the SNP effects from GEBVs for MY305, DO, and milk FPR are shown in Figure 2. The peaks of the Manhattan plots highlight impactful SNPs, with positive values at the top and negative values at the bottom. Figure 3 presents Manhattan plots illustrating the percentage of additive genetic variance accounted for by the five SNP moving windows. Using a threshold of at least 5% of the total genetic variance, 14 SNPs were significantly associated with MY305 across seven chromosomes (BTA 4, 6, 13, 18, 24, and 25), 19 SNPs with DO across five chromosomes (BTA 2, 5, 13, 18, and 25), and 16 SNPs with milk FPR across five chromosomes (BTA 5, 18, 19, 21, and 25), all within a distance constraint of less than 50 kb (Table 6 and Table 7).
After comparing the results with NCBI databases, we identified 24 candidate genes associated with MY305, 46 genes associated with DO, and 33 genes associated with milk FPR. However, four genes from MY305 (LOC618297, LOC101906304, LOC529511, and LOC786948), nine genes from DO (LOC101905166, LOC107133069, LOC101902869, LOC100138951, LOC618463, LOC100141212, LOC101905312, C25H16orf58, and LOC783313), and six genes from milk FPR (LOC101905166, LOC100138951, LOC618463, LOC100141212, LOC100196898, and LOC786948) have not been characterized (Table 6 and Table 7). We also identified seven genes from MY305 (LOC618297, LOC101906304, PPARGC1A, VSIG10L, PARD6G, and LMF1), ten genes from DO (TNS1, SOX10, LOC101905166, BAIAP2L2, PDYN, LOC618463, VSIG10L, LMF1, ITGAD, and SEPT14), and nine genes from milk FPR (SOX10, LOC101905166, BAIAP2L2, LOC618463, VSIG10L, LOC100196898, MGAT5B, MGAT5B, and LINGO1) at the target SNP location. Hajihosseinlo et al. [80] found that the r2 value tends to decrease as the distance between SNP pairs increases, implying that SNPs positioned within 1 Mb are more likely to exhibit strong and consistent associations with QTLs.
Candidate gene analysis revealed genes in the same genomic region across all three traits, suggesting potential pleiotropic effects (Figure 4). Specifically, 14 genes were shared among MY305, DO, and milk FPR; 17 genes were shared between MY305 and DO; 14 genes were shared between MY305 and milk FPR; and 26 genes were shared between DO and milk FPR. According to Gratten and Visscher [81], pleiotropy is a genetic phenomenon whereby a single DNA variant influences multiple traits. This indicates that when selection targets one trait, other traits often change over generations. This response is driven by genetic correlations that reflect the combined genome-wide effects of pleiotropy at shared genetic loci. Identifying pleiotropic genes associated with MY305, DO, and milk FPR is challenging, owing to the complexity of these traits. Although limited research has directly connected these traits to pleiotropic genes, some studies have examined genes that affect multiple production traits [35,82] and multiple fertility traits in dairy cattle [83,84].
4.5. Pleiotropy and Candidate Genes for MY305, DO, and Milk FPR
Pleiotropy occurs when one gene affects multiple phenotypic traits. This study identified genes influencing MY305, DO, and milk FPR simultaneously. The pleiotropic effects of these genes are shown through their SNP variance and effect relationships (Figure 5, Figure 6 and Figure 7).
Figure 5A, Figure 6A, and Figure 7A show positive relationships between SNP variance for MY305 and DO, FPR and DO, and MY305 and FPR, respectively. Most SNP variances are clustered near the origin, indicating that most SNPs have low variances across all traits, while a few SNPs exhibit strong pleiotropic genetic influences. Influential SNPs can be found in all figures, indicating significant pleiotropic SNPs for all three relationships. It can be observed that SNPs affecting high MY305 variation also affect high DO variation (Figure 5A). SNPs influencing high FPR variation affect moderate DO variation (Figure 6A). However, SNPs influencing high DO and FPR variation might contribute independently (Figure 7A).
Figure 5B, Figure 6B, and Figure 7B demonstrate strong relationships between SNP effects for MY305 and DO, FPR and DO, and MY305 and FPR, respectively. Positive collinearity can be observed in SNPs affecting MY305 and DO, indicating that SNPs with large effects on MY305 also extend to DO (Figure 5B). A negative relationship can be found in SNPs affecting FPR and DO, showing that SNPs with large negative effects on FPR tend to increase DO (Figure 6B). Similarly, SNPs influencing MY305 and FPR have strong negative correlations, suggesting that high MY305 effects decrease the FPR (Figure 7B). This implies that selecting cows for high milk yield may lower the FPR and prolong DO, posing a challenge, as a greater value for DO is linked to extended calving intervals and reduced fertility. These finding confirm previous studies reporting antagonistic genetic correlations between milk yield and fertility [85,86], indicating that higher milk yield might reduce the fat-to-protein ratio, affecting milk composition and metabolic efficiency.
These findings highlight the need for multi-trait selection strategies to balance milk yield, fertility, and metabolic efficiency. Breeding programs should use optimal selection indices to improve MY305, DO, and milk FPR. High-variance SNPs in these traits suggest strong genetic influences, making them key targets for marker-assisted selection (MAS). Identifying beneficial SNPs for all three traits can refine breeding strategies. Additionally, pleiotropic genes associated with all three traits highlight the genetic connections among production, fertility, and health traits.
The results reveal fourteen genes within the same region, indicating pleiotropy among MY305, DO, and milk FPR (SIGLECL1, IGLON5, VSIG10L, ETFB, NKG7, CLDND2, LIM2, SOX8, SSTR5, TEKT4, C1QTNF8, CACNA1H, LOC786948, and TPSB2). Some genes have been reported to be pleiotropic and influence production, reproduction, and health traits across various species. Recent studies highlight SIGLECL1’s role in animal reproduction and immune regulation, with its expression detected in the male reproductive tracts of mice, rats, and bovine sperm [87,88]. Additionally, SIGLEC family polymorphisms have been associated with milk yield, DO, and calving intervals in cattle [89,90].
The following genes play a role in reproduction in some species. LIM2 plays a role in spermatogenesis, with studies in mice showing that Limk2-deficient individuals exhibit impaired testicular development and increased germ cell apoptosis [91]. This gene also protects spermatogenic cells from stress-induced damage [91]. TEKT4 is expressed in male germ cells, and is essential for sperm motility in mice [92,93]. Additionally, TEKT4 has been identified as a key gene associated with sperm motility in Brahman cattle, based on proteomic studies [94].
Three genes, SSTR5, ETFB, and SOX8, have been linked to animal production and growth traits. SSTR5 polymorphisms in sheep are associated with growth traits, making them potential molecular markers for selective breeding [95]. At the same time, ETFB has been identified as a key gene influencing meat quality in Qinchuan cattle [96]. Copy number variations (CNVs) of SOX8 genes in yaks are significantly associated with growth traits such as withers height and chest girth [97].
Among the genes related to immunity and behavior, IGLON5 plays a critical role in immune function in cattle [98,99]. NKG7 enhances cytotoxic activity in CD8+ T cells and NK cells, promoting T cell accumulation [100,101]. CLDND2 plays a role in immune responses in cattle [102]. CACNA1H knockout in mice leads to autistic-like behaviors [103]. TPSB2 is linked to immune cells in adipose tissue in Holstein–Friesian cows [104]. Additionally, three genes (PDYN, SIRPA, and LMF1) were found to be shared between MY305 and DO. PDYN shows signs of positive selection in dairy cattle, indicating its role in reproductive traits [105]. SIRPA is a marker of spermatogonial stem cells, embryogenesis, and gametogenesis in mice [106,107,108]. LMF1 is essential for the post-translational activation of lipoprotein lipase and other enzymes [109,110].
The results indicate that DO and milk FPR share 17 common genes (MICALL1, C5H22orf23, SOX10, POLR2F, LOC101905166, PICK1, SLC16A8, BAIAP2L2, PLA2G6, LOC100138951, LOC618463, and LOC100141212); most genes influence health and immunity, while others impact fertility. BAIAP2L2 genes are essential for mechanotransduction [111]. PLA2G6 encodes iPLA2β, related to immunity and membrane homeostasis [112]. LOC618463 is associated with livability in Holstein cattle [113], and LOC100138951 is linked to calf survival in Nordic Holstein cattle [114]. The SLC16A8 gene family includes 14 monocarboxylate transporters vital for metabolic processes [115]. LOC618463 is also a target gene for calving traits [116]. SOX10 is involved in sex determination [117], and PICK1 is essential for male fertility [118].
Candidate genes in MY305 include LOC618297, SHFM1, LOC101906304, PPARGC1A, LOC529511, PARD6G, and ADNP2. PPARGC1A polymorphisms are linked to milk fat yield [119] and birth weight in Holsteins [120], with specific SNPs affecting milk traits in Iranian Holsteins [121]. PARD6 is associated with the Hippo signaling pathway [122,123], and ADNP2 is highly expressed in embryonic brain tissues [124].
Seventeen candidate genes influencing DO were identified, with key roles in reproduction (TNS1, MAFF, LOC107133069, LOC101902869, ITGAD, COX6A2, ARMC5, LOC101905312, TGFB1I1, SLC5A2, RUSF1, AHSP, LOC783313, OR7D4, SEPT14, ZNF713, and MRPS17). ITGAD is linked to fertility and tick resistance [124,125,126], and TGFB1I1 is important for ovarian development [127]. Genes from the TGF-β pathway are essential for embryogenesis [128]. The SLC5 family, especially SLC5A1 and SLC5A2, affects glucose transport and litter size in sheep [129]. OR7D4 in horses and stallion testes plays a role in reproductive functions [130]. MRPs like MRPS17 are vital for embryogenesis [131], and MRPS17 and SEPTIN14 are candidates for fertility traits in cattle [83]. C25H16orf58 and SLC45A2 are associated with clinical ketosis in Holsteins and heat tolerance in Chinese cattle, respectively [132,133].
Finally, five genes (LOC100196898, MGAT5B, MFSD11, LINGO1, and ODF3L10) were associated with milk FPR. In dairy cattle, milk FPR is linked to NEB and the overall health condition. MGAT5 is crucial for the synthesis of complex N-glycans and is essential for various biological processes. MGAT5-deficient mice exhibit complex phenotypes, including susceptibility to autoimmune diseases and reduced cancer progression [134,135]. Additionally, MGAT5 has been associated with mastitis resistance [136]. MFSD11 is widely expressed in the brain and periphery, particularly in the neurons, and its expression is altered by changes in energy balance in mice [137]. LINGO1, a protein selectively expressed in the central nervous system, functions as a negative regulator of oligodendrocyte differentiation, myelination, neuronal survival, and axonal regeneration and plays a key role in neural health [138]. Research on agonistic behavior in Lidia cattle has also identified genomic regions containing LINGO2 that are linked to behavioral traits [139]. At the same time, ODF3L10 (also known as Odf3) is primarily associated with sperm tail outer dense fibers (ODFs) in animals with internal fertilization [140]. Most of these genes contribute to health-related traits in cattle, including neurological function, energy balance, immune response, and structural integrity.
5. Conclusions
In conclusion, this study highlights the potential of wssGBLUP to improve GEBV accuracy in Thai-Holstein crossbred dairy cattle. The identification of pleiotropic genes provides valuable insights into the genetic relationships between milk yield, fertility, and health traits, enabling more informed breeding decisions. Heritability estimates suggest a moderate genetic influence on milk yield, whereas fertility and milk FPR exhibit lower heritability, emphasizing the challenges of genetic improvement in these traits. Furthermore, genetic correlations reveal trade-offs in selection, particularly a negative relationship between milk yield and fertility, which must be carefully managed in breeding strategies. wssGBLUP outperformed ssGBLUP, making it a promising alternative for multi-trait genomic prediction. Breeding strategies should integrate genomic markers associated with desirable pleiotropic effects, enabling balanced selection for high milk yields without compromising fertility and health traits. To ensure sustainable genetic progress, further functional validation of the candidate genes is essential. Incorporating these validated markers into selection programs can optimize breeding decisions, improve reproductive efficiency, and enhance the overall productivity of tropical dairy cattle. Ultimately, this approach could contribute to long-term genetic gains and economic sustainability in the dairy industry.
Author Contributions
Conceptualization, A.F., S.B., M.D., V.C., and W.B.; methodology, A.F., M.D., and W.B.; formal analysis, A.F., M.D., and W.B.; original draft preparation and writing, A.F., M.D., W.B., and V.C.; review and editing, A.F., M.D., W.B., and V.C.; supervision, M.D. and W.B.; project administration, A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Program of Khon Kaen University, Thailand (Grant Number: RP68-1-RCRI-001), and the KKU Scholarship for ASEAN and GMS Countries (No. 588/2021).
Institutional Review Board Statement
This study was approved by the Institutional Animal Care and Use Committee of Khon Kaen University (no. IACUC-KKU-120/64, 30 November 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
Additional data are available upon request from the corresponding author.
Conflicts of Interest
The authors declare that they have no known competing financial or personal interests that could have influenced the work reported in this study.
References
- Fathoni, A.; Boonkum, W.; Chankitisakul, V.; Duangjinda, M. An Appropriate Genetic Approach for Improving Reproductive Traits in Crossbred Thai-Holstein Cattle under Heat Stress Conditions. Vet. Sci. 2022, 9, 163. [Google Scholar] [CrossRef]
- Barkema, H.W.; von Keyserlingk, M.A.G.; Kastelic, J.P.; Lam, T.J.G.M.; Luby, C.; Roy, J.-P.; LeBlanc, S.J.; Keefe, G.P.; Kelton, D.F. Invited Review: Changes in the Dairy Industry Affecting Dairy Cattle Health and Welfare. J. Dairy Sci. 2015, 98, 7426–7445. [Google Scholar] [CrossRef]
- Fricke, P.M.; Wiltbank, M.C.; Pursley, J.R. The High Fertility Cycle. JDS Commun. 2023, 4, 127–131. [Google Scholar] [CrossRef]
- Liu, Z.; Jaitner, J.; Reinhardt, F.; Pasman, E.; Rensing, S.; Reents, R. Genetic Evaluation of Fertility Traits of Dairy Cattle Using a Multiple-Trait Animal Model. J. Dairy Sci. 2008, 91, 4333–4343. [Google Scholar] [CrossRef] [PubMed]
- Collard, B.L.; Boettcher, P.J.; Dekkers, J.C.M.; Petitclerc, D.; Schaeffer, L.R. Relationships Between Energy Balance and Health Traits of Dairy Cattle in Early Lactation. J. Dairy Sci. 2000, 83, 2683–2690. [Google Scholar] [CrossRef] [PubMed]
- Veerkamp, R.F.; Koenen, E.P.C.; De Jong, G. Genetic Correlations Among Body Condition Score, Yield, and Fertility in First-Parity Cows Estimated by Random Regression Models. J. Dairy Sci. 2001, 84, 2327–2335. [Google Scholar] [CrossRef]
- Ingvartsen, K.L.; Dewhurst, R.J.; Friggens, N.C. On the Relationship between Lactational Performance and Health: Is It Yield or Metabolic Imbalance That Cause Production Diseases in Dairy Cattle? A Position Paperq. Livest. Prod. Sci. 2003, 83, 277–308. [Google Scholar] [CrossRef]
- Puangdee, S.; Duangjinda, M.; Boonkum, W.; Buaban, S.; Katawatin, S. Effect of Milk Fat to Protein Ratio on Genetic Variance for Milk Yield in Thai Tropical Holstein Cattle. Can. J. Anim. Sci. 2016, 96, 410–415. [Google Scholar] [CrossRef]
- Klein, M.S.; Buttchereit, N.; Miemczyk, S.P.; Immervoll, A.-K.; Louis, C.; Wiedemann, S.; Junge, W.; Thaller, G.; Oefner, P.J.; Gronwald, W. NMR Metabolomic Analysis of Dairy Cows Reveals Milk Glycerophosphocholine to Phosphocholine Ratio as Prognostic Biomarker for Risk of Ketosis. J. Proteome Res. 2012, 11, 1373–1381. [Google Scholar] [CrossRef]
- Tetens, J.; Seidenspinner, T.; Buttchereit, N.; Thaller, G. Whole-Genome Association Study for Energy Balance and Fat/Protein Ratio in German Holstein Bull Dams. Anim. Genet. 2013, 44, 1–8. [Google Scholar] [CrossRef]
- Buttchereit, N.; Stamer, E.; Junge, W.; Thaller, G. Genetic Parameters for Energy Balance, Fat/Protein Ratio, Body Condition Score and Disease Traits in German Holstein Cows: Genetics of Energy Balance and Disease Traits. J. Anim. Breed. Genet. 2012, 129, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Vallejo-Timarán, D.; Reyes-Vélez, J.; VanLeeuwen, J.; Maldonado-Estrada, J.; Astaiza-Martínez, J. Incidence and Effects of Subacute Ruminal Acidosis and Subclinical Ketosis with Respect to Postpartum Anestrus in Grazing Dairy Cows. Heliyon 2020, 6, e03712. [Google Scholar] [CrossRef]
- Mekuriaw, Y. Negative Energy Balance and Its Implication on Productive and Reproductive Performance of Early Lactating Dairy Cows: Review Paper. J. Appl. Anim. Res. 2023, 51, 220–228. [Google Scholar] [CrossRef]
- Butler, W.R. Energy Balance Relationships with Follicular Development, Ovulation and Fertility in Postpartum Dairy Cowsq. Livest. Prod. Sci. 2003, 83, 211–218. [Google Scholar] [CrossRef]
- Abdela, N. Sub-Acute Ruminal Acidosis (SARA) and Its Consequence in Dairy Cattle: A Review of Past and Recent Research at Global Prospective. Achiev. Life Sci. 2016, 10, 187–196. [Google Scholar] [CrossRef]
- Plaizier, J.C.; Krause, D.O.; Gozho, G.N.; McBride, B.W. Subacute Ruminal Acidosis in Dairy Cows: The Physiological Causes, Incidence and Consequences. Vet. J. 2008, 176, 21–31. [Google Scholar] [CrossRef]
- Buaban, S.; Duangjinda, M.; Suzuki, M.; Masuda, Y.; Sanpote, J.; Kuchida, K. Genetic Relationships of Fertility Traits with Test-Day Milk Yield and Fat-to-Protein Ratio in Tropical Smallholder Dairy Farms: Relationships of Fertility and Fat-To-Protein Ratio. Anim. Sci. J. 2016, 87, 627–637. [Google Scholar] [CrossRef]
- Jamrozik, J.; Schaeffer, L.R. Test-Day Somatic Cell Score, Fat-to-Protein Ratio and Milk Yield as Indicator Traits for Sub-Clinical Mastitis in Dairy Cattle. J. Anim. Breed. Genet. 2012, 129, 11–19. [Google Scholar] [CrossRef]
- Negussie, E.; Strandén, I.; Mäntysaari, E.A. Genetic Associations of Test-Day Fat:Protein Ratio with Milk Yield, Fertility, and Udder Health Traits in Nordic Red Cattle. J. Dairy Sci. 2013, 96, 1237–1250. [Google Scholar] [CrossRef]
- Matilainen, K.; Strandén, I.; Aamand, G.P.; Mäntysaari, E.A. Single Step Genomic Evaluation for Female Fertility in Nordic Red Dairy Cattle. J. Anim. Breed. Genet. 2018, 135, 337–348. [Google Scholar] [CrossRef]
- Hutchison, J.L.; VanRaden, P.M.; Null, D.J.; Cole, J.B.; Bickhart, D.M. Genomic Evaluation of Age at First Calving. J. Dairy Sci. 2017, 100, 6853–6861. [Google Scholar] [CrossRef]
- Fathoni, A.; Boonkum, W.; Chankitisakul, V.; Buaban, S.; Duangjinda, M. Integrating Genomic Selection and a Genome-Wide Association Study to Improve Days Open in Thai Dairy Holstein Cattle: A Comprehensive Genetic Analysis. Animals 2025, 15, 43. [Google Scholar] [CrossRef] [PubMed]
- Buaban, S.; Prempree, S.; Sumreddee, P.; Duangjinda, M.; Masuda, Y. Genomic Prediction of Milk-Production Traits and Somatic Cell Score Using Single-Step Genomic Best Linear Unbiased Predictor with Random Regression Test-Day Model in Thai Dairy Cattle. J. Dairy Sci. 2021, 104, 12713–12723. [Google Scholar] [CrossRef]
- Bekele, R.; Taye, M.; Abebe, G.; Meseret, S. Genetic and Non-Genetic Factors Affecting Test Day Milk Yield and Milk Composition Traits in Crossbred Dairy Cattle in Ethiopia. Vet. Integr. Sci. 2023, 21, 717–733. [Google Scholar] [CrossRef]
- Zhang, H.; Gao, Q.; Wang, A.; Wang, Z.; Liang, Y.; Guo, M.; Mao, Y.; Wang, Y. Estimation of Genetic Parameters for Milk Production Rate and Its Stability in Holstein Population. Animals 2024, 14, 2761. [Google Scholar] [CrossRef]
- Moncur, V.S.; Hardie, L.C.; Dechow, C.D. Genetic Analysis of Daily Milk Yield Variability in Holstein Dairy Cattle in an Experimental Herd. Livest. Sci. 2021, 244, 104397. [Google Scholar] [CrossRef]
- Hayes, B.J.; Bowman, P.J.; Chamberlain, A.J.; Goddard, M.E. Invited Review: Genomic Selection in Dairy Cattle: Progress and Challenges. J. Dairy Sci. 2009, 92, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Burrow, H.M.; Mrode, R.; Mwai, A.O.; Coffey, M.P.; Hayes, B.J. Challenges and Opportunities in Applying Genomic Selection to Ruminants Owned by Smallholder Farmers. Agriculture 2021, 11, 1172. [Google Scholar] [CrossRef]
- Misztal, I.; Lourenco, D.; Aguilar, I.; Legarra, A.; Vitezica, Z. Manual for BLUPF90 Family of Programs; University of Georgia: Athens, GA, USA.
- Ravagnolo, O.; Misztal, I. Effect of Heat Stress on Nonreturn Rate in Holsteins: Fixed-Model Analyses. J. Dairy Sci. 2002, 85, 3101–3106. [Google Scholar] [CrossRef]
- VanRaden, P.M. Efficient Methods to Compute Genomic Predictions. J. Dairy Sci. 2008, 91, 4414–4423. [Google Scholar] [CrossRef]
- Wang, H.; Misztal, I.; Aguilar, I.; Legarra, A.; Fernando, R.L.; Vitezica, Z.; Okimoto, R.; Wing, T.; Hawken, R.; Muir, W.M. Genome-Wide Association Mapping Including Phenotypes from Relatives without Genotypes in a Single-Step (ssGWAS) for 6-Week Body Weight in Broiler Chickens. Front. Genet. 2014, 5, 134. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, I.; Misztal, I.; Tsuruta, S.; Legarra, A.; Wang, H. PREGSF90—POSTGSF90: Computational Tools for the Implementation of Single-Step Genomic Selection and Genome-Wide Association with Ungenotyped Individuals in BLUPF90 Programs. In Proceedings of the 10th World Congress of Genetics Applied to Livestock Production, Vancouver, BC, Canada, 17–22 August 2014. [Google Scholar]
- Zhang, Z.; Liu, J.; Ding, X.; Bijma, P.; De Koning, D.-J.; Zhang, Q. Best Linear Unbiased Prediction of Genomic Breeding Values Using a Trait-Specific Marker-Derived Relationship Matrix. PLoS ONE 2010, 5, e12648. [Google Scholar] [CrossRef]
- Buaban, S.; Lengnudum, K.; Boonkum, W.; Phakdeedindan, P. Genome-Wide Association Study on Milk Production and Somatic Cell Score for Thai Dairy Cattle Using Weighted Single-Step Approach with Random Regression Test-Day Model. J. Dairy Sci. 2022, 105, 468–494. [Google Scholar] [CrossRef]
- Lee, S.; Dang, C.; Choy, Y.; Do, C.; Cho, K.; Kim, J.; Kim, Y.; Lee, J. Comparison of Genome-Wide Association and Genomic Prediction Methods for Milk Production Traits in Korean Holstein Cattle. Asian-Australas J. Anim. Sci. 2019, 32, 913–921. [Google Scholar] [CrossRef]
- Zhou, C.; Li, C.; Cai, W.; Liu, S.; Yin, H.; Shi, S.; Zhang, Q.; Zhang, S. Genome-Wide Association Study for Milk Protein Composition Traits in a Chinese Holstein Population Using a Single-Step Approach. Front. Genet. 2019, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Medeiros De Oliveira Silva, R.; Bonvino Stafuzza, N.; De Oliveira Fragomeni, B.; Miguel Ferreira De Camargo, G.; Matos Ceacero, T.; Noely Dos Santos Gonçalves Cyrillo, J.; Baldi, F.; Augusti Boligon, A.; Zerlotti Mercadante, M.E.; Lino Lourenco, D.; et al. Genome-Wide Association Study for Carcass Traits in an Experimental Nelore Cattle Population. PLoS ONE 2017, 12, e0169860. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Iso-Touru, T.; Sanchez, M.-P.; Kadri, N.; Bouwman, A.C.; Chitneedi, P.K.; MacLeod, I.M.; Vander Jagt, C.J.; Chamberlain, A.J.; Gredler-Grandl, B.; et al. Meta-Analysis of Six Dairy Cattle Breeds Reveals Biologically Relevant Candidate Genes for Mastitis Resistance. Genet. Sel. Evol. 2024, 56, 54. [Google Scholar] [CrossRef]
- Huang, M.; Liu, X.; Zhou, Y.; Summers, R.M.; Zhang, Z. BLINK: A Package for the next Level of Genome-Wide Association Studies with Both Individuals and Markers in the Millions. GigaScience 2019, 8, giy154. [Google Scholar] [CrossRef]
- Sudrajad, P.; Suhada, H.; Prasetyo, D.; Gariri, P.N.; Eddianto, E.; Abiyoga, A.F.; Kusminanto, R.Y.; Sukaryo, S.; Bramastya, T.A.; Volkandari, S.D.; et al. Genome-Wide Association Study of Birth Weight in Bali Cattle (Bos Javanicus). Trop. Anim. Sci. J. 2023, 46, 151–156. [Google Scholar] [CrossRef]
- Santos, D.J.A.; Peixoto, M.G.C.D.; Borquis, R.R.A.; Verneque, R.S.; Panetto, J.C.C.; Tonhati, H. Genetic Parameters for Test-Day Milk Yield, 305-Day Milk Yield, and Lactation Length in Guzerat Cows. Livest. Sci. 2013, 152, 114–119. [Google Scholar] [CrossRef][Green Version]
- Paiva, J.T.; Peixoto, M.G.C.D.; Bruneli, F.A.T.; Alvarenga, A.B.; Oliveira, H.R.; Silva, A.A.; Silva, D.A.; Veroneze, R.; Silva, F.F.; Lopes, P.S. Genetic Parameters, Genome-Wide Association and Gene Networks for Milk and Reproductive Traits in Guzerá Cattle. Livest. Sci. 2020, 242, 104273. [Google Scholar] [CrossRef]
- Pangmao, S.; Thomson, P.C.; Khatkar, M.S. Genetic Parameters of Milk and Lactation Curve Traits of Dairy Cattle from Research Farms in Thailand. Anim. Biosci. 2022, 35, 1499–1511. [Google Scholar] [CrossRef]
- Wu, X.-L.; Wiggans, G.R.; Norman, H.D.; Caputo, M.J.; Miles, A.M.; Van Tassell, C.P.; Baldwin, R.L.; Sievert, S.; Mattison, J.; Burchard, J.; et al. Updating Test-Day Milk Yield Factors for Use in Genetic Evaluations and Dairy Production Systems: A Comprehensive Review. Front. Genet. 2023, 14, 1298114. [Google Scholar] [CrossRef]
- Ayalew, W.; Aliy, M.; Negussie, E. Estimation of Genetic Parameters of the Productive and Reproductive Traits in Ethiopian Holsteinusing Multi-Trait Models. Asian-Australas J. Anim. Sci. 2017, 30, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- González-Recio, O.; Alenda, R. Genetic Parameters for Female Fertility Traits and a Fertility Index in Spanish Dairy Cattle. J. Dairy Sci. 2005, 88, 3282–3289. [Google Scholar] [CrossRef]
- Ghiasi, H.; Pakdel, A.; Nejati-Javaremi, A.; Mehrabani-Yeganeh, H.; Honarvar, M.; González-Recio, O.; Carabaño, M.J.; Alenda, R. Genetic Variance Components for Female Fertility in Iranian Holstein Cows. Livest. Sci. 2011, 139, 277–280. [Google Scholar] [CrossRef]
- Abe, H.; Masuda, Y.; Suzuki, M. Relationships between Reproductive Traits of Heifers and Cows and Yield Traits for Holsteins in Japan. J. Dairy Sci. 2009, 92, 4055–4062. [Google Scholar] [CrossRef] [PubMed]
- Sharko, F.S.; Khatib, A.; Prokhortchouk, E.B. Genomic Estimated Breeding Valueof Milk Performance and Fertility Traits in the Russian Black-and-White Cattle Population. Acta Naturae 2022, 14, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Buaban, S.; Duangjinda, M.; Suzuki, M.; Masuda, Y.; Sanpote, J.; Kuchida, K. Short Communication: Genetic Analysis for Fertility Traits of Heifers and Cows from Smallholder Dairy Farms in a Tropical Environment. J. Dairy Sci. 2015, 98, 4990–4998. [Google Scholar] [CrossRef]
- Boonkum, W.; Misztal, I.; Duangjinda, M.; Pattarajinda, V.; Tumwasorn, S.; Buaban, S. Short Communication: Genetic Effects of Heat Stress on Days Open for Thai Holstein Crossbreds. J. Dairy Sci. 2011, 94, 1592–1596. [Google Scholar] [CrossRef]
- Oseni, S.; Misztal, I.; Tsuruta, S.; Rekaya, R. Genetic Components of Days Open Under Heat Stress. J. Dairy Sci. 2004, 87, 3022–3028. [Google Scholar] [CrossRef] [PubMed]
- Boonkum, W.; Chankitisakul, V.; Duangjinda, M.; Buaban, S.; Sumreddee, P.; Sungkhapreecha, P. Genomic Selection Using Single-Step Genomic BLUP on the Number of Services per Conception Trait in Thai-Holstein Crossbreeds. Animals 2023, 13, 3609. [Google Scholar] [CrossRef] [PubMed]
- Toghiani, S.; Hay, E.; Sumreddee, P.; Geary, T.W.; Rekaya, R.; Roberts, A.J. Genomic Prediction of Continuous and Binary Fertility Traits of Females in a Composite Beef Cattle Breed. J. Anim. Sci. 2017, 95, 4787–4795. [Google Scholar] [CrossRef] [PubMed]
- Boonkum, W.; Teawyoneyong, W.; Chankitisakul, V.; Duangjinda, M.; Buaban, S. Impact of Heat Stress on Milk Yield, Milk Fat-to-Protein Ratio, and Conception Rate in Thai-Holstein Dairy Cattle: A Phenotypic and Genetic Perspective. Animals 2024, 14, 3026. [Google Scholar] [CrossRef]
- Maskal, J.M.; Pedrosa, V.B.; Rojas de Oliveira, H.; Brito, L.F. A Comprehensive Meta-Analysis of Genetic Parameters for Resilience and Productivity Indicator Traits in Holstein Cattle. J. Dairy Sci. 2024, 107, 3062–3079. [Google Scholar] [CrossRef]
- Schneider, H.; Segelke, D.; Tetens, J.; Thaller, G.; Bennewitz, J. A Genomic Assessment of the Correlation between Milk Production Traits and Claw and Udder Health Traits in Holstein Dairy Cattle. J. Dairy Sci. 2023, 106, 1190–1205. [Google Scholar] [CrossRef]
- Satoła, A.; Ptak, E. Genetic Parameters of Milk Fat-to-Protein Ratio in First Threelactations of Polish Holstein-Friesian Cows. J. Anim. Feed Sci. 2019, 28, 97–109. [Google Scholar] [CrossRef]
- Kadarmideen, H.N.; Thompson, R.; Coffey, M.P.; Kossaibati, M.A. Genetic Parameters and Evaluations from Single- and Multiple-Trait Analysis of Dairy Cow Fertility and Milk Production. Livest. Prod. Sci. 2003, 81, 183–195. [Google Scholar] [CrossRef]
- González-Recio, O.; Alenda, R.; Chang, Y.M.; Weigel, K.A.; Gianola, D. Selection for Female Fertility Using Censored Fertility Traits and Investigation of the Relationship with Milk Production. J. Dairy Sci. 2006, 89, 4438–4444. [Google Scholar] [CrossRef]
- Dematawewa, C.M.B.; Berger, P.J. Genetic and Phenotypic Parameters for 305-Day Yield, Fertility, and Survival in Holsteins. J. Dairy Sci. 1998, 81, 2700–2709. [Google Scholar] [CrossRef]
- Walsh, S.W.; Williams, E.J.; Evans, A.C.O. A Review of the Causes of Poor Fertility in High Milk Producing Dairy Cows. Anim. Reprod. Sci. 2011, 123, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Wall, E.; Brotherstone, S.; Woolliams, J.A.; Banos, G.; Coffey, M.P. Genetic Evaluation of Fertility Using Direct and Correlated Traits. J. Dairy Sci. 2003, 86, 4093–4102. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, M.A.; Llewellyn, S.; Fitzpatrick, R.; Kenny, D.A.; Murphy, J.J.; Patton, J.; Wathes, D.C. Negative Energy Balance in Dairy Cows Is Associated with Specific Changes in IGF-Binding Protein Expression in the Oviduct. Reproduction 2008, 135, 63–75. [Google Scholar] [CrossRef]
- Buttchereit, N.; Stamer, E.; Junge, W.; Thaller, G. Short Communication: Genetic Relationships among Daily Energy Balance, Feed Intake, Body Condition Score, and Fat to Protein Ratio of Milk in Dairy Cows. J. Dairy Sci. 2011, 94, 1586–1591. [Google Scholar] [CrossRef]
- Puangdee, S.; Duangjinda, M.; Boonkum, W.; Katawatin, S.; Buaban, S.; Thepparat, M. Genetic Associations between Milk Fat-to-Protein Ratio, Milk Production and Fertility in the First Two Lactations of Thai Holsteins Dairy Cattle. Anim. Sci. J. 2017, 88, 723–730. [Google Scholar] [CrossRef]
- Chaidate, I.; Somchai, C.; Jos, N.; Henk, H. A Cow-Level Association of Ruminal pH on Body Condition Score, Serum Beta-Hydroxybutyrate and Postpartum Disorders in Thai Dairy Cattle. Anim. Sci. J. 2014, 85, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Pongpiachan, P.; Rodtian, P.; Ōta, K. Reproduction of Cross- and Purebred Friesian Cattle in Northern Thailand with Special Reference to Their Milk Production. Asian Australas J. Anim. Sci. 2003, 16, 1093–1101. [Google Scholar] [CrossRef]
- Ghazy, A.A.; El-Enin, A.S.A.; Badr, A.A.E.-S.A.E.-A.; El-Awady, H.G.; El-Naser, I.A.M.A. Genetic Assessment of Productive and Reproductive Traits in Friesian, Native, and Crossbred Cattle in Egypt. Trop. Anim. Health Prod. 2024, 56, 344. [Google Scholar] [CrossRef]
- Coffey, E.L.; Horan, B.; Evans, R.D.; Berry, D.P. Milk Production and Fertility Performance of Holstein, Friesian, and Jersey Purebred Cows and Their Respective Crosses in Seasonal-Calving Commercial Farms. J. Dairy Sci. 2016, 99, 5681–5689. [Google Scholar] [CrossRef]
- Buckley, F.; Lopez-Villalobos, N.; Heins, B.J. Crossbreeding: Implications for Dairy Cow Fertility and Survival. Animal 2014, 8, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.A.F.; Hussein, M.A.; Alkhedaide, A.Q.; El-Tarabany, M.S.; Roushdy, E.M. Reproductive Performance and Culling Rate of Purebred Holstein Cows and Their Crosses With Fleckvieh and Brown Swiss Cows Under Subtropical Conditions. Front. Vet. Sci. 2021, 8, 752941. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, M.A.; Heins, B.J.; Dechow, C.; Huson, H.J. The Impact of Using Different Ancestral Reference Populations in Assessing Crossbred Population Admixture and Influence on Performance. Front. Genet. 2022, 13, 910998. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lourenco, D.; Aguilar, I.; Legarra, A.; Misztal, I. Weighting Strategies for Single-Step Genomic BLUP: An Iterative Approach for Accurate Calculation of GEBV and GWAS. Front. Genet. 2016, 7, 151. [Google Scholar] [CrossRef]
- Alvarenga, A.B.; Veroneze, R.; Oliveira, H.R.; Marques, D.B.D.; Lopes, P.S.; Silva, F.F.; Brito, L.F. Comparing Alternative Single-Step GBLUP Approaches and Training Population Designs for Genomic Evaluation of Crossbred Animals. Front. Genet. 2020, 11, 263. [Google Scholar] [CrossRef]
- Mehrban, H.; Naserkheil, M.; Lee, D.H.; Cho, C.; Choi, T.; Park, M.; Ibáñez-Escriche, N. Genomic Prediction Using Alternative Strategies of Weighted Single-Step Genomic BLUP for Yearling Weight and Carcass Traits in Hanwoo Beef Cattle. Genes 2021, 12, 266. [Google Scholar] [CrossRef]
- Kabanov, A.; Melnikova, E.; Nikitin, S.; Somova, M.; Fomenko, O.; Volkova, V.; Kostyunina, O.; Karpushkina, T.; Martynova, E.; Trebunskikh, E. Weighted Single-Step Genomic Best Linear Unbiased Prediction Method Application for Assessing Pigs on Meat Productivity and Reproduction Traits. Animals 2022, 12, 1693. [Google Scholar] [CrossRef]
- Teissier, M.; Larroque, H.; Robert-Granié, C. Weighted Single-Step Genomic BLUP Improves Accuracy of Genomic Breeding Values for Protein Content in French Dairy Goats: A Quantitative Trait Influenced by a Major Gene. Genet. Sel. Evol. 2018, 50, 31. [Google Scholar] [CrossRef]
- Hajihosseinlo, A.; Nejati-Javaremi, A.; Miraei-Ashtiani, S.R. Genetic Structure Analysis in Several Populations of Cattle Using SNP Genotypes. Anim. Biotechnol. 2023, 34, 288–300. [Google Scholar] [CrossRef]
- Gratten, J.; Visscher, P.M. Genetic Pleiotropy in Complex Traits and Diseases: Implications for Genomic Medicine. Genome Med. 2016, 8, 78. [Google Scholar] [CrossRef]
- Cai, Z.; Dusza, M.; Guldbrandtsen, B.; Lund, M.S.; Sahana, G. Distinguishing Pleiotropy from Linked QTL between Milk Production Traits and Mastitis Resistance in Nordic Holstein Cattle. Genet. Sel. Evol. 2020, 52, 19. [Google Scholar] [CrossRef]
- Chen, S.-Y.; Schenkel, F.S.; Melo, A.L.P.; Oliveira, H.R.; Pedrosa, V.B.; Araujo, A.C.; Melka, M.G.; Brito, L.F. Identifying Pleiotropic Variants and Candidate Genes for Fertility and Reproduction Traits in Holstein Cattle via Association Studies Based on Imputed Whole-Genome Sequence Genotypes. BMC Genom. 2022, 23, 331. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Wang, Y.; Sahana, G.; Zhang, Q.; Liu, L.; Lund, M.S.; Su, G. Genome-Wide Association Studies for Female Fertility Traits in Chinese and Nordic Holsteins. Sci. Rep. 2017, 7, 8487. [Google Scholar] [CrossRef] [PubMed]
- Windig, J.J.; Calus, M.P.L.; Beerda, B.; Veerkamp, R.F. Genetic Correlations between Milk Production and Health and Fertility Depending on Herd Environment. J. Dairy Sci. 2006, 89, 1765–1775. [Google Scholar] [CrossRef] [PubMed]
- Jayawardana, J.M.D.R.; Lopez-Villalobos, N.; McNaughton, L.R.; Hickson, R.E. Heritabilities and Genetic and Phenotypic Correlations for Milk Production and Fertility Traits of Spring-Calved Once-Daily or Twice-Daily Milking Cows in New Zealand. J. Dairy Sci. 2023, 106, 1910–1924. [Google Scholar] [CrossRef]
- Almhanna, H.; Kumar, A.H.; Kilroy, D.; Duggan, G.; Irwin, J.A.; Hogg, B.; Reid, C. Comparison of Siglec-1 Protein Networks and Expression Patterns in Sperm and Male Reproductive Tracts of Mice, Rats, and Humans. Vet. World 2024, 17, 645–657. [Google Scholar] [CrossRef]
- Alkhodair, K.; Almhanna, H.; McGetrick, J.; Gedair, S.; Gallagher, M.E.; Fernandez-Fuertes, B.; Tharmalingam, T.; Larsen, P.B.; Fitzpatrick, E.; Lonergan, P.; et al. Siglec Expression on the Surface of Human, Bull and Ram Sperm. Reproduction 2018, 155, 361–371. [Google Scholar] [CrossRef]
- Puckowska, P.; Borowska, A.; Szwaczkowski, T.; Oleński, K.; Kamiński, S. Effects of a Novel Missense Polymorphism within the SIGLEC5 Gene on Fertility Traits in Holstein-Friesian Cattle. Reprod. Domest. Anim. 2019, 54, 1163–1168. [Google Scholar] [CrossRef]
- Poulsen, N.A.; Robinson, R.C.; Barile, D.; Larsen, L.B.; Buitenhuis, B. A Genome-Wide Association Study Reveals Specific Transferases as Candidate Loci for Bovine Milk Oligosaccharides Synthesis. BMC Genom. 2019, 20, 404. [Google Scholar] [CrossRef]
- Takahashi, H.; Koshimizu, U.; Miyazaki, J.; Nakamura, T. Impaired Spermatogenic Ability of Testicular Germ Cells in Mice Deficient in the LIM-Kinase 2 Gene. Dev. Biol. 2002, 241, 259–272. [Google Scholar] [CrossRef]
- Roy, A.; Lin, Y.-N.; Agno, J.E.; DeMayo, F.J.; Matzuk, M.M. Tektin 3 Is Required for Progressive Sperm Motility in Mice. Mol. Reprod. Dev. 2009, 76, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, T.; Honda, Y.; Doiguchi, M.; Iida, H. Molecular Cloning of a New Member of TEKTIN Family, Tektin4, Located to the Flagella of Rat Spermatozoa. Mol. Reprod. Dev. 2005, 72, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Thepparat, T.; Katawatin, S.; Vongpralub, T.; Duangjinda, M.; Thammasirirak, S.; Utha, A. Separation of Bovine Spermatozoa Proteins Using 2D-PAGE Revealed the Relationship between Tektin-4 Expression Patterns and Spermatozoa Motility. Theriogenology 2012, 77, 1816–1821. [Google Scholar] [CrossRef]
- Li, X.; Ding, N.; Zhang, Z.; Tian, D.; Han, B.; Liu, D.; Liu, S.; Tian, F.; Fu, D.; Song, X.; et al. Identification of SSTR5 Gene Polymorphisms and Their Association With Growth Traits in Hulun Buir Sheep. Front. Genet. 2022, 13, 831599. [Google Scholar] [CrossRef]
- Yu, H.; Yang, Z.; Wang, J.; Li, H.; Li, X.; Liang, E.; Mei, C.; Zan, L. Identification of Key Genes and Metabolites Involved in Meat Quality Performance in Qinchuan Cattle by WGCNA. J. Integr. Agric. 2024, 23, 3923–3937. [Google Scholar] [CrossRef]
- Xia, W.; Osorio, J.S.; Yang, Y.; Liu, D.; Jiang, M.F. Short Communication: Characterization of Gene Expression Profiles Related to Yak Milk Protein Synthesis during the Lactation Cycle. J. Dairy Sci. 2018, 101, 11150–11158. [Google Scholar] [CrossRef]
- Gonzalez-Recio, O.; Scrobota, N.; López-Paredes, J.; Saborío-Montero, A.; Fernández, A.; López de Maturana, E.; Villanueva, B.; Goiri, I.; Atxaerandio, R.; García-Rodríguez, A. Review: Diving into the Cow Hologenome to Reduce Methane Emissions and Increase Sustainability. Animal 2023, 17, 100780. [Google Scholar] [CrossRef]
- Landa, J.; Serafim, A.B.; Alba, M.; Maudes, E.; Molina-Porcel, L.; Garcia-Serra, A.; Mannara, F.; Dalmau, J.; Graus, F.; Sabater, L. IgLON5 Deficiency Produces Behavioral Alterations in a Knockout Mouse Model. Front. Immunol. 2024, 15, 1347948. [Google Scholar] [CrossRef]
- Li, X.-Y.; Corvino, D.; Nowlan, B.; Aguilera, A.R.; Ng, S.S.; Braun, M.; Cillo, A.R.; Bald, T.; Smyth, M.J.; Engwerda, C.R. NKG7 Is Required for Optimal Antitumor T-Cell Immunity. Cancer Immunol. Res. 2022, 10, 154–161. [Google Scholar] [CrossRef]
- Ng, S.S.; De Labastida Rivera, F.; Yan, J.; Corvino, D.; Das, I.; Zhang, P.; Kuns, R.; Chauhan, S.B.; Hou, J.; Li, X.-Y.; et al. The NK Cell Granule Protein NKG7 Regulates Cytotoxic Granule Exocytosis and Inflammation. Nat. Immunol. 2020, 21, 1205–1218. [Google Scholar] [CrossRef]
- Congiu, M.; Cesarani, A.; Falchi, L.; Macciotta, N.P.P.; Dimauro, C. Combined Use of Univariate and Multivariate Approaches to Detect Selection Signatures Associated with Milk or Meat Production in Cattle. Genes. 2024, 15, 1516. [Google Scholar] [CrossRef] [PubMed]
- Jiao, C.; Wang, J.; Kuang, H.; Wu, Z.; Liu, T. Effects of CACNA1H Gene Knockout on Autistic-like Behaviors and the Morphology of Hippocampal Neurons in Mice. Beijing Da Xue Xue Bao Yi Xue Ban = J. Peking Univ. Health Sci. 2022, 54, 209–216. [Google Scholar]
- Oliveira, B.M.; Pinto, A.; Correia, A.; Ferreira, P.G.; Vilanova, M.; Teixeira, L. Characterization of Myeloid Cellular Populations in Mesenteric and Subcutaneous Adipose Tissue of Holstein-Friesian Cows. Sci. Rep. 2020, 10, 1771. [Google Scholar] [CrossRef]
- Suqueli García, M.F.; Castellote, M.A.; Corva, P.M. Sequence Analysis Suggests Positive Selection on the Bovine Prodynorphin Gene. J. Basic Appl. Genet. 2020, 31, 13–25. [Google Scholar] [CrossRef]
- Sharifi, N.; Ament, M.; Brennan, M.B.; Hochgeschwender, U. Isolation and Characterization of the Mouse Homolog of the Preprodynorphin (Pdyn) Gene. Neuropeptides 1999, 33, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Kanatsu-Shinohara, M.; Ema, M.; Shinohara, T. Signal Regulatory Protein Alpha Is a Conserved Marker for Mouse and Rat Spermatogonial Stem Cells. Biol. Reprod. 2023, 108, 682–693. [Google Scholar] [CrossRef]
- McBurney, M.W.; Yang, X.; Jardine, K.; Hixon, M.; Boekelheide, K.; Webb, J.R.; Lansdorp, P.M.; Lemieux, M. The Mammalian SIR2α Protein Has a Role in Embryogenesis and Gametogenesis. Mol. Cell Biol. 2003, 23, 38–54. [Google Scholar] [CrossRef]
- Hosseini, M.; Ehrhardt, N.; Weissglas-Volkov, D.; Lai, C.-M.; Mao, H.Z.; Liao, J.-L.; Nikkola, E.; Bensadoun, A.; Taskinen, M.-R.; Doolittle, M.H.; et al. Transgenic Expression and Genetic Variation of Lmf1 Affect LPL Activity in Mice and Humans. ATVB 2012, 32, 1204–1210. [Google Scholar] [CrossRef]
- Ehrhardt, N.; Bedoya, C.; Péterfy, M. Embryonic Viability, Lipase Deficiency, Hypertriglyceridemia and Neonatal Lethality in a Novel LMF1-Deficient Mouse Model. Nutr. Metab. 2014, 11, 37. [Google Scholar] [CrossRef]
- Yan, K.; Zong, W.; Du, H.; Zhai, X.; Ren, R.; Liu, S.; Xiong, W.; Wang, Y.; Xu, Z. BAIAP2L2 Is Required for the Maintenance of Mechanotransducing Stereocilia of Cochlear Hair Cells. J. Cell Physiol. 2022, 237, 774–788. [Google Scholar] [CrossRef]
- Wada, H.; Yasuda, T.; Miura, I.; Watabe, K.; Sawa, C.; Kamijuku, H.; Kojo, S.; Taniguchi, M.; Nishino, I.; Wakana, S.; et al. Establishment of an Improved Mouse Model for Infantile Neuroaxonal Dystrophy That Shows Early Disease Onset and Bears a Point Mutation in Pla2g6. Am. J. Pathol. 2009, 175, 2257–2263. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Freebern, E.; Santos, D.J.A.; Fang, L.; Jiang, J.; Parker Gaddis, K.L.; Liu, G.E.; VanRaden, P.M.; Maltecca, C.; Cole, J.B.; Ma, L. GWAS and Fine-Mapping of Livability and Six Disease Traits in Holstein Cattle. BMC Genom. 2020, 21, 41. [Google Scholar] [CrossRef]
- Wu, X.; Guldbrandtsen, B.; Nielsen, U.S.; Lund, M.S.; Sahana, G. Association Analysis for Young Stock Survival Index with Imputed Whole-Genome Sequence Variants in Nordic Holstein Cattle. J. Dairy Sci. 2017, 100, 6356–6370. [Google Scholar] [CrossRef]
- Halestrap, A.P. The SLC16 Gene Family—Structure, Role and Regulation in Health and Disease. Mol. Asp. Med. 2013, 34, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.B.; VanRaden, P.M.; O’Connell, J.R.; Van Tassell, C.P.; Sonstegard, T.S.; Schnabel, R.D.; Taylor, J.F.; Wiggans, G.R. Distribution and Location of Genetic Effects for Dairy Traits. J. Dairy Sci. 2009, 92, 2931–2946. [Google Scholar] [CrossRef] [PubMed]
- Polanco, J.C.; Wilhelm, D.; Davidson, T.-L.; Knight, D.; Koopman, P. Sox10 Gain-of-Function Causes XX Sex Reversal in Mice: Implications for Human 22q-Linked Disorders of Sex Development. Hum. Mol. Genet. 2010, 19, 506–516. [Google Scholar] [CrossRef]
- Xiao, N.; Kam, C.; Shen, C.; Jin, W.; Wang, J.; Lee, K.M.; Jiang, L.; Xia, J. PICK1 Deficiency Causes Male Infertility in Mice by Disrupting Acrosome Formation. J. Clin. Investig. 2009, 119, 802–812. [Google Scholar] [CrossRef]
- Weikard, R.; Kühn, C.; Goldammer, T.; Freyer, G.; Schwerin, M. The Bovine PPARGC1A Gene: Molecular Characterization and Association of an SNP with Variation of Milk Fat Synthesis. Physiol. Genom. 2005, 21, 1–13. [Google Scholar] [CrossRef]
- Pasandideh, M. Two SNPs in the Bovine PPARGC1A Gene Are Associated with the Birth Weight of Holstein Calves. Meta Gene 2020, 25, 100732. [Google Scholar] [CrossRef]
- Pasandideh, M.; Mohammadabadi, M.R.; Esmailizadeh, A.K.; Tarang, A. Association of Bovine PPARGC1A and OPN Genes with Milk Production and Composition in Holstein Cattle. Czech J. Anim. Sci. 2015, 60, 97–104. [Google Scholar] [CrossRef]
- Marques, E.; Klefström, J. Par6 Family Proteins in Cancer. Oncoscience 2015, 2, 894–895. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Carmelo, V.A.O.; Kadarmideen, H.N. Genome-Wide Epistatic Interaction Networks Affecting Feed Efficiency in Duroc and Landrace Pigs. Front. Genet. 2020, 11, 121. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, M.; Dresner, E.; Mandel, S.; Gozes, I. Silencing of the ADNP-Family Member, ADNP2, Results in Changes in Cellular Viability under Oxidative Stress. J. Neurochem. 2008, 105, 537–545. [Google Scholar] [CrossRef]
- Zhao, J.; Li, J.; Jiang, F.; Song, E.; Lan, X.; Zhao, H. Fertility-Associated Polymorphism within Bovine ITGβ5 and Its Significant Correlations with Ovarian and Luteal Traits. Animals 2021, 11, 1579. [Google Scholar] [CrossRef]
- Porto Neto, L.R.; Bunch, R.J.; Harrison, B.E.; Prayaga, K.C.; Barendse, W. Haplotypes That Include the Integrin Alpha 11 Gene Are Associated with Tick Burden in Cattle. BMC Genet. 2010, 11, 55. [Google Scholar] [CrossRef]
- Hatzirodos, N.; Hummitzsch, K.; Irving-Rodgers, H.F.; Breen, J.; Perry, V.E.A.; Anderson, R.A.; Rodgers, R.J. Transcript Abundance of Stromal and Thecal Cell Related Genes during Bovine Ovarian Development. PLoS ONE 2019, 14, e0213575. [Google Scholar] [CrossRef]
- Li, G.; Khateeb, K.; Schaeffer, E.; Zhang, B.; Khatib, H. Genes of the Transforming Growth Factor-Beta Signalling Pathway Are Associated with Pre-Implantation Embryonic Development in Cattle. J. Dairy Res. 2012, 79, 310–317. [Google Scholar] [CrossRef]
- La, Y.; Liu, Q.; Zhang, L.; Chu, M. Single Nucleotide Polymorphisms in SLC5A1, CCNA1, and ABCC1 and the Association with Litter Size in Small-Tail Han Sheep. Animals 2019, 9, 432. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jung, Y.; Jung, H.; Shakeel, M.; Yoon, M. Olfactory Receptor (OR7D4 and OR1I1) Expression in Stallion Testes. J. Anim. Reprod. Biotechnol. 2021, 36, 292–298. [Google Scholar] [CrossRef]
- Cheong, A.; Lingutla, R.; Mager, J. Expression Analysis of Mammalian Mitochondrial Ribosomal Protein Genes. Gene Expr. Patterns 2020, 38, 119147. [Google Scholar] [CrossRef]
- Soares, R.A.N.; Vargas, G.; Duffield, T.; Schenkel, F.; Squires, E.J. Genome-Wide Association Study and Functional Analyses for Clinical and Subclinical Ketosis in Holstein Cattle. J. Dairy Sci. 2021, 104, 10076–10089. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Ma, J.; Yan, H.; Meng, Y.; Qi, X.; Qu, K.; Li, F.; Zhang, J.; Zhuzha, B.; Quji, S.; et al. Distribution of a Missense Mutation (Rs525805167) within the SLC45A2 Gene Associated with Climatic Conditions in Chinese Cattle. Gene 2022, 835, 146643. [Google Scholar] [CrossRef] [PubMed]
- Dennis, J.; Pawling, J.; Cheung, P.; Partridge, E.; Demetriou, M. UDP-N-Acetylglucosamine:Alpha-6-D-Mannoside Beta1,6 N-Acetylglucosaminyltransferase V (Mgat5) Deficient Mice. Biochim. Biophys. Acta 2002, 1573, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, L.; Roder, J.C.; Dennis, J.W.; Lipina, T. Beta N-acetylglucosaminyltransferase V (Mgat5) Deficiency Reduces the Depression-like Phenotype in Mice. Genes Brain Behav. 2008, 7, 334–343. [Google Scholar] [CrossRef]
- Zhong, L.; Ma, S.; Wang, D.; Zhang, M.; Tian, Y.; He, J.; Zhang, X.; Xu, L.; Wu, C.; Dong, M.; et al. Methylation Levels in the Promoter Region of FHIT and PIAS1 Genes Associated with Mastitis Resistance in Xinjiang Brown Cattle. Genes 2023, 14, 1189. [Google Scholar] [CrossRef]
- Perland, E.; Lekholm, E.; Eriksson, M.M.; Bagchi, S.; Arapi, V.; Fredriksson, R. The Putative SLC Transporters Mfsd5 and Mfsd11 Are Abundantly Expressed in the Mouse Brain and Have a Potential Role in Energy Homeostasis. PLoS ONE 2016, 11, e0156912. [Google Scholar] [CrossRef]
- Mi, S.; Sandrock, A.; Miller, R.H. LINGO-1 and Its Role in CNS Repair. Int. J. Biochem. Cell Biol. 2008, 40, 1971–1978. [Google Scholar] [CrossRef]
- Eusebi, P.G.; Cortés, O.; Carleos, C.; Dunner, S.; Cañon, J. Detection of Selection Signatures for Agonistic Behaviour in Cattle. J. Anim. Breed. Genet. 2018, 135, 170–177. [Google Scholar] [CrossRef]
- Petersen, C.; Aumüller, G.; Bahrami, M.; Hoyer-Fender, S. Molecular Cloning of Odf3 Encoding a Novel Coiled-coil Protein of Sperm Tail Outer Dense Fibers. Mol. Reprod. Dev. 2002, 61, 102–112. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).