Uncovering Human Tooth Marks in the Search for Dog Domestication: The Case of Coímbre Cave
Simple Summary
Abstract
1. Introduction
2. Archaeological and Geological Context
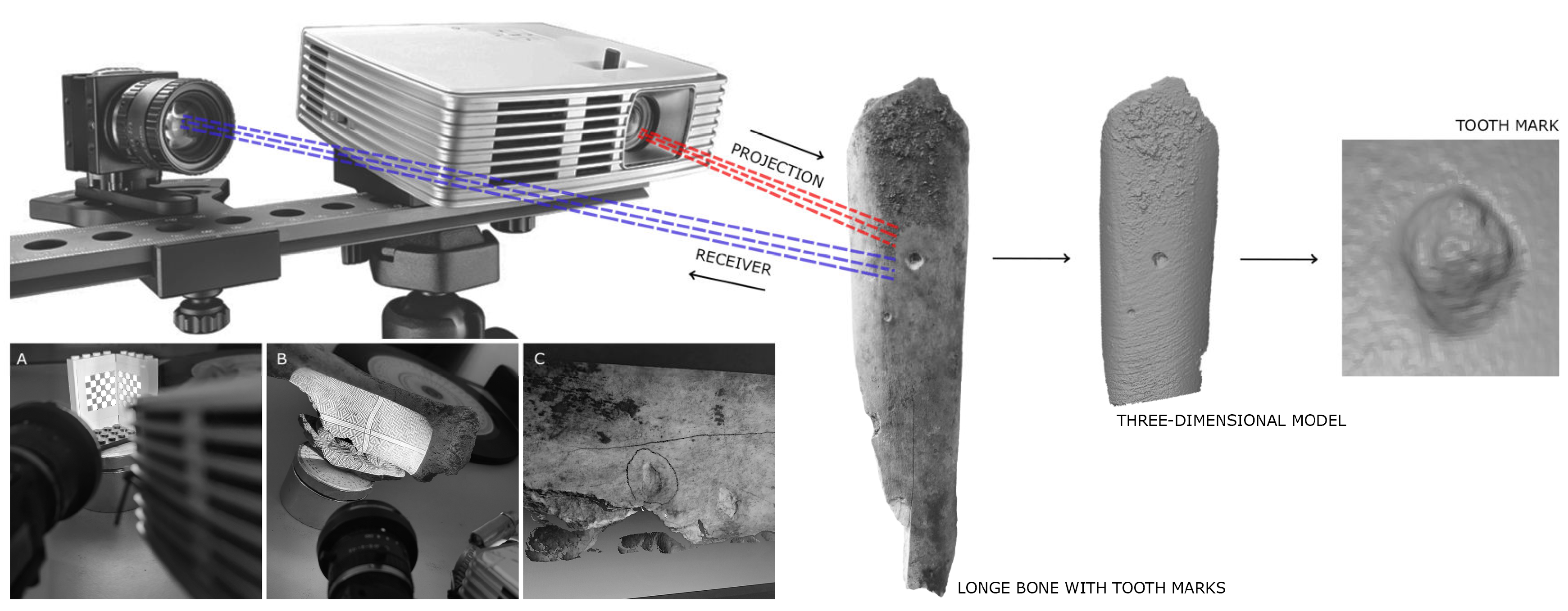
3. Materials and Methods
- 1.
- Generalized Procrustes Analysis (GPA): The package was used to align the landmark points through translation, rotation, and scaling [71,72,73,74,75]. This analysis allowed for the extraction of morphological information from the samples, producing configurations based on shape (with scaling) and form (without scaling). As sample size does not influence comparative results, the decision was made to work with data in its original scale using the function . Additionally, scaled analyses were conducted with to ensure that the results did not show significant variation.
- 2.
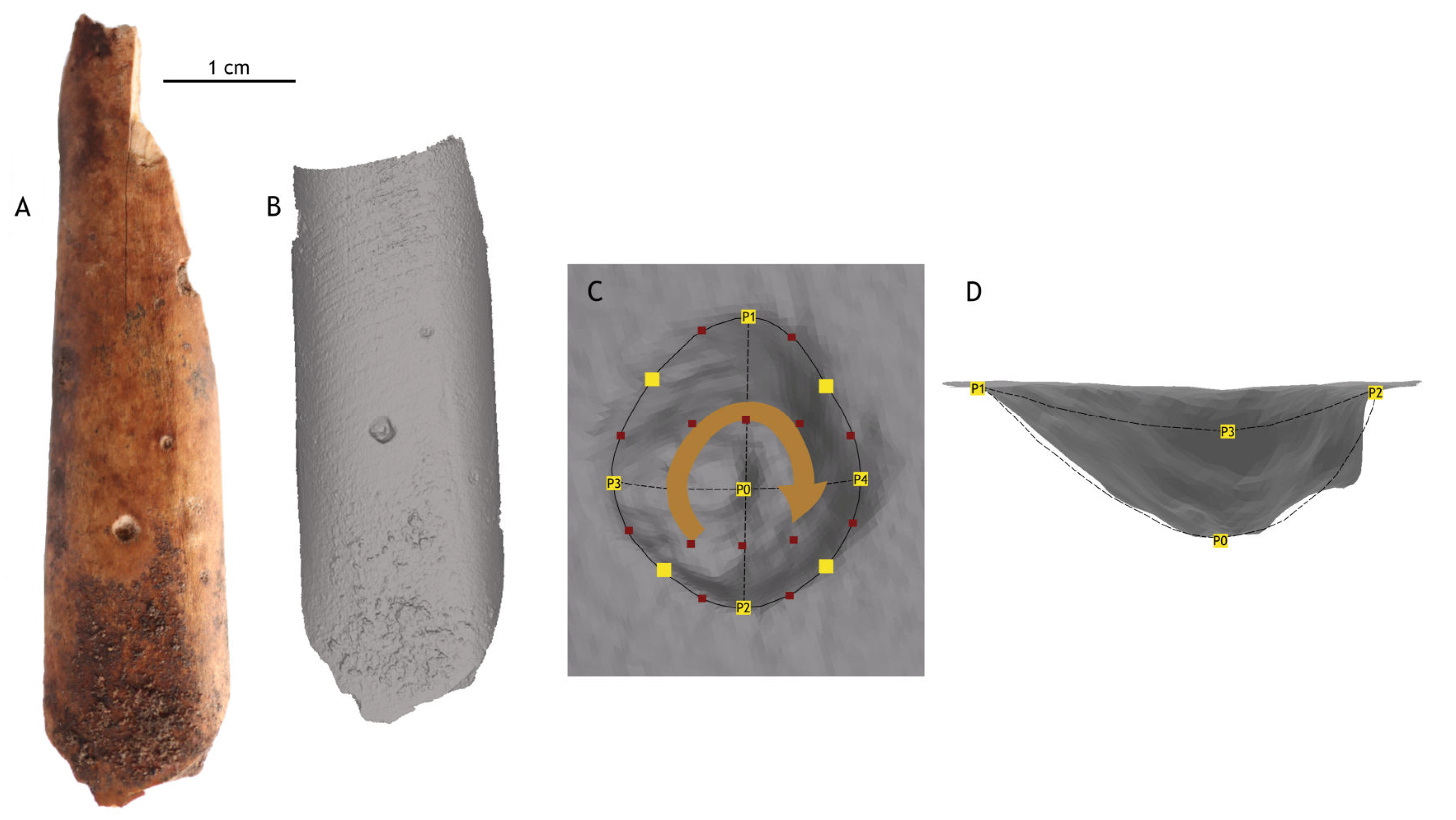
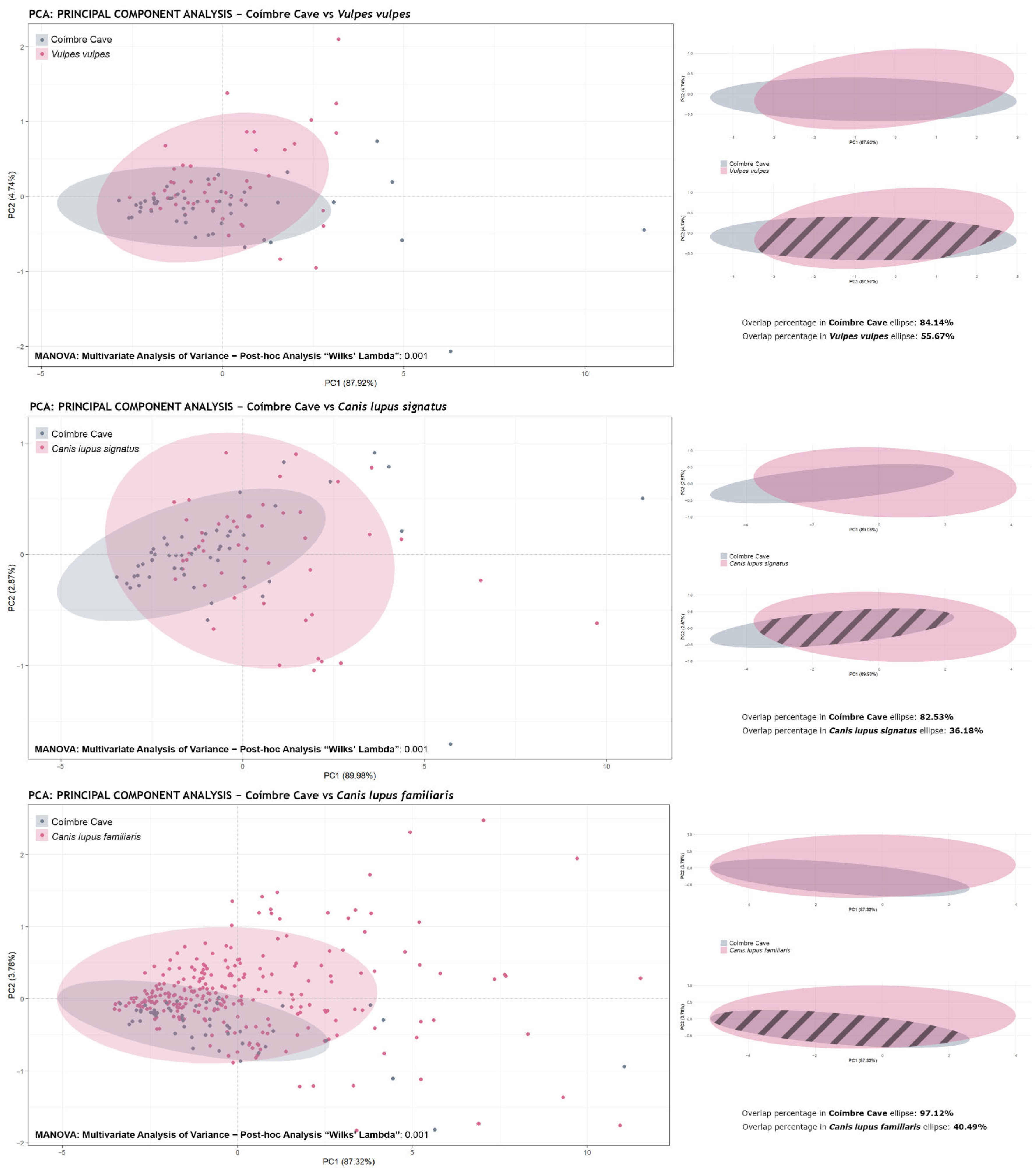
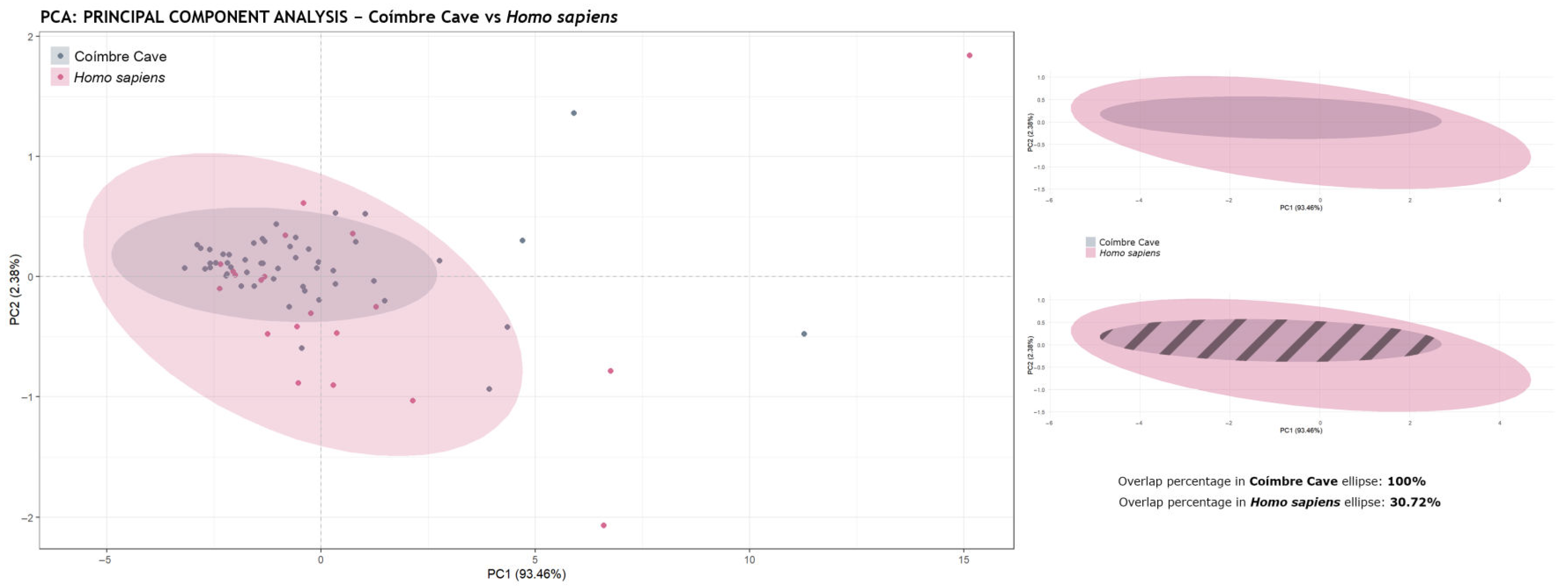
- Principal Component Analysis (PCA): Based on the GPA results, principal component (PCs) values were calculated and graphically represented using . Confidence ellipses at 95% were included for each group (species or subfamily).
- 3.
- Ellipse Overlap Percentage Calculation: The and packages were used to assess the intersection between the confidence regions of the ellipses. For this purpose, the ellipse coordinates were extracted and converted into spatial geometry objects. The individual and intersection areas were then calculated using the functions and . Finally, the percentage of overlap was obtained using the following formulas:
- 4.
- Multivariate Analysis of Variance (MANOVA): Based on the PCs values obtained from the PCA, differences between groups across multiple dependent variables were evaluated using the function . This analysis made it possible to determine whether there were significant morphological differences between the groups studied.
- 5.
- Post-hoc Pairwise Comparisons: In cases where the MANOVA indicated significant differences, pairwise comparisons were carried out using permutations via the function , from the package. This non-parametric approach is robust to non-normal distributions and allows for the identification of which groups differ significantly. In this study, a more stringent threshold of p < 0.003 (equivalent to 3σ) was adopted instead of the traditional p < 0.05, to obtain more robust and reliable results indicating the absence of significant differences between the samples compared. The traditional p-value of 0.05 carries a high risk of type I errors (false positives), with up to a 28.9% probability of incorrectly accepting the alternative hypothesis when the null hypothesis is true. Conversely, using the more conservative threshold of p < 0.003 significantly reduces the risk of type I error, with only a 4.5% probability of making this type of error. Moreover, this stricter value increases the reliability of the results, reflecting stronger evidence in favor of the alternative hypothesis. In this way, using p < 0.003 ensures more robust conclusions and minimizes the likelihood of false positives, which is essential for ensuring the validity of findings in contexts with high variability or uncertainty (according to [76]). Before choosing which test to apply (Wilks’ lambda/ “Wilks” or Hotelling–Lawley Trace/ “Hotelling”), the Shapiro–Wilk test was performed using the function .
- 6.
- Procrustes ANOVA: To assess the relationship between shape and size (allometric analysis), the package [69] was used. A data frame was created from the GPA data using the function , and then the function was applied, taking into account centroid size and the groups analyzed. Results were visualised graphically with , using the function with the methods “PredLine”, “RegScore”, “size.shape”, and “CAC”.
4. Results
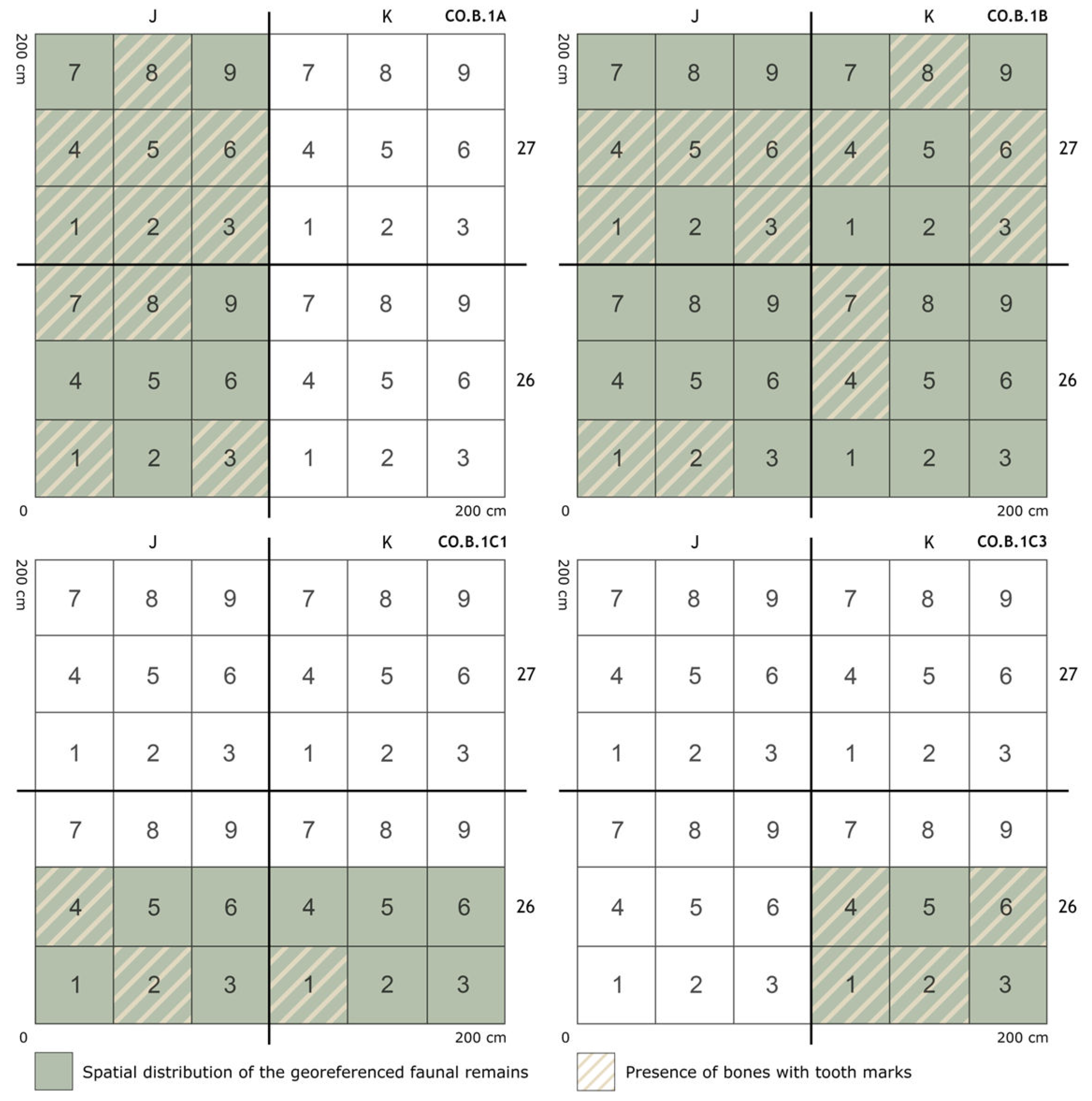
4.1. Taphonomic Study of Tooth Marks
4.2. Statistical Analysis: Humans
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benecke, N. Studies on early dog remains from Nothern Europe. J. Archaeol. Sci. 1987, 14, 31–49. [Google Scholar] [CrossRef]
- Linares-Matás, G.J. La Domesticación del Perro y sus Orígenes. Rev. Soc. Estud. Hist. Etnográficos 2019, 13, 42–49. [Google Scholar]
- Vigne, J.D. L’humérus de chien magdalénien de Erralla (Gipuzkoa, Espagne) et la domestication tardiglaciaire du loup en Europe. Munibe 2006, 57, 279–287. [Google Scholar]
- Germonpré, M.; Sablin, M.V.; Stevens, R.E.; Hedges, R.E.M.; Hofreiter, M.; Stiller, M.; Després, V.R. Fossil dogs and wolves from Palaeolithic sites in Belgium, the Ukraine and Russia: Osteometry, ancient DNA and stable isotopes. J. Archaeol. Sci. 2009, 36, 473–490. [Google Scholar] [CrossRef]
- Shipman, P. The Invaders: How Humans and Their Dogs Drove Neanderthals of Extinction, 1st ed.; The Belknap Press of Harvard University Press: Cambridge, MA, USA; London, UK, 2015. [Google Scholar]
- Wang, X.; Wei, W.; Kang, N.; Zhang, N.; Tang, Z.; Chen, Q.; Wang, L.; Zhang, L.; Tian, H.; Liu, W.; et al. Ancient DNA reveals the origin and history of early dogs in northeastern China. J. Archaeol. Sci. 2024, 168, 106010. [Google Scholar] [CrossRef]
- Germonpré, M.; Lázničková-Galetová, M.; Losey, R.J.; Räikkönen, J.; Sablin, M.V. Large canids at the Gravettian Předmostí site, the Czech Republic: The mandible. Quat. Int. 2015, 359–360, 261–279. [Google Scholar] [CrossRef]
- Valadez, R. El origen del perro, primera parte (entre el lobo y el perro). AMMVEPE 2000, 11, 75–84. [Google Scholar]
- Botigué, L.R.; Song, S.; Scheu, A.; Gopalan, S.; Pendleton, A.L.; Oetjens, M.; Taravella, A.M.; Seregély, T.; Zeeb-Lanz, A.; Arbogast, R.M.; et al. Ancient European dog genomes reveal continuity since the Early Neolithic. Nat. Commun. 2017, 8, 16082. [Google Scholar] [CrossRef] [PubMed]
- Thalmann, O.; Shapiro, B.; Cui, P.; Schuenemann, V.J.; Sawjer, S.K.; Reinfield, D.L.; Germonpré, M.B.; Sablin, M.V.; López-Giráldez, F.; Domingo-Roura, X.; et al. Complete mitochondrial genomes of ancient canids suggest a European origin of domestic dogs. Science 2013, 342, 871–874. [Google Scholar] [CrossRef]
- Savolainen, P.; Zhang, Y.-P.; Luo, J.; Lundeberg, J.; Leitner, T. Genetic evidence for an East Asian origin of domestic dogs. Science 2002, 298, 1610–1613. [Google Scholar]
- Pang, J.F.; Kluetsch, C.; Zou, X.J.; Zhang, A.B.; Luo, L.Y.; Angleby, H.; Ardalan, A.; Ekström, C.; Sköllermo, A.; Lundeberg, J.; et al. mtDNA Indicate a single origin for dogs south of Yangtze River, less than 16.300 years ago, from numerous wolves. Mol. Biol. Evol. 2009, 26, 2849–2864. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.K.; Pedersen, N.C.; Jafarishorijeh, S.; Bannasch, D.L.; Ahrens, K.D.; Wu, J.T.; Okon, M.; Sacks, B. La distinción filogenética de los cromosomas y del perro de aldea de Oriente Medio y el sudeste asiático ilumina los orígenes del perro. PLoS ONE 2011, 6, e28496. [Google Scholar]
- Pionnier-Capitan, M.; Bemilli, C.; Bodu, P.; Célérier, G.; Fierrié, J.G.; Fosse, P.; Garcià, M.; Vigne, J.D. New Evidence for Upper Palaeolithic small domestic dogs in South-Western. J. Archaeol. Sci. 2011, 38, 2123–2140. [Google Scholar]
- Ding, Z.L.; Oskarsson, M.; Ardalan, A.; Angleby, H.; Daghlgren, L.G.; Tepeli, C.; Kirkness, E.; Savolainen, P.; Zhang, Y.P. Origins of domestic dog in Southern East Asia is supported by analysis of Y-chromosome DNA. Nat. Hered. 2012, 108, 507–514. [Google Scholar] [CrossRef]
- Skoglund, P.; Ersmark, E.; Palkopoulou, E.; Dalén, L. Ancient wolf genome reveals an early divergence of domestic dog ancestors and admixture into high-latitude breeds. Curr. Biol. 2015, 25, 1515–1519. [Google Scholar] [CrossRef] [PubMed]
- Loog, L.; Thalmann, O.; Sinding, M.H.S.; Schuenemann, V.J.; Perri, A.; Germonpré, M.; Bocherens, H.; Wittm, K.E.; Samaniego-Castruita, J.A.; Velasco, M.S.; et al. Ancient DNA suggests modern wolves trace their origin to a Late Pleistocene Expansion from Beringia. Mol. Ecol. 2019, 29, 1596–1610. [Google Scholar] [CrossRef]
- Crockford, S.J.; Juzmin, Y.V. Comments on Germonpré et al. Journal of Archaeological Science 36, 2009 “Fossil dogs and wolves from Palaeolithic sites in Belgium, the Ukraine and Russia: Osteometry, ancient DNA and stable isotopes”, and Germonpré, Lázničková-Galetová and Sablin, Journal of Archaeological Science 39, 2021 “Palaeolithic dog skull at the Gravettian Predmostí site, the Czech Republic”. J. Archaeol. Sci. 2012, 39, 2797–2801. [Google Scholar]
- Courtenay, L.A.; Yravedra, J.; Huguet, R.; Aramendi, J.; Maté-González, M.A.; González-Aguilera, D.; Arriaza, M.C. Combining machine learning algorithms and geometric morphometrics: A study of carnivore tooth marks. Palaeogeogr. Palaeoclimatol. Palaecol. 2019, 522, 28–39. [Google Scholar] [CrossRef]
- Larson, G.; Karlsson, E.K.; Perri, A.; Webster, M.T.; Ho, S.Y.W.; Peters, J.; Skoglund, P. Rethinking dog domestication by integrating genetics, archaeology, and biogeography. Proc. Natl. Acad. Sci. USA 2012, 109, 8878–8883. [Google Scholar] [CrossRef]
- Morey, D.F. The early evolution of the domestic dog. Am. Sci. 1994, 82, 336–347. [Google Scholar]
- Coppinger, R.; Coppinger, L. Dogs: A New Understanding of Canine Origin, Behavior and Evolution; University of Chicago Press: Chicago, IL, USA, 2001. [Google Scholar]
- Miklósi, Á. Dog Behaviour, Evolution, and Cognition; Oxford University Press: Oxford, UK, 2007. [Google Scholar]
- Larson, G. The origins of animal domestication and husbandry: A biological perspective. In The Oxford Handbook of Zooarchaeology; Sykes, N., Baker, J., Higham, T., Eds.; Oxford University Press: Oxford, UK, 2012; pp. 1–12. [Google Scholar]
- Vilà, C.; Savolainen, P.; Maldonado, J.E.; Amorim, I.R.; Rice, J.E.; Honeycutt, R.L.; Crandall, K.A.; Lundeberg, J.; Wayne, R.K. Multiple and ancient origins of the domestic dog. Science 1997, 276, 1687–1689. [Google Scholar] [CrossRef]
- Gifford-Gonzalez, D. The Role of Taphonomy in the Study of Human-Animal Interactions; McDonald, T.R.H., McDonald, J.S.R.G., Eds.; Archaeozoology of the Near East: Los Angeles, CA, USA, 1991; pp. 45–61. [Google Scholar]
- Gifford-Gonzalez, D. An Introduction to Zooarchaeology; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Haynes, G. A guide for differentiating mammalian carnivore taxa responsible for gnaw damage to herbivore limb bones. Paleobiology 1983, 9, 164–172. [Google Scholar]
- Pickering, T.R.; Egeland, C.P. Experimental patterns of tooth mark production by large carnivores and their implications for the interpretation of hominin-controlled bone accumulations. J. Archaeol. Sci. 2006, 33, 82–97. [Google Scholar]
- Yravedra, J.; Maté-González, M.A.; Courtenay, L.A.; González-Aguilera, D.; Fernández-Fernández, M. The use of canid tooth marks on bone for the identification of livestock predation. Sci. Rep. 2019, 9, 16301. [Google Scholar]
- Yravedra, J.; Herranz-Rodrigo, D.; Mendoza, C.; Aragón-Poza, P.; Courtenay, L.A. The use of tooth marks for new research into identifying and understanding the first domestic dogs in Palaeolithic populations. J. Archaeol. Sci. Rep. 2021, 40, 103252. [Google Scholar] [CrossRef]
- Courtenay, L.A.; Herranz-Rodrigo, D.; Huguet, R.; Maté-González, M.A.; González-Aguilera, D.; Yravedra, J. Obtaining new resolutions in carnivore tooth pit morphological analyses: A methodological update for digital taphonomy. PLoS ONE 2020, 15, e0240328. [Google Scholar]
- Courtenay, L.A.; Herranz-Rodrigo, D.; González-Aguilera, D.; Yravedra, J. Developments in data science solutions for carnivore tooth pit classification. Sci. Rep. 2021, 11, 10209. [Google Scholar]
- Courtenay, L.A.; Herranz-Rodrigo, D.; Yravedra, J.; Vázquez-Rodríguez, J.M.; Huguet, R.; Barja, I.; Maté González, M.A.; Fernández, M.; Muñoz-Nieto, A.L.; González-Aguilera, D. 3D Insights into the Effects of Captivity on Wolf Mastication and Their Tooth Marks; Implications in Ecological Studies of Both the Past and Present. Animals 2021, 11, 2323. [Google Scholar] [CrossRef]
- Dimitrejevic, V.; Vukovic, S. Was the Dog Locally Domesticated in the Danube Gorges? Morphometric Study of Dog Cranial Remains From Four Mesolithic–Early Neolithic Archaeological Sites by Comparison With Contemporary Wolves. Int. J. Osteoarchaeol. 2015, 25, 1–30. [Google Scholar] [CrossRef]
- Claver, I.; Estaca-Gómez, V.; Linares-Matás, G.; Arenas-Esteban, J.A.; Yravedra, J. Taphonomy as a Methodological Approach for the Study of Dog Domestication: Application to the Prehistoric Site of Peña Moñuz (Guadalajara, Spain). Heritage 2025, 8, 34. [Google Scholar] [CrossRef]
- Hervella, M.; San-Juan-Nó, A.; Aldasoro-Zabala, A.; Mariezkurrena, K.; Altuna, J.; de-la-Rua, C. The domestic dog that lived ∼17,000 years ago in the Lower Magdalenian of Erralla site (Basque Country): A radiometric and genetic analysis. J. Archaeol. Sci. Rep. 2022, 46, 103706. [Google Scholar]
- Altuna, J. Fauna de mamíferos de los yacimientos prehistóricos de Guipúzcoa. Munibe 1972, 24, XXIV. [Google Scholar]
- Altuna, J. Hallazgo de un cuon (Cuon alpinus pallas) en Obarreta, Gorbea Vizcaya. Kobie 1983, 13, XIII. [Google Scholar]
- Álvarez-Alonso, D.; Yravedra, J. La excavación arqueológica en la zona B de la Cueva de Coímbre (Asturias, España): Campañas 2008–2012. In La cueva de Coímbre (Peñamellera Alta, Asturias): Ocupaciones Humanas en el Valle del Cares Durante el Paleolítico Superior; Álvarez-Alonso, D., Yravedra, J., Eds.; Fundación María Cristina Masaveu Peterson: Madrid, Spain, 2017; pp. 130–156. [Google Scholar]
- Álvarez-Alonso, D.; Yravedra, J.; Jordá Pardo, J.F.; Arrizabalaga, A. The Magdalenian sequence at Coímbre cave (Asturias, Northern Iberian Peninsula): Adaptative strategies of hunter-gatherer groups in montane environments. Quat. Int. 2016, 402, 100–111. [Google Scholar]
- Álvarez-Alonso, D. La Cueva de Coímbre (Asturias, España): Localización e historia de su investigación. In La Cueva de Coímbre (Peñamellera Alta, Asturias): Ocupaciones Humanas en el Valle del Cares Durante el Paleolítico Superior; Álvarez-Alonso, D., Yravedra, J., Eds.; Fundación María Cristina Masaveu Peterson: Madrid, Spain, 2017; pp. 74–100. [Google Scholar]
- Meléndez-Asensio, M.; Ballesteros, D. La geomorfología de la Cueva de Coímbre (Asturias, España): Ubicación del yacimiento arqueológico en la evolución del karst. In La Cueva de Coímbre (Peñamellera Alta, Asturias): Ocupaciones Humanas en el Valle del Cares Durante el Paleolítico Superior; Álvarez-Alonso, D., Yravedra, J., Eds.; Fundación María Cristina Masaveu Peterson: Madrid, Spain, 2017; pp. 158–169. [Google Scholar]
- Álvarez-Alonso, D.; Yravedra, J.; Arrizabalaga, A.; Jordá Pardo, J.F.; Heredia, N. La cueva de Coímbre (Peñamellera Alta, Asturias, España): Su yacimiento arqueológico y su santuario rupestre. Un estado de la cuestión en 2008. Munibe 2009, 60, 139–155. [Google Scholar]
- Álvarez-Alonso, D.; Jordá-Pardo, J. Secuencia estratigráfica, radiocarbono y cronoestratigrafía del registro del Pleistoceno superior de la Zona B de la Cueva de Coímbre (Asturias, España). In La cueva de Coímbre (Peñamellera Alta, Asturias): Ocupaciones humanas en el Valle del Cares Durante el Paleolítico Superior; Álvarez-Alonso, D., Yravedra, J., Eds.; Fundación María Cristina Masaveu Peterson: Madrid, Spain, 2017; pp. 194–218. [Google Scholar]
- Yravedra, J.; Álvarez-Alonso, D.; Estaca-Gómez, V.; López-Cisneros, P.; Arrizabalaga, Á.; Elorza, M.; Iriarte, M.J.; Pardo, J.F.J.; Sesé, C.; Uzquiano, P. New evidence of bones used as fuel in the Gravettian level at Coímbre cave, northern Iberian Peninsula. Archaeol. Anthropol. Sci. 2016, 9, 1153–1168. [Google Scholar] [CrossRef]
- Yravedra, J.; Álvarez-Alonso, D.; Estaca, V.; López-Cisneros, P.; Andrés-Chaín, M.; Arrizabalaga, Á.; Jordá Pardo, J.F.; Elorza, M.; Iriarte-Chiapusso, M.-J.; Sesé, C.; et al. Selection and Exploitation of Macro-Vertebrate Resources During the Upper Palaeolithic in Northern Spain. New Evidence from Coímbre Cave (Peñamellera Alta, Asturias). Oxf. J. Archaeol. 2017, 36, 331–354. [Google Scholar]
- López-Cisneros, P.; Linares-Matás, G.; Yravedra, J.; Maté-González, M.Á.; Estaca-Gómez, V.; Mora, R.; Aramendi, J.; Rodríguez Asensio, J.A.; Barrera-Logares, J.M.; González Aguilera, D. Applying new technologies to the taphonomic study of La Lluera (Asturias, Spain). Geometric morphometrics and the study of bone surface modifications (BSM). Quat. Int. 2019, 517, 107–117. [Google Scholar]
- Sutclife, A.J. Spotted hyaena: Crusher, gnawer, digester and collector of bones. Nature 1970, 227, 1110–1113. [Google Scholar]
- Haynes, G. Evidence of carnivore gnawing on Pleistocene and recent mammalian bones. Paleobiology 1980, 6, 341–351. [Google Scholar]
- Maguire, J.M.; Pemberton, D.; Collet, M.H. The Makapansgat limeworks grey breccia: Hominids, hyenas, hystricids or hillwash? Paleontol. Afr. 1980, 23, 75–98. [Google Scholar]
- Binford, L.R. Bones: Ancient Men and Modern Myths; Academic Press: New York, NY, USA, 1981. [Google Scholar]
- Brain, C.K. The Hunters or the Hunted? Introduction to African Cave Taphonomy; University of Chicago Press: Chicago, IL, USA, 1983. [Google Scholar]
- Horwitz, L.K.; Smith, P. The effects of striped hyaena activity on human remains. J. Archaeol. Sci. 1988, 15, 471–481. [Google Scholar]
- Selvaggio, M.M. Carnivore tooth marks and stone tool butchery marks on scavenged bones: Archaeological implications. J. Hum. Evol. 1994, 27, 215–228. [Google Scholar] [CrossRef]
- Blumenschine, R.J. Percussion marks, tooth marks and the experimental determinations of the timing of hominin and carnivore access to long bones at FLK Zinjanthropus, Olduvai Gorge, Tanzania. J. Hum. Evol. 1995, 29, 21–51. [Google Scholar]
- Fisher, J. Bone surface modifications in zooarchaeology. J. Archaeol. Method Theory 1995, 2, 7–68. [Google Scholar]
- D’Errico, F.; Villa, P. Hooles and Grooves: The Contribution of microscopy and taphonomy to the problem of art origins. J. Hum. Evol. 1997, 33, 1–31. [Google Scholar] [CrossRef]
- Villa, P.; Bartram, L. Flaked bone from a hyena den. Paleo 1996, 8, 143–159. [Google Scholar]
- Yravedra, J.; Lagos, L.; Bárcena, F. A taphonomic study of wild wolf (Canis lupus) modification of horse bones in northwestern Spain. J. Taphon. 2011, 9, 37–66. [Google Scholar]
- Courtenay, L.A.; Yravedra, J.; Maté-González, M.A.; Vázquez-Rodríguez, J.M.; Fernández-Fernández, M.; González-Aguilera, D. The effects of prey size on carnivore tooth mark morphologies on bone: The case study of Canis lupus signatus. Hist. Biol. 2020, 33, 2760–2772. [Google Scholar] [CrossRef]
- Gidna, A.; Yravedra, J.; Domínguez-Rodrigo, M. A cautionary note on the use of captive carnivores to model wild predator behavior: A comparison of bone modification patterns on long bones by captive and wild lions. J. Archaeol. Sci. 2013, 40, 1903–1910. [Google Scholar] [CrossRef]
- Sala, N.; Arsuaga, J.L.; Haynes, G. Taphonomic comparison of bone modifications caused by wild and captive wolves Canis lupus. Quat. Int. 2014, 330, 126–135. [Google Scholar] [CrossRef]
- Mora, R.; Aramendi, J.; Courtenay, L.A.; González-Aguilera, D.; Yravedra, J.; Maté-González, M.A.; Prieto-Herráez, D.; Vázquez-Rodríguez, J.M.; Barja, I. Ikhnos: A Novel Software to Register and Analyze Bone Surface Modifications Based on Three-Dimensional Documentation. Animals 2022, 12, 2861. [Google Scholar] [CrossRef]
- Courtenay, L.; Yravedra, J.; Herranz-Rodrigo, D.; Rodríguez, J.J.; Serrano-Ramos, A.; Estaca-Gómez, V.; Gonzálo-Aguilera, D.; Solano, J.A.; Jiménez-Arenas, J.M. Deciphering carnivoran competition for animal resources at the 1.46 Ma early Pleistocene site of Barranco León (Orce, Granada, Spain). Quat. Sci. Rev. 2022, 300, 107912. [Google Scholar] [CrossRef]
- Herranz-Rodrigo, D.; Tardáguila-Giacomozzi, S.J.; Courtenay, L.A.; Rodríguez-Alba, J.-J.; Garrucho, A.; Recuero, J.; Yravedra, J. New Geometric Morphometric Insights in Digital Taphonomy: Analyses into the Sexual Dimorphism of Felids through Their Tooth Pits. Appl. Sci. 2021, 11, 7848. [Google Scholar] [CrossRef]
- Yravedra, J.; Fosse, P.; Andrés, M.; Besson, J.P. Taphonomic analysis of small ungulates modified by fox (Vulpes vulpes) in Southwestern Europe. J. Taphono. 2014, 12, 37–67. [Google Scholar]
- Rodríguez Alba, J.J.; Linares-Matás, G.; Yravedra, J. First assessments of the taphonomic behaviour of jaguar (Panthera onca). Quat. International. 2019, 517, 88–96. [Google Scholar] [CrossRef]
- Adams, D.C.; Collyer, M.; Otarola-Castillo, E.; Sherratt, E. Geometric Morphometric Analyses of 2D/3D Landmarks Data. Package ‘Geomorph’. CRAN. 2014. Available online: https://geomorphr.r-universe.dev/geomorph (accessed on 20 October 2024).
- O’Higgins, P.; Jones, N. Facial growth in Cercocebus torquatus: An application of three-dimensional geometric morphometry techniques to the study of morphological variation. J. Anat. 1998, 193, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Gower, J.C. Generalized Procrustes analysis. Psychometrika 1975, 40, 33–50. [Google Scholar] [CrossRef]
- Ten-Berge, J.M.F. Orthogonal Procrustes rotation for two or more matrices. Psychometrika 1977, 42, 267–276. [Google Scholar] [CrossRef]
- Goodall, C.R. Procrustes methods in the statistical analysis of shape (with discussion). J. R. Stat. Soc. 1991, 53, 285–339. [Google Scholar] [CrossRef]
- Kent, J.T. The complex Bingham distribution and shape analysis. J. R. Stat. Soc. 1994, 56, 285–299. [Google Scholar] [CrossRef]
- Dryden, I.L.; Mardia, K.V. Statistical Shape Analysis, with Applications in R, 2nd ed.; John Wiley & Sons: Chichester, UK, 2016; pp. 125–173. [Google Scholar]
- Courtenay, L.; González-Aguilera, D.; Lagüela, S.; del Pozo, S.; Ruiz-Mendez, C.; Barbero-García, I.; Román-Curto, C.; Cañueto, J.; Santos-Durán, C.; Cardeñoso-Álvarez, M.E.; et al. Hyperspectral imaging and robust statistics in non-melanoma skin cancer analysis. Biomed. Opt. Express 2021, 12, 5107–5127. [Google Scholar] [CrossRef]
- Brain, C.K. Some principles in the interpretation of bone accumulations associated with man. In Human Origins; Isaac, G.L., McCown, E.R., Eds.; W.A. Benjamin: Menlo Park, CA, USA, 1976; pp. 97–116. [Google Scholar]
- Gifford-González, D.P. Ethnographic analogues for interpreting modified bones: Some cases East Africa. In Bone Modifications; Bonnicsen, R., Sorg, M.H., Eds.; Center for the study for the first Americans: Orono, ME, USA, 1989; pp. 43–52. [Google Scholar]
- Saladié, P.; Rosas, A.; García-Tabernero, A.; Fidalgo, D.; Fero Meñe, M.; Ebana Ebana, C. An actualistic taphonomic modelo f human tooth marks on bone remains: A sample recovered in villages of continental Equatorial Guinea. J. Archaeol. Sci. Rep. 2024, 55, 104514. [Google Scholar]
- Oliver, J.S. Carcass processing by the Hadza: Bone breakage from butchery to consumption. In From Bones to Behavior: Ethnoarchaeological and Experimental Contributions to the Interpretation of Faunal Remains; Hudson, E.J., Ed.; Southern Illinois University, Center for Archaeological Investigations: Carbondale, IL, USA, 1993; pp. 200–227. [Google Scholar]
- Landt, M.J. Tooth marks and human consumption: Ethnoarchaeological mastication research among foragers of the Central African Republic. J. Archaeol. Sci. 2007, 34, 1629–1640. [Google Scholar] [CrossRef]
- Fernández-Jalvo, Y.; Andrews, P. When humans chew bones. J. Hum. Evol. 2011, 60, 117–123. [Google Scholar] [CrossRef]
- Saladié, P.; Rodríguez-Hidalgo, A.; Díez, C.; Martín-Rodríguez, P.; Carbonell, E. Range of bone modifications by human chewing. J. Archaeol. Sci. 2013, 40, 380–397. [Google Scholar] [CrossRef]
- White, T.D. Prehistoric Cannibalism at Mancos 5MTUMR-2346; Princeton University Press: Princeton, NJ, USA, 1992. [Google Scholar]
- White, T.D.; Toth, N. Carnivora and carnivory: Assessing hominid toothmarks in zooarchaeology. In Breathing Life into Fossils: Taphonomic Studies in Honor of C.K. (Bob) Brain; En, T.R., Pickering, K., Toth, S.M., Eds.; Stone Age Institute Press: Gosport, IN, USA, 2007; pp. 281–297. [Google Scholar]
- Laroulandie, V. Anthropogenic versus non-anthropogenic bird bone assemblages: New criteria for their distinction. In Biosphere to Lithosphere: New Studies in Vertebrate Taphonomy; O’Connor, T., Ed.; Proceedings of the 9th conference of ICAZ; Oxbow Books: Durham, UK, 2005; pp. 23–28. [Google Scholar]
- Sanchis Serra, A.; Morales Pérez, J.V.; Pérez Ripoll, M. Creación de un referente experimental para el estudio de las alteraciones causadas por dientes humanos sobre huesos de conejo. In La Investigación Experimental Aplicada a la Arqueología; Rodríguez, M.E.A., Baena Preysler, J., García González, D., Eds.; Imprenta Galindo: Ronda, Spain, 2011; pp. 343–349. [Google Scholar]
- Romero, A.J.; Díez, J.C.; Saladié, P. Mammal bone Surface alteration during human consumption: An experimental approach. J. Archaeol. Sci. Rep. 2016, 8, 82–89. [Google Scholar] [CrossRef]


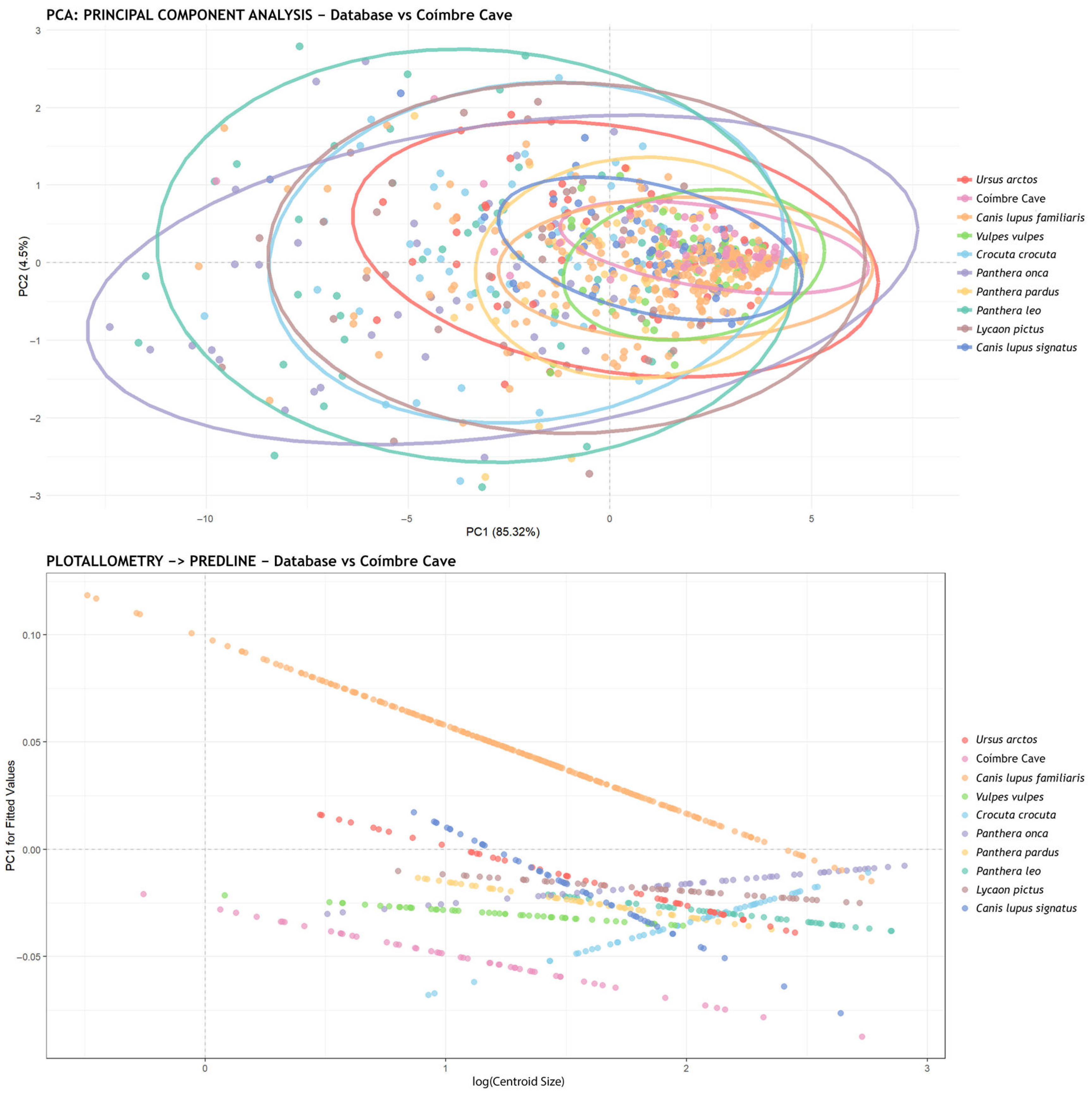
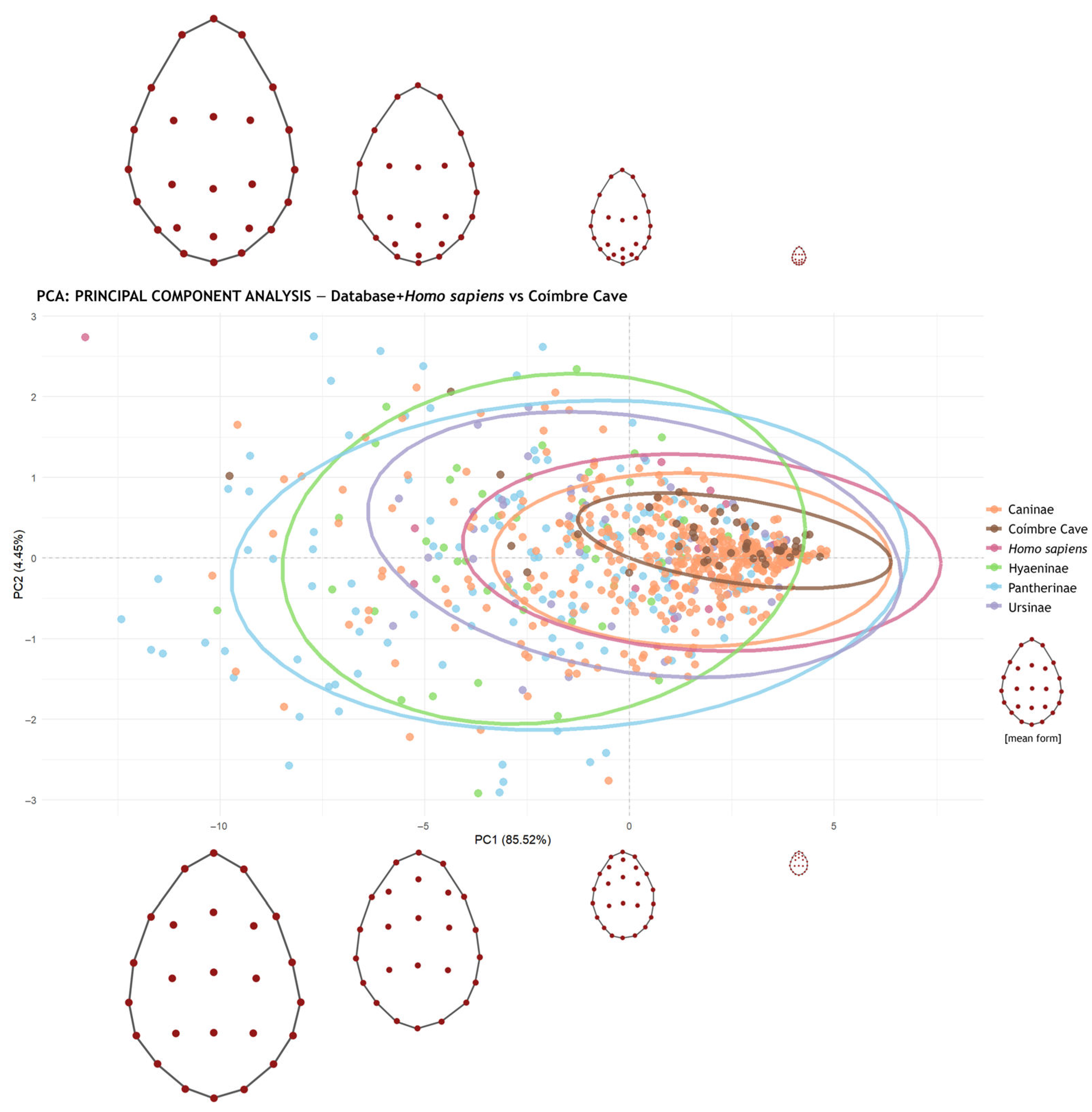
| Ursus arctos | Coímbre Cave | Canis lupus familiaris | Vulpes vulpes | Crocuta crocuta | Panthera onca | Panthera pardus | Panthera leo | Lycaon pictus | |
|---|---|---|---|---|---|---|---|---|---|
| Coímbre Cave | 0.001 | - | - | - | - | - | - | - | - |
| Canis lupus familiaris | 0.001 | 0.001 | - | - | - | - | - | - | - |
| Vulpes vulpes | 0.001 | 0.001 | 0.001 | - | - | - | - | - | - |
| Crocuta crocuta | 0.001 | 0.001 | 0.001 | 0.001 | - | - | - | - | - |
| Panthera onca | 0.001 | 0.001 | 0.001 | 0.001 | 0.617 | - | - | - | - |
| Panthera pardus | 0.001 | 0.001 | 0.001 | 0.014 | 0.001 | 0.001 | - | - | - |
| Panthera leo | 0.001 | 0.001 | 0.001 | 0.001 | 0.415 | 0.748 | 0.001 | - | - |
| Lycaon pictus | 0.045 | 0.001 | 0.001 | 0.001 | 0.486 | 0.253 | 0.006 | 0.024 | - |
| Canis lupus signatus | 0.066 | 0.001 | 0.004 | 0.001 | 0.001 | 0.001 | 0.005 | 0.001 | 0.004 |
| Caninae | Coímbre Cave | Homo sapiens | Hyaeninae | Pantherinae | |
|---|---|---|---|---|---|
| Coímbre Cave | 0.001 | - | - | - | - |
| Homo sapiens | 0.362 | 0.086 | - | - | - |
| Hyaeninae | 0.001 | 0.001 | 0.001 | - | - |
| Pantherinae | 0.001 | 0.001 | 0.001 | 0.951 | - |
| Ursinae | 0.001 | 0.001 | 0.066 | 0.001 | 0.009 |
| Coímbre Cave | |
|---|---|
| Canis lupus signatus | 0.001 |
| Canis lupus familiaris | 0.001 |
| Vulpes vulpes | 0.001 |
| Lycaon pictus | 0.001 |
| Crocuta crocuta | 0.001 |
| Panthera onca | 0.001 |
| Panthera pardus | 0.001 |
| Panthera leo | 0.001 |
| Ursus arctos | 0.001 |
| Homo sapiens | 0.086 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claver, I.; Estaca, V.; de Andrés-Herrero, M.; Herranz-Rodrigo, D.; Álvarez-Alonso, D.; Yravedra, J. Uncovering Human Tooth Marks in the Search for Dog Domestication: The Case of Coímbre Cave. Animals 2025, 15, 1319. https://doi.org/10.3390/ani15091319
Claver I, Estaca V, de Andrés-Herrero M, Herranz-Rodrigo D, Álvarez-Alonso D, Yravedra J. Uncovering Human Tooth Marks in the Search for Dog Domestication: The Case of Coímbre Cave. Animals. 2025; 15(9):1319. https://doi.org/10.3390/ani15091319
Chicago/Turabian StyleClaver, Idoia, Verónica Estaca, María de Andrés-Herrero, Darío Herranz-Rodrigo, David Álvarez-Alonso, and José Yravedra. 2025. "Uncovering Human Tooth Marks in the Search for Dog Domestication: The Case of Coímbre Cave" Animals 15, no. 9: 1319. https://doi.org/10.3390/ani15091319
APA StyleClaver, I., Estaca, V., de Andrés-Herrero, M., Herranz-Rodrigo, D., Álvarez-Alonso, D., & Yravedra, J. (2025). Uncovering Human Tooth Marks in the Search for Dog Domestication: The Case of Coímbre Cave. Animals, 15(9), 1319. https://doi.org/10.3390/ani15091319








