D-Glucuronolactone Supplementation Enhances Production Performance, Eggshell Quality, and Liver Health in Laying Hens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Design and Animal Husbandry
2.2. Laying Performance
2.3. Egg Quality
2.4. Sample Collection
2.5. Clinical Blood Parameters
2.6. Liver Index
2.7. Hepatic Lipid Accumulation
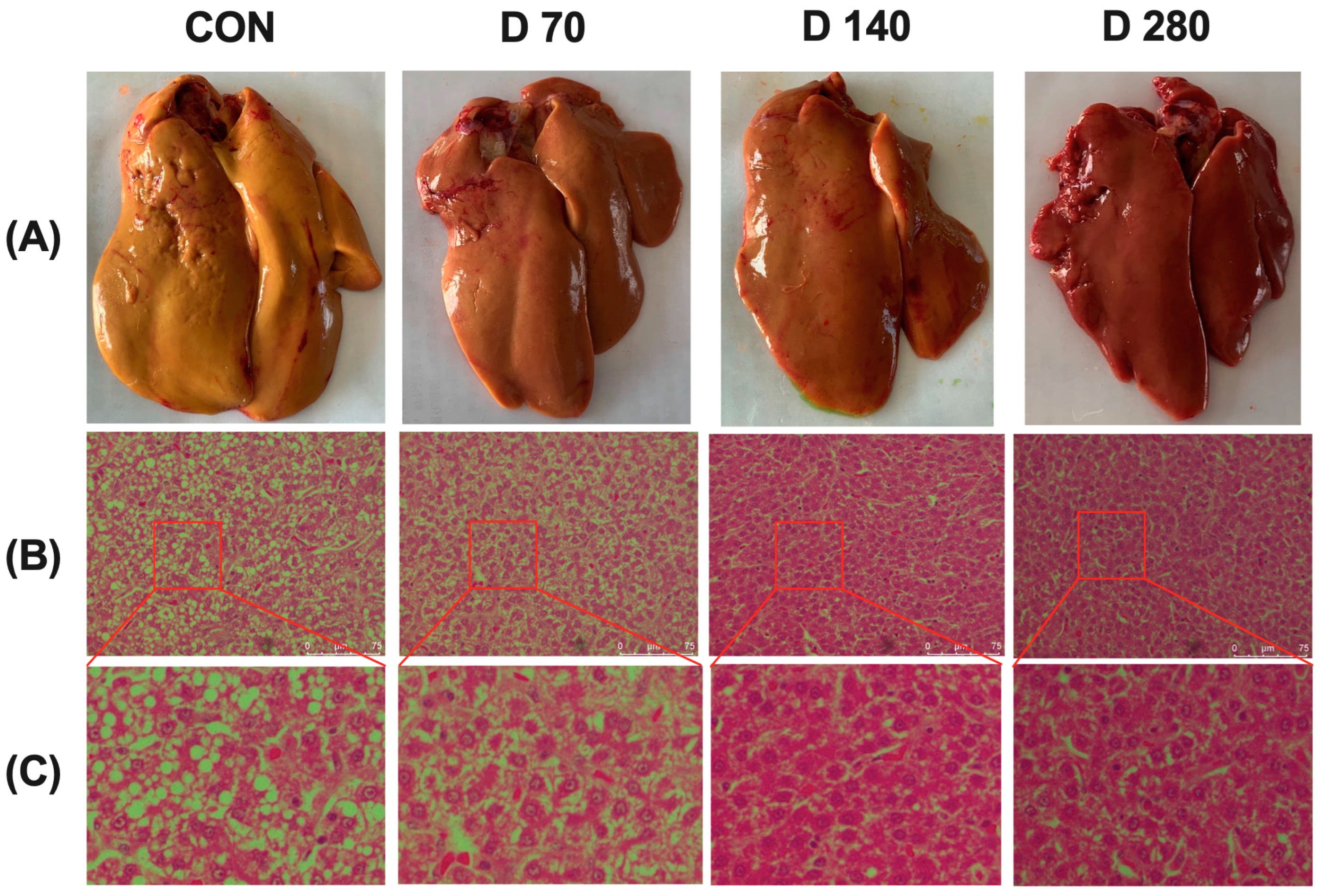
2.8. Histological Examination of the Liver Tissue
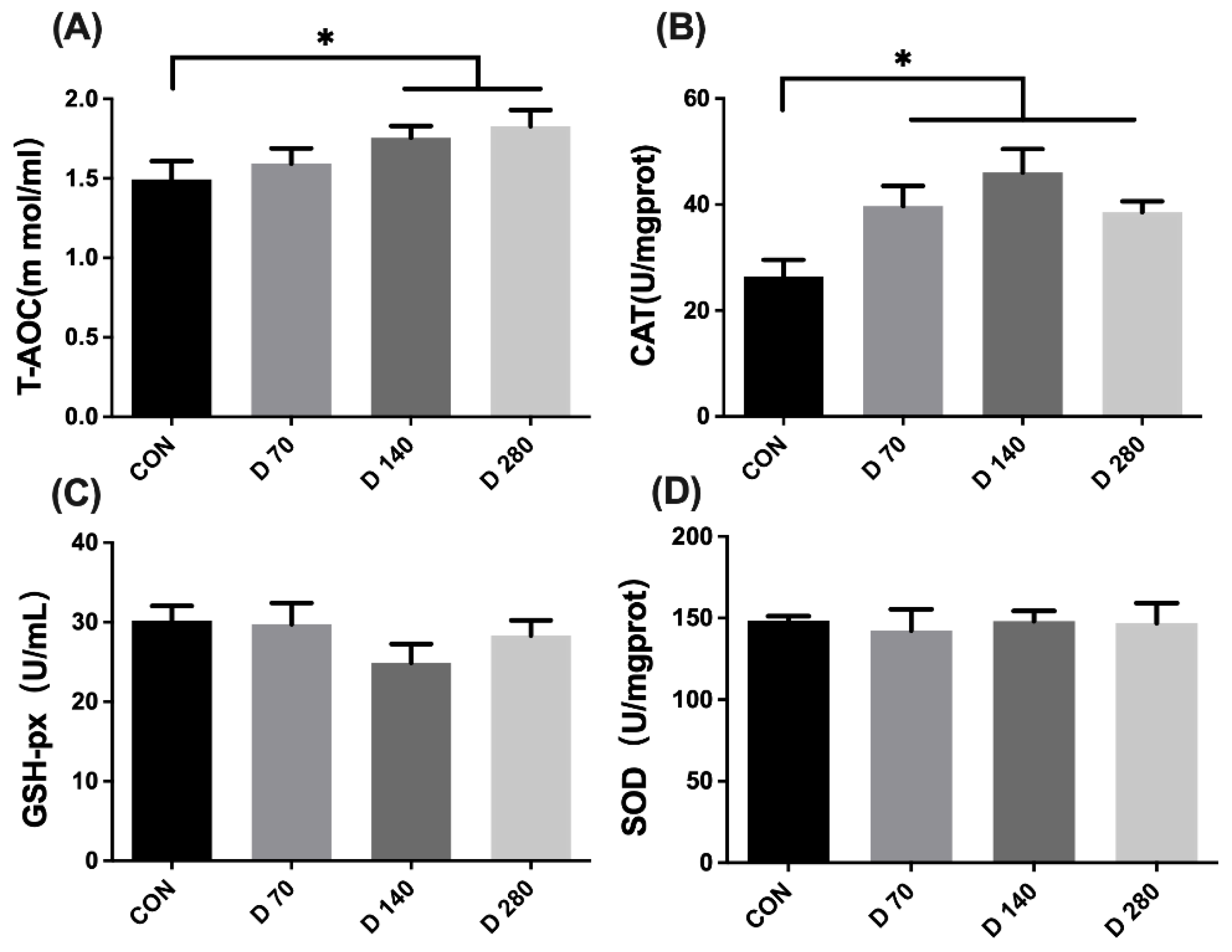
2.9. Antioxidant Capacity
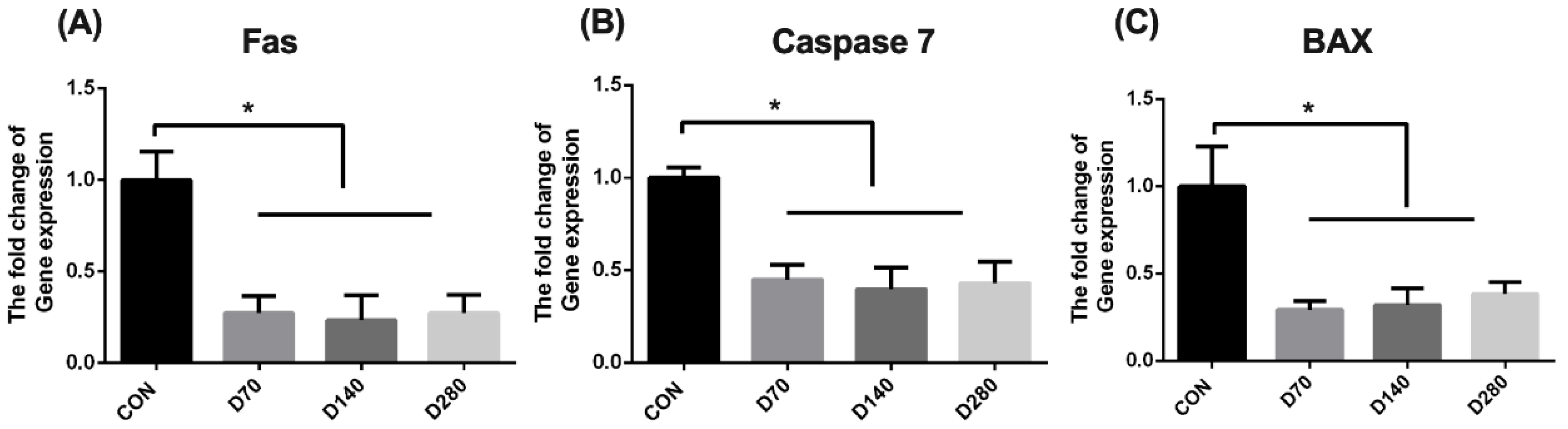
2.10. Hepatic Gene Expression Analysis
2.11. Statistical Analysis
3. Results
3.1. Effect of DGL on Laying Performance
3.2. Effect of DGL on Egg Quality
3.3. Effect of DGL on Blood Biochemical Parameters
3.4. Effect of DGL on Liver Indexes and Liver Lipids
3.5. Effect of DGL on Liver Histopathological Changes
3.6. Effect of DGL on Antioxidant Capacity
3.7. Effect of DGL on Inflammatory Response
3.8. Effect of DGL on Cell Apoptosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADFI | average daily feed intake |
| AEW | average egg weight |
| ANOVA | a one-way analysis of variance |
| ALP | alkaline phosphatase |
| ALT | alanine aminotransferase |
| AST | aspartate aminotransferase |
| BAX | BCL2 Associated X Protein |
| Caspase 7 | Cysteine aspartic acid specific protease 7 |
| CAT | catalase |
| DGL | D-glucuronolactone |
| Fas | factor-related apoptosis |
| FCR | feed conversion ratio |
| GGT | glutamyl transferase |
| GSH-Px | glutathione peroxidase |
| HE | hematoxylin–eosin |
| IFN-γ | interferon-gamma |
| IL-10 | interleukin-10 |
| IL-1β | interleukin 1beta |
| IL-6 | interleukin-6 |
| IL-8 | interleukin-8 |
| ROS | reactive oxygen species |
| SEM | standard error of mean |
| SOD | superoxide dismutase |
| T-AOC | total antioxidant capacity |
| TBIL | total bilirubin |
| TC | total cholesterol |
| TG | triglyceride |
| TNF-α | tumor necrosis factor-alpha |
| β-actin | beta-actin |
References
- Zaefarian, F.; Abdollahi, M.R.; Cowieson, A.; Ravindran, V. Avian liver: The forgotten organ. Animals 2019, 9, 63. [Google Scholar] [CrossRef] [PubMed]
- May-Panloup, P.; Boucret, L.; De La Barca, J.M.C.; Desquiret-Dumas, V.; Ferré-L’Hotellier, V.; Morinière, C.; Descamps, P.; Procaccio, V.; Reynier, P. Ovarian ageing: The role of mitochondria in oocytes and follicles. Hum. Reprod. Update 2016, 22, 725–743. [Google Scholar] [CrossRef] [PubMed]
- Różewicz, M. Effect of Age on Egg Quality of Lakenvelder Hens Kept Under Extensive Rearing Conditions. Int. J. Poult.-Ornam. Birds Sci. Technol. 2023, 4, 1–7. [Google Scholar]
- Gu, Y.F.; Chen, Y.P.; Jin, R.; Wang, C.; Wen, C.; Zhou, Y.M. A comparison of intestinal integrity, digestive function, and egg quality in laying hens with different ages. Poult. Sci. 2021, 100, 100949. [Google Scholar] [CrossRef]
- Abbas, A.O.; Alaqil, A.A.; El-Beltagi, H.S.; El-Atty, H.K.A.; Kamel, N.N. Modulating Laying Hens Productivity and Immune Performance in Response to Oxidative Stress Induced by E. coli Challenge Using Dietary Propolis Supplementation. Antioxidants 2020, 9, 893. [Google Scholar] [CrossRef]
- Wang, J.; Jia, R.; Gong, H.; Celi, P.; Zhuo, Y.; Ding, X.; Bai, S.; Zeng, Q.; Yin, H.; Xu, S.; et al. The Effect of Oxidative Stress on the Chicken Ovary: Involvement of Microbiota and Melatonin Interventions. Antioxidants 2021, 10, 1422. [Google Scholar] [CrossRef]
- Gloux, A.; Duclos, M.J.; Brionne, A.; Bourin, M.; Nys, Y.; Réhault-Godbert, S. Integrative analysis of transcriptomic data related to the liver of laying hens: From physiological basics to newly identified functions. BMC Genom. 2019, 20, 821. [Google Scholar] [CrossRef]
- Shini, A.; Shini, S.; Bryden, W.L. Fatty liver haemorrhagic syndrome occurrence in laying hens: Impact of production system. Avian Pathol. 2019, 48, 25–34. [Google Scholar] [CrossRef]
- Muñoz-Solano, B.; Pérez, E.L.; González-Peñas, E. Monitoring Mycotoxin Exposure in Food-Producing Animals (Cattle, Pig, Poultry, and Sheep). Toxins 2024, 16, 218. [Google Scholar] [CrossRef]
- Ricciutelli, M.; Caprioli, G.; Cortese, M.; Lombardozzi, A.; Strano, M.; Vittori, S.; Sagratini, G. Simultaneous determination of taurine, glucuronolactone and glucuronic acid in energy drinks by ultra high performance liquid chromatography-tandem mass spectrometry (triple quadrupole). J. Chromatogr. A 2014, 1364, 303–307. [Google Scholar] [CrossRef]
- Zółtaszek, R.; Hanausek, M.; Kiliańska, Z.M.; Walaszek, Z. The biological role of D-glucaric acid and its derivatives: Potential use in medicine. Postep. Hig. Med. Dosw. 2008, 62, 451–462. [Google Scholar]
- Serfling, S.E.; Buck, A.; Rowe, S.P.; Higuchi, T.; Werner, R. Red Bull PET/CT. Nuklearmedizin 2024, 63, 76–77. [Google Scholar] [CrossRef] [PubMed]
- Rubio, C.; Cámara, M.; Giner, R.M.; González-Muñoz, M.J.; López-García, E.; Morales, F.J.; Moreno-Arribas, M.V.; Portillo, M.P.; Bethencourt, E. Caffeine, D-glucuronolactone and Taurine Content in Energy Drinks: Exposure and Risk Assessment. Nutrients 2022, 14, 5103. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.M.; Church, R.J.; Lewander, W. Energy drinks: The new eye-opener for adolescents. Clin. Pediatr. Emerg. Med. 2008, 9, 35–42. [Google Scholar] [CrossRef]
- Yu, Z.; Wu, F.; Tian, J.; Guo, X.; An, R. Protective effects of compound ammonium glycyrrhizin, L-arginine, silymarin and glucurolactone against liver damage induced by ochratoxin A in primary chicken hepatocytes. Mol. Med. Rep. 2018, 18, 2551–2560. [Google Scholar] [CrossRef]
- Kratzer, F.H.; Almquist, H.J.; Vohra, P. Effect of diet on growth and plasma ascorbic acid in chicks. Poult. Sci. 1996, 75, 82–89. [Google Scholar] [CrossRef]
- Aguilar, F.; Charrondiere, U.; Dusemund, B.; Galtier, P.; Gilbert, J.; Gott, D.; Grilli, S.; Guertler, R.; Kass, G.; Koenig, J.; et al. The use of taurine and D-glucurono-γ-lactone as constituents of the so-called “energy” drinks. EFSA J. 2009, 935, 1–31. [Google Scholar]
- Berbert; Queiroz, D.M.; Melo, E.C. Official Methods of Analysis of AOAC International, 18th ed.; AOAC: Washington, DC, USA, 2005. [Google Scholar]
- Ma, G.; Ayalew, H.; Mahmood, T.; Mercier, Y.; Wang, J.; Lin, J.; Wu, S.; Qiu, K.; Qi, G.; Zhang, H. Methionine and vitamin E supplementation improve production performance, antioxidant potential, and liver health in aged laying hens. Poult. Sci. 2024, 103, 104415. [Google Scholar] [CrossRef]
- Yao, H.; Hu, Y.; Wang, Q.; Zhang, Y.; Rao, K.; Shi, S. Effects of dietary dimethylglycine supplementation on laying performance, egg quality, and tissue index of hens during late laying period. Poult. Sci. 2022, 101, 101610. [Google Scholar] [CrossRef]
- Dai, H.; Lv, Z.; Hu, C.; Shi, Z.; Wei, X.; Jin, S.; Yuan, Y.; Yu, D.; Shi, F. Alpha-lipoic acid improves the reproduction performance of breeder hens during the late egg-laying period. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1788–1797. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, X.; Wang, B.; Zhou, H.; Dang, S.; Shi, Y.; Hao, L.; Luo, Q.; Jin, M.; Zhou, Q.; et al. Aging-associated oxidative stress inhibits liver progenitor cell activation in mice. Aging 2017, 9, 1359–1374. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Huang, T.W.; Peng, Y.J.; Lin, Y.Y.; Mersmann, H.J.; Ding, S.T. A novel chicken model of fatty liver disease induced by high cholesterol and low choline diets. Poult. Sci. 2021, 100, 100869. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, J.; Obianwuna, U.E.; Long, C.; Qiu, K.; Zhang, H.; Qi, X.; Wu, S. Optimizing selenium-enriched yeast supplementation in laying hens: Enhancing egg quality, selenium concentration in eggs, antioxidant defense, and liver health. Poult Sci. 2025, 104, 104584. [Google Scholar] [CrossRef]
- Yang, Y.; Shu, X.; Javed, H.U.; Wu, Q.; Liu, H.; Han, J.; Zhou, H. Dietary supplementation of poly-dihydromyricetin-fused zinc nanoparticles alleviates fatty liver hemorrhagic syndrome by improving antioxidant capacity, intestinal health and lipid metabolism of laying hens. Poult. Sci. 2024, 103, 104301. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, J.; Zhou, Q.; Yu, D. Tolerance and safety evaluation of sodium sulfate: A subchronic study in laying hens. Anim. Nutr. 2021, 7, 576–586. [Google Scholar] [CrossRef]
- Chen, N.N.; Liu, B.; Xiong, P.W.; Guo, Y.; He, J.N.; Hou, C.C.; Ma, L.X.; Yu, D.Y. Safety evaluation of zinc methionine in laying hens: Effects on laying performance, clinical blood parameters, organ development, and histopathology—ScienceDirect. Poult. Sci. 2018, 97, 1120–1126. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhong, F.Y.; Wu, M.; Zhang, X.P. Efficacy of Jian’ganle versus Hugan Pian, glucuronolactone and reduced glutathione in prevention of antituberculosis drug-induced liver injury. J. Huazhong Univ. Sci. Technol. Med. Sci. 2014, 34, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Zhou, G.; Zou, S.; Yang, W.; Liu, A.; Sun, S.; Xie, B. Metabonomics of d-glucaro-1,4-lactone in preventing diethylnitrosamine-induced liver cancer in rats. Pharm. Biol. 2018, 56, 643–648. [Google Scholar] [CrossRef]
- Cai, H.; Zhang, Y.; Wang, J.; Deng, Y.; Liu, J.; Wu, Z.; Cao, D.; Song, Z.; Wang, L.; Xie, B. D-glucaro-1,4-lactone improves Diethylnitrosamine induced hepatocellular carcinoma in rats via the uric acid-ROS pathway. J. Ethnopharmacol. 2024, 334, 118569. [Google Scholar] [CrossRef]
- Yunusa, I.; Ahmad, I. Energy-Drinks: Composition and Health Benefits. Bayero J. Pure Appl. Sci. 2011, 4, 186–191. [Google Scholar] [CrossRef]
- Xie, B.; Liu, A.; Zhan, X.; Ye, X.; Wei, J. Alteration of gut bacteria and metabolomes after glucaro-1,4-lactone treatment contributes to the prevention of hypercholesterolemia. J. Agric. Food Chem. 2014, 62, 7444–7451. [Google Scholar] [CrossRef] [PubMed]
- Marsh, C.A. Metabolism of D-glucuronolactone in mammalian systems. Inhibitory properties of the products of D-glucuronolactone-dehydrogenase action. Biochem. J. 1966, 99, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Chu, N.H.; Li, L.; Zhang, X.; Gu, J.; Du, Y.D.; Cai, C.; Xiao, H.P. Role of bicyclol in preventing drug-induced liver injury in tuberculosis patients with liver disease. Int. J. Tuberc. Lung Dis. 2015, 19, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Milne, G.L.; Nogueira, M.S.; Gao, B.; Sanchez, S.C.; Amin, W.; Thomas, S.; Oger, C.; Galano, J.M.; Murff, H.J.; Yang, G.; et al. Identification of novel F(2)-isoprostane metabolites by specific UDP-glucuronosyltransferases. Redox Biol. 2024, 70, 103020. [Google Scholar] [CrossRef]
- Trott, K.A.; Giannitti, F.; Rimoldi, G.; Hill, A.; Woods, L.; Barr, B.; Anderson, M.; Mete, A. Fatty liver hemorrhagic syndrome in the backyard chicken: A retrospective histopathologic case series. Vet. Pathol. 2014, 51, 787–795. [Google Scholar] [CrossRef]
- Shini, S.; Shini, A.; Bryden, W.L. Unravelling fatty liver haemorrhagic syndrome: 2. Inflammation and pathophysiology. Avian Pathol. 2020, 49, 131–143. [Google Scholar] [CrossRef]
- Engin, A. Lipid Storage, Lipolysis, and Lipotoxicity in Obesity. Adv. Exp. Med. Biol. 2024, 1460, 97–129. [Google Scholar]
- Gao, J.; Ruan, H.; Qi, X.; Guo, X.; Zheng, J.; Liu, C.; Fang, Y.; Huang, M.; Xu, M.; Shen, W. Increased apoptosis and abnormal visual behavior by histone modifications with exposure to para-xylene in developing Xenopus. Neuroscience 2016, 331, 177–185. [Google Scholar] [CrossRef]
- Li, H.; Hou, Y.; Chen, J.; Wu, H.; Huang, L.; Hu, J.; Zhang, Z.; Lu, Y.; Liu, X. Dietary naringin supplementation on laying performance and antioxidant capacity of Three-Yellow breeder hens during the late laying period. Poult. Sci. 2022, 101, 102023. [Google Scholar] [CrossRef]
- Xiaodi, E.; Shao, D.; Li, M.; Shi, S.; Xiao, Y. Supplemental dietary genistein improves the laying performance and antioxidant capacity of Hy-Line brown hens during the late laying period. Poult. Sci. 2023, 102, 102573. [Google Scholar]
- Samiullah, S.; Roberts, J.R.; Chousalkar, K. Eggshell color in brown-egg laying hens—A review. Poult Sci. 2015, 94, 2566–2575. [Google Scholar] [CrossRef] [PubMed]
- Samiullah, S.; Roberts, J.R. The location of protoporphyrin in the eggshell of brown-shelled eggs. Poult. Sci. 2013, 92, 2783–2788. [Google Scholar] [CrossRef] [PubMed]
- Hargitai, R.; Boross, N.; Nyiri, Z.; Eke, Z. Biliverdin- and protoporphyrin-based eggshell pigmentation in relation to antioxidant supplementation, female characteristics and egg traits in the canary (Serinus canaria). Behav. Ecol. Sociobiol. 2016, 70, 2093–2110. [Google Scholar] [CrossRef]
- Wang, X.T.; Zhao, C.J.; Li, J.Y.; Xu, G.Y.; Lian, L.S.; Wu, C.X.; Deng, X.M. Comparison of the total amount of eggshell pigments in Dongxiang brown-shelled eggs and Dongxiang blue-shelled eggs. Poult. Sci. 2009, 88, 1735–1739. [Google Scholar] [CrossRef]
- Lu, M.Y.; Xu, L.; Qi, G.H.; Zhang, H.J.; Qiu, K.; Wang, J.; Wu, S.G. Mechanisms associated with the depigmentation of brown eggshells: A review. Poult. Sci. 2021, 100, 101273. [Google Scholar] [CrossRef]

| Ingredients | Content (%) | Nutrient Levels 3 | Content (%) |
|---|---|---|---|
| Corn | 60.00 | Metabolizable energy (MJ/kg) | 11.34 |
| 43% Soybean meal | 28.43 | Crude protein | 17.05 |
| Soybean oil | 1.00 | Calcium | 3.52 |
| Limestone | 8.30 | Available phosphorus | 0.44 |
| Dicalcium phosphate | 1.50 | Lysine | 0.83 |
| DL-methionine(99%) | 0.15 | Cystine | 0.24 |
| NaCL | 0.30 | Methionine | 0.41 |
| 70% Choline chloride | 0.09 | ||
| Mineral premix 1 | 0.20 | ||
| Vitamin premix 2 | 0.03 | ||
| Total | 100.00 |
| Items | Genes | Primer Sequences (5′-3′) | Gene Bank No. |
|---|---|---|---|
| Inflammatory response | IL-1β | F: CCGAGGAGCAGGGACTTT R: AGGACTGTGAGCGGGTGT | NM_204524.1 |
| TNF-α | F: CAGGACAGCCTATGCCAACAAG R: GGTTACAGGAAGGGCAACTCATC | NM_204267.1 | |
| IFN-γ | F: CAAGCTCCCGATGAACGACTT R: AGTTGAGCACAGGAGGTCAT | NM_205149.1 | |
| IL-6 | F: TTTATGGAGAAGACCGTGAGG R: TGTGGCAGATTGGTAACAGAG | NM_204628.1 | |
| IL-8 | F: ATGAACGGCAAGCTTGGAGCTG R: TCCAAGCACACCTCTCTTCCATCC | NM_205498.1 | |
| IL-10 | F: GCTGAGGGTGAAGTTTGAG R: CAGGTGAAGAAGCGGTGA | NM_001004414.2 | |
| Cell apoptosis | Fas | F: TTGGACGAGTGTATGAGATG R:ACAGTGTCTGAAGTTGAAGT | XM_015288392.4 |
| Caspase 7 | F: TCTCTGTGCTCTGTGCTA R:AAGGAATCTGCTTCTTCTCA | XM_046920622.1 | |
| BAX | F:GTGATGGAGGTGACTGAAG R:AATCTGGTCCTGGCTGTT | XM_040693909.2 | |
| Reference gene | β-actin | F: TCAGGGTGTGATGGTTGGTATG R: TGTTCAATGGGGTACTTCAGGG | NM_205518.1 |
| Items | Groups | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | D 70 | D 140 | D 280 | ANOVA | Linear | Quadratic | ||
| Laying rate, % | ||||||||
| 1–4 week | 94.20 | 94.46 | 95.01 | 95.16 | 0.312 | 0.688 | 0.253 | 0.862 |
| 5–8 week | 91.88 | 92.57 | 93.83 | 93.51 | 0.483 | 0.483 | 0.803 | 0.522 |
| 9–12 week | 89.15 b | 91.92 ab | 91.98 ab | 95.16 a | 0.971 | 0.042 | 0.021 | 0.506 |
| 1–12 week | 91.74 b | 92.98 ab | 93.71 ab | 94.61 a | 0.432 | 0.022 | 0.002 | 0.689 |
| ADFI, g/hen/day | ||||||||
| 1–4 week | 119.95 | 119.29 | 122.50 | 120.33 | 0.338 | 0.101 | 0.712 | 0.388 |
| 5–8 week | 114.93 | 114.25 | 116.40 | 114.75 | 0.452 | 0.686 | 0.445 | 0.627 |
| 9–12 week | 124.10 | 120.01 | 122.62 | 125.80 | 0.701 | 0.538 | 0.508 | 0.606 |
| 1–12 week | 119.75 | 117.50 | 120.53 | 118.96 | 0.312 | 0.199 | 0.891 | 0.762 |
| AEW, g | ||||||||
| 1–4 week | 59.23 | 57.93 | 59.41 | 59.09 | 0.174 | 0.056 | 0.321 | 0.038 |
| 5–8 week | 59.85 a | 58.22 b | 59.93 ab | 59.01 ab | 0.173 | 0.016 | 0.112 | 0.029 |
| 9–12 week | 59.74 a | 57.77 b | 59.51 ab | 59.02 ab | 0.304 | 0.010 | 0.443 | 0.076 |
| 1–12 week | 59.60 a | 57.97 b | 59.55 a | 59.03 ab | 0.155 | 0.008 | 0.199 | 0.015 |
| Egg mass, g/day/hen | ||||||||
| 1–4 week | 55.79 ab | 54.72 b | 56.45 a | 56.23 a | 0.230 | 0.031 | 0.218 | 0.090 |
| 5–8 week | 54.99 ab | 53.89 b | 56.23 b | 55.17 ab | 0.307 | 0.044 | 0.349 | 0.452 |
| 9–12 week | 53.26 | 53.10 | 54.74 | 56.16 | 1.191 | 0.156 | 0.054 | 0.238 |
| 1–12 week | 54.68 ab | 53.90 b | 55.80 a | 55.85 a | 0.282 | 0.022 | 0.039 | 0.109 |
| FCR, feed(g)/egg(g) | ||||||||
| 1–4 week | 2.15 | 2.18 | 2.17 | 2.14 | 0.010 | 0.273 | 0.943 | 0.053 |
| 5–8 week | 2.09 | 2.12 | 2.07 | 2.08 | 0.012 | 0.454 | 0.481 | 0.396 |
| 9–12 week | 2.33 | 2.26 | 2.24 | 2.24 | 0.022 | 0.555 | 0.180 | 0.646 |
| 1–12 week | 2.19 | 2.18 | 2.16 | 2.13 | 0.011 | 0.210 | 0.056 | 0.384 |
| Mortality rate, % | 0 | 0 | 0 | 0 | —— | —— | —— | —— |
| Items | Groups | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | D 70 | D 140 | D 280 | ANOVA | Linear | Quadratic | ||
| Egg shape Index 1 | 1.33 | 1.33 | 1.32 | 1.32 | 0.004 | 0.545 | 0.285 | 0.647 |
| Shell strength, kg/cm2 | 3.84 | 4.33 | 4.37 | 4.60 | 0.097 | 0.082 | 0.091 | 0.452 |
| Shell ratio 2, % | 9.92 | 10.03 | 10.21 | 9.98 | 0.082 | 0.764 | 0.624 | 0.524 |
| Shell thickness, μm | 335.03 | 332.93 | 338.99 | 338.61 | 2.791 | 0.858 | 0.577 | 0.759 |
| Shell color | 27.79 a | 25.37 b | 25.40 b | 24.15 b | 0.392 | 0.004 | <0.001 | 0.669 |
| Yolk ratio 3, % | 28.75 | 28.08 | 28.89 | 36.58 | 1.924 | 0.365 | 0.228 | 0.220 |
| Yolk color | 6.48 | 6.55 | 6.35 | 6.05 | 0.078 | 0.256 | 0.128 | 0.190 |
| Albumen Ratio 4,% | 61.33 | 61.89 | 60.91 | 53.44 | 1.920 | 0.371 | 0.218 | 0.232 |
| Haugh unit | 76.75 | 79.48 | 79.80 | 79.18 | 1.101 | 0.770 | 0.389 | 0.549 |
| Items | Groups | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | D 70 | D 140 | D 280 | ANOVA | Linear | Quadratic | ||
| TBIL, U/L | 0.45 | 0.39 | 0.46 | 0.56 | 0.104 | 0.953 | 0.733 | 0.650 |
| ALT, U/L | 2.88 | 3.25 | 4.50 | 3.38 | 0.445 | 0.623 | 0.462 | 0.598 |
| AST, U/L | 182.88 a | 150.75 ab | 142.63 b | 134.50 b | 5.868 | 0.013 | 0.001 | 0.536 |
| ALP, U/L | 29.00 | 28.50 | 41.71 | 25.29 | 2.030 | 0.424 | 0.534 | 0.862 |
| AST/ALT | 76.95 | 75.40 | 58.04 | 66.72 | 2.353 | 0.894 | 0.586 | 0.946 |
| GGT, U/L | 56.88 a | 44.25 ab | 42.75 b | 32.88 b | 3.503 | 0.029 | <0.001 | 0.218 |
| Items | Groups | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| CON | D 70 | D 140 | D 280 | ANOVA | Linear | Quadratic | ||
| Liver index,% | 2.32 a | 2.19 ab | 1.94 b | 2.02 b | 0.051 | 0.030 | 0.198 | 0.007 |
| Liver fat, % | 20.13 a | 18.67 ab | 17.29 ab | 17.07 b | 0.429 | 0.018 | 0.001 | 0.179 |
| TG, mmol/g prot | 0.31 a | 0.30 a | 0.29 ab | 0.26 b | 0.009 | 0.036 | 0.231 | 0.425 |
| TC, mmol/g prot | 0.078 | 0.080 | 0.083 | 0.077 | 0.001 | 0.363 | 0.209 | 0.281 |
| Histopathology score | 1.50 | 1.63 | 2.01 | 2.62 | 0.151 | 0.078 | 0.018 | 0.264 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.; Miao, Z.; Zheng, Y.; Dong, Y.; Han, M.; Huang, C.; Bai, R.; Xia, C.; Shi, S.; Li, J. D-Glucuronolactone Supplementation Enhances Production Performance, Eggshell Quality, and Liver Health in Laying Hens. Animals 2025, 15, 1317. https://doi.org/10.3390/ani15091317
Shen Y, Miao Z, Zheng Y, Dong Y, Han M, Huang C, Bai R, Xia C, Shi S, Li J. D-Glucuronolactone Supplementation Enhances Production Performance, Eggshell Quality, and Liver Health in Laying Hens. Animals. 2025; 15(9):1317. https://doi.org/10.3390/ani15091317
Chicago/Turabian StyleShen, Yiru, Zhiqiang Miao, Yuqi Zheng, Yuanyang Dong, Miaomiao Han, Chenxuan Huang, Rui Bai, Chengqiang Xia, Shourong Shi, and Jianhui Li. 2025. "D-Glucuronolactone Supplementation Enhances Production Performance, Eggshell Quality, and Liver Health in Laying Hens" Animals 15, no. 9: 1317. https://doi.org/10.3390/ani15091317
APA StyleShen, Y., Miao, Z., Zheng, Y., Dong, Y., Han, M., Huang, C., Bai, R., Xia, C., Shi, S., & Li, J. (2025). D-Glucuronolactone Supplementation Enhances Production Performance, Eggshell Quality, and Liver Health in Laying Hens. Animals, 15(9), 1317. https://doi.org/10.3390/ani15091317







