Sectional Anatomy with Micro-Computed Tomography and Magnetic Resonance Imaging Correlation of the Middle and Caudal Abdominal Regions in the Syrian Hamster (Mesocricetus auratus)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Examined Animals
2.2. Radiological Procedures
2.3. Micro-CT Procedures
2.4. MRI Procedures
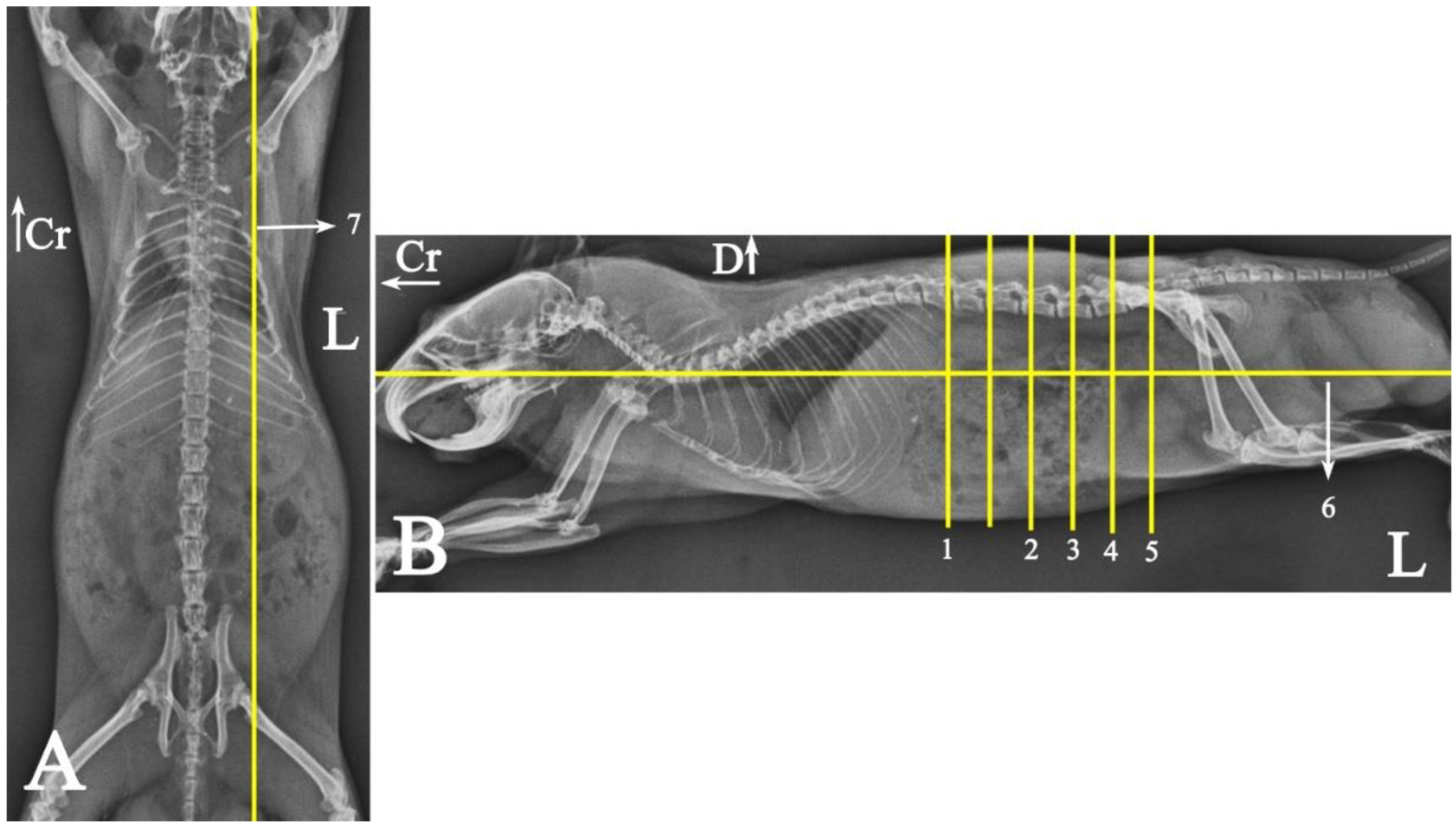
2.5. Anatomical Examinations
2.5.1. Topographic Anatomy
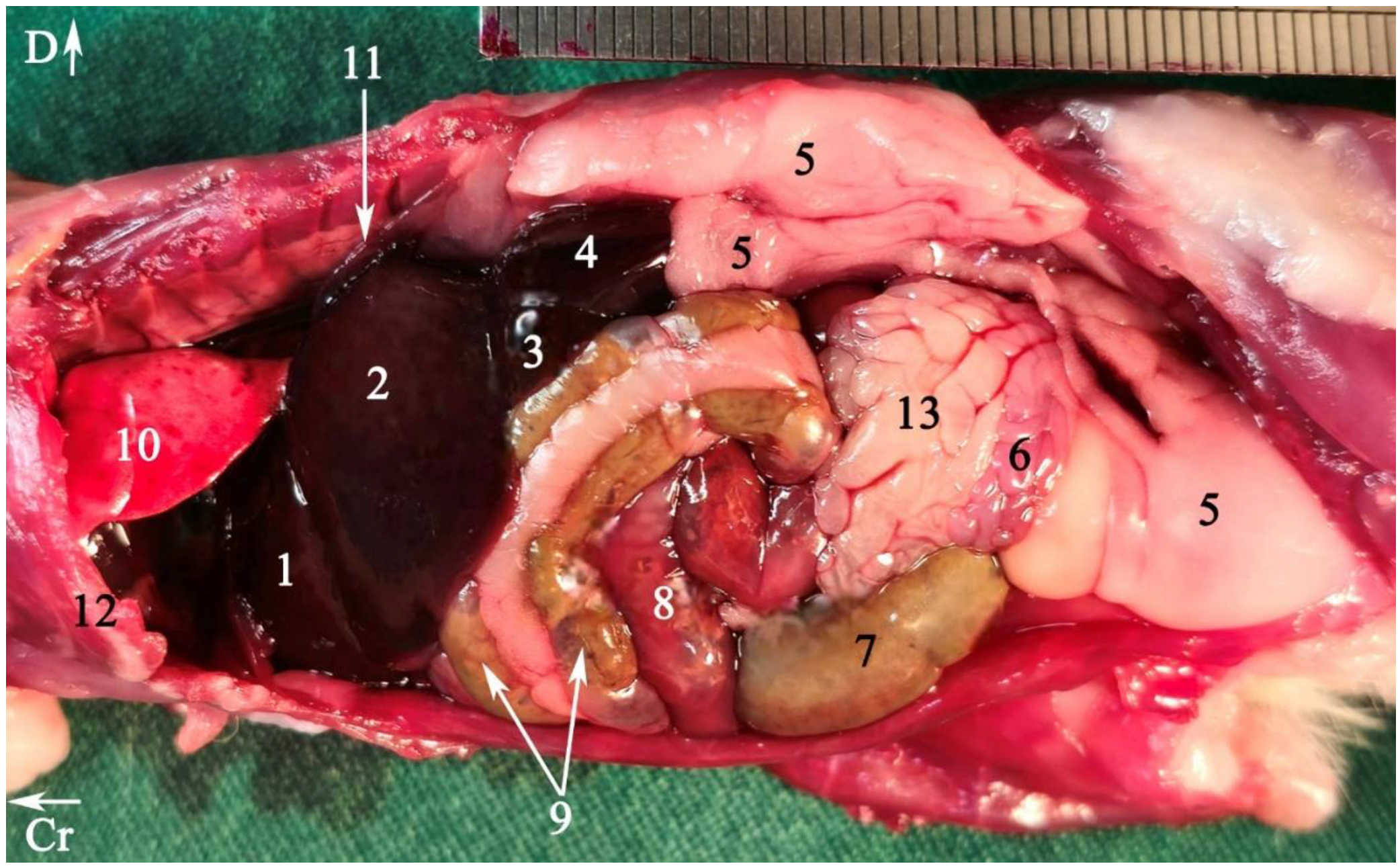
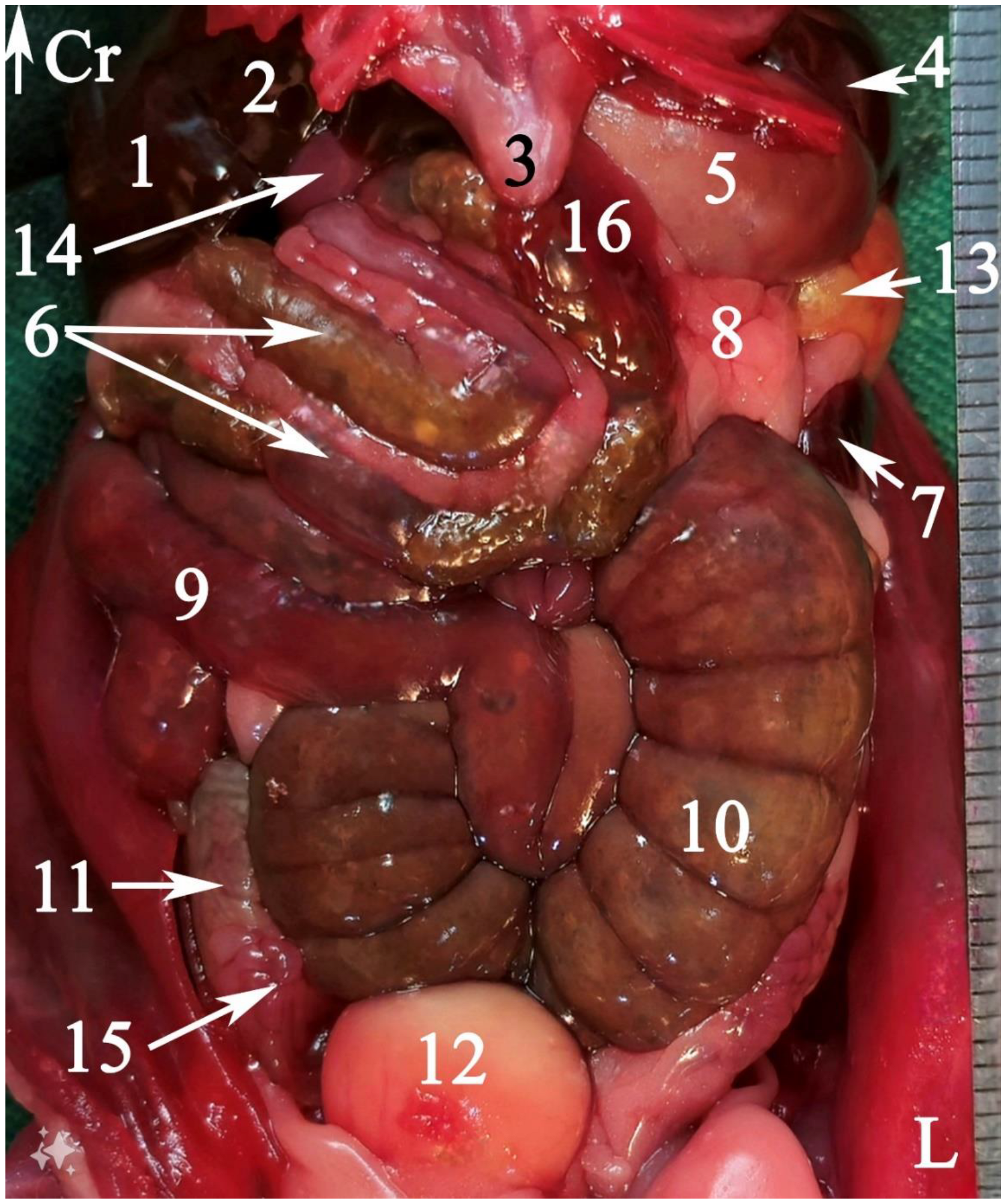
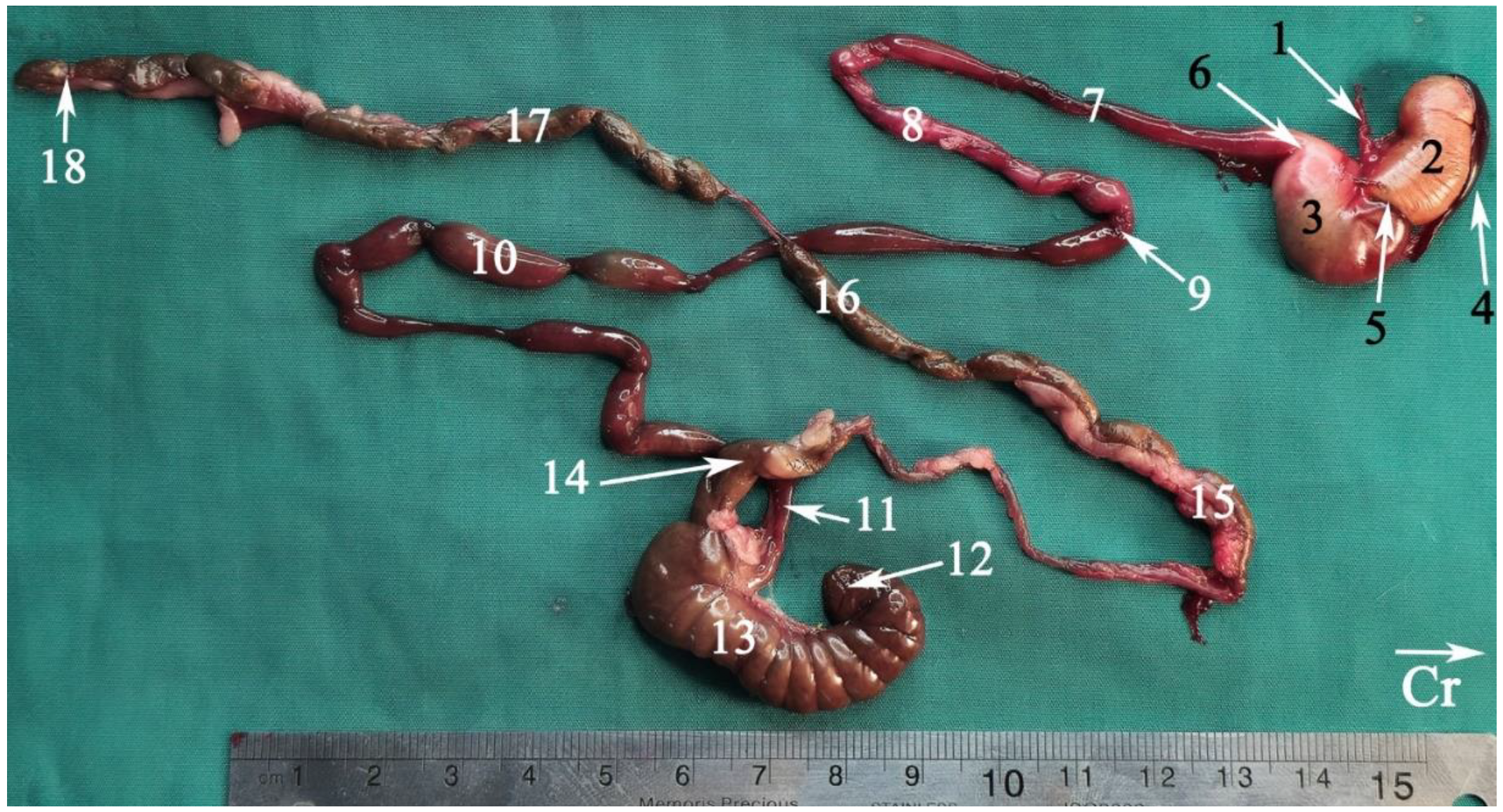
2.5.2. Anatomical Sectioning
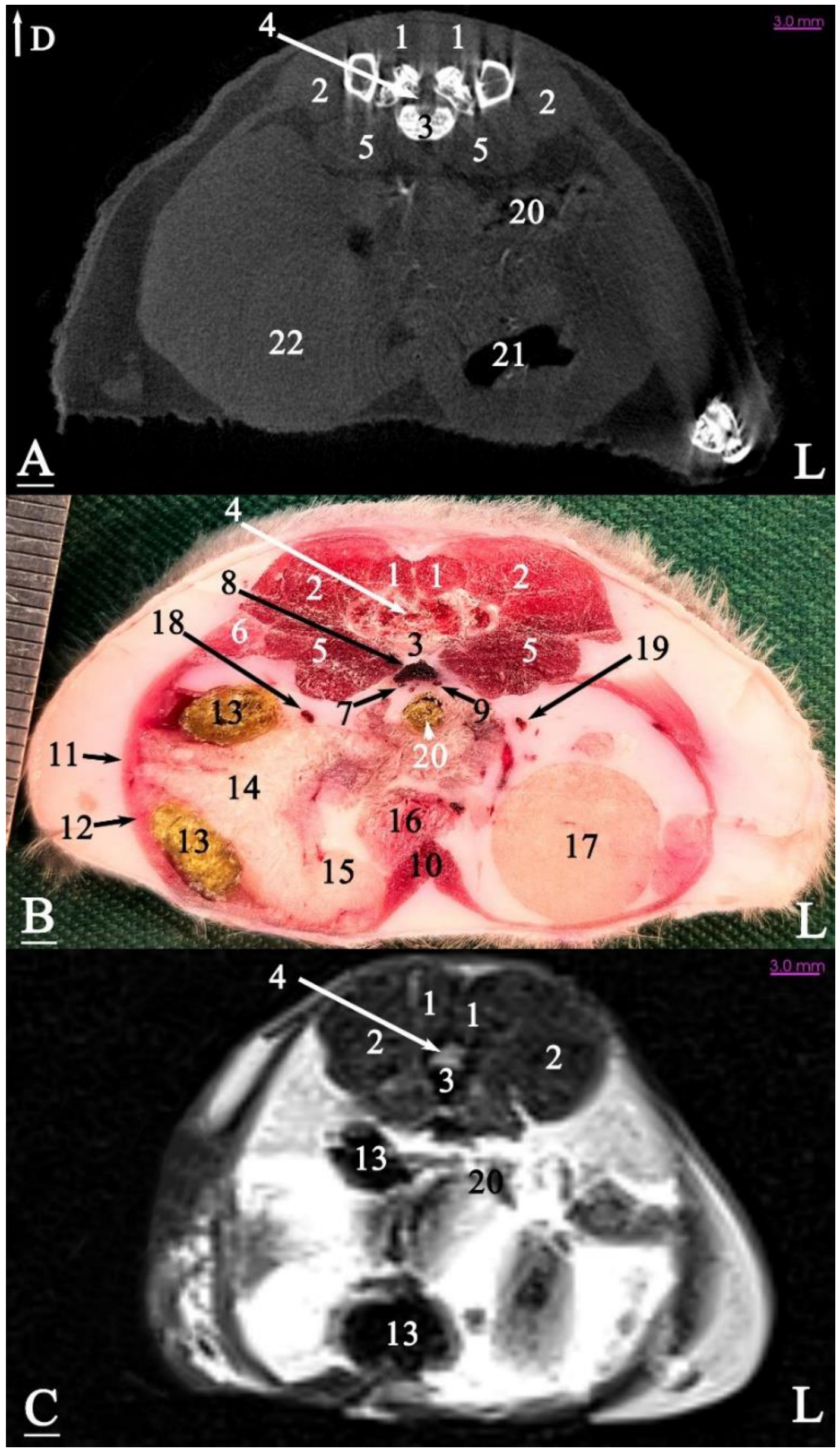
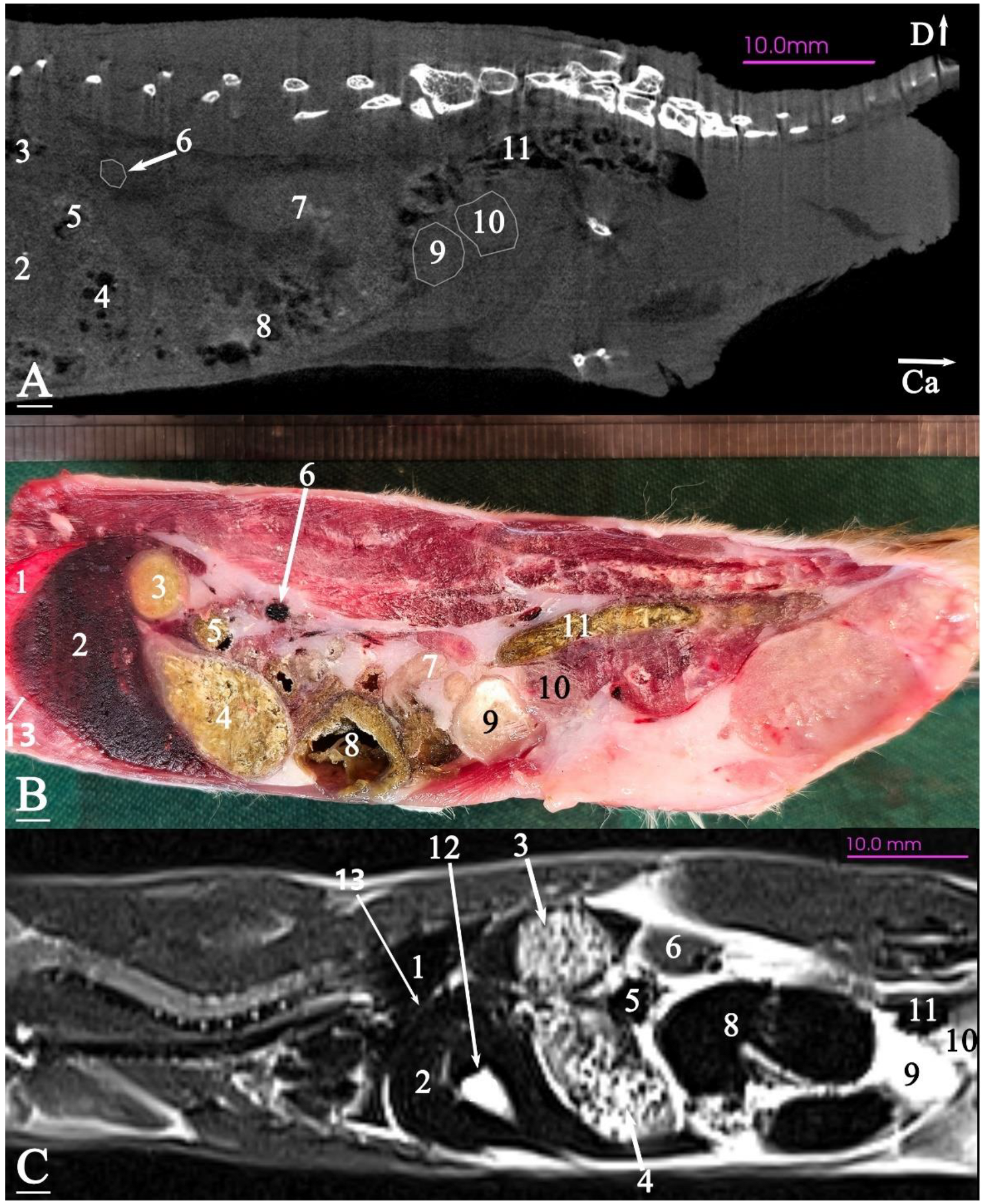
3. Results
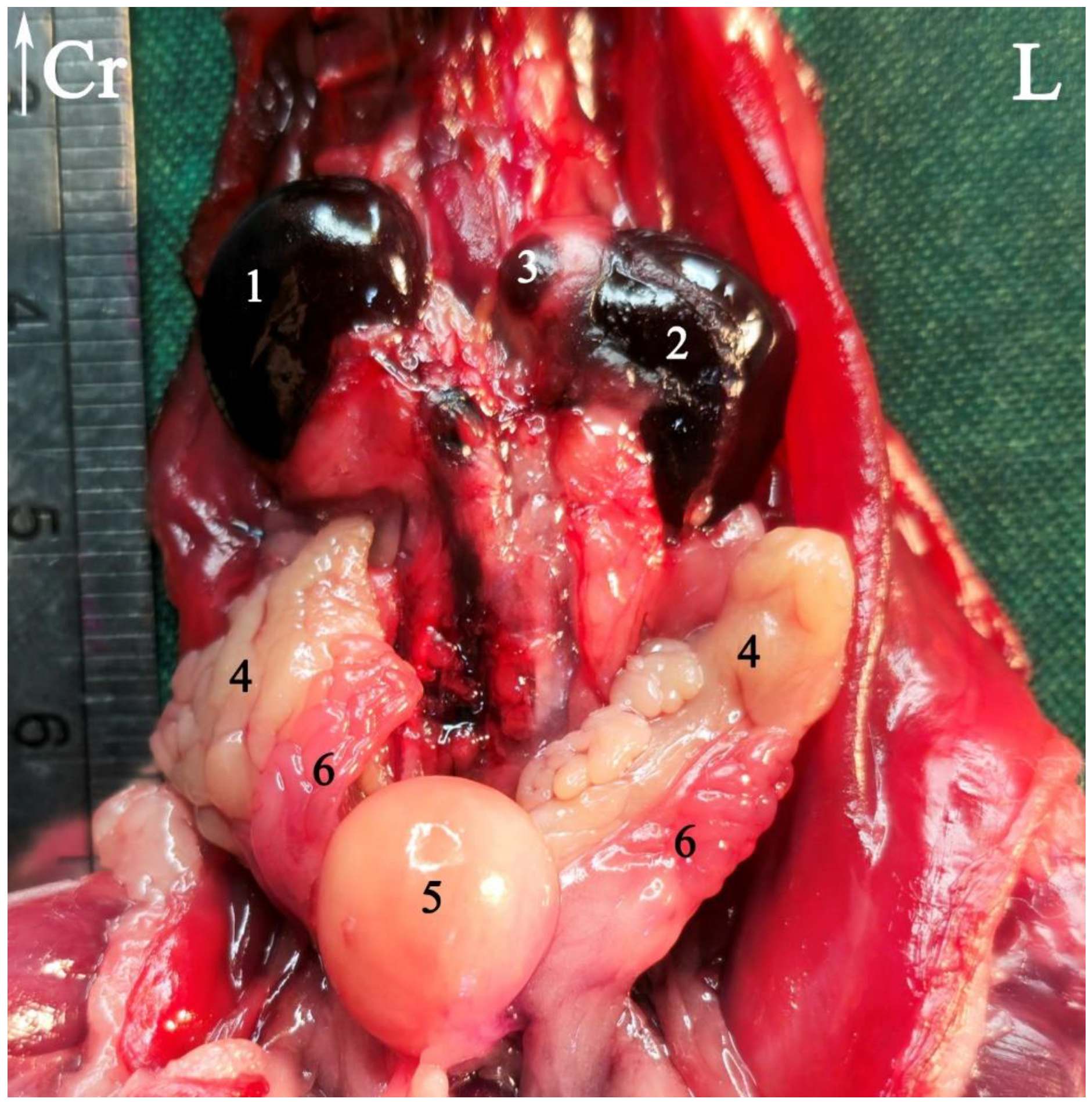
3.1. Topographic Anatomy
3.2. Anatomical Sectioning
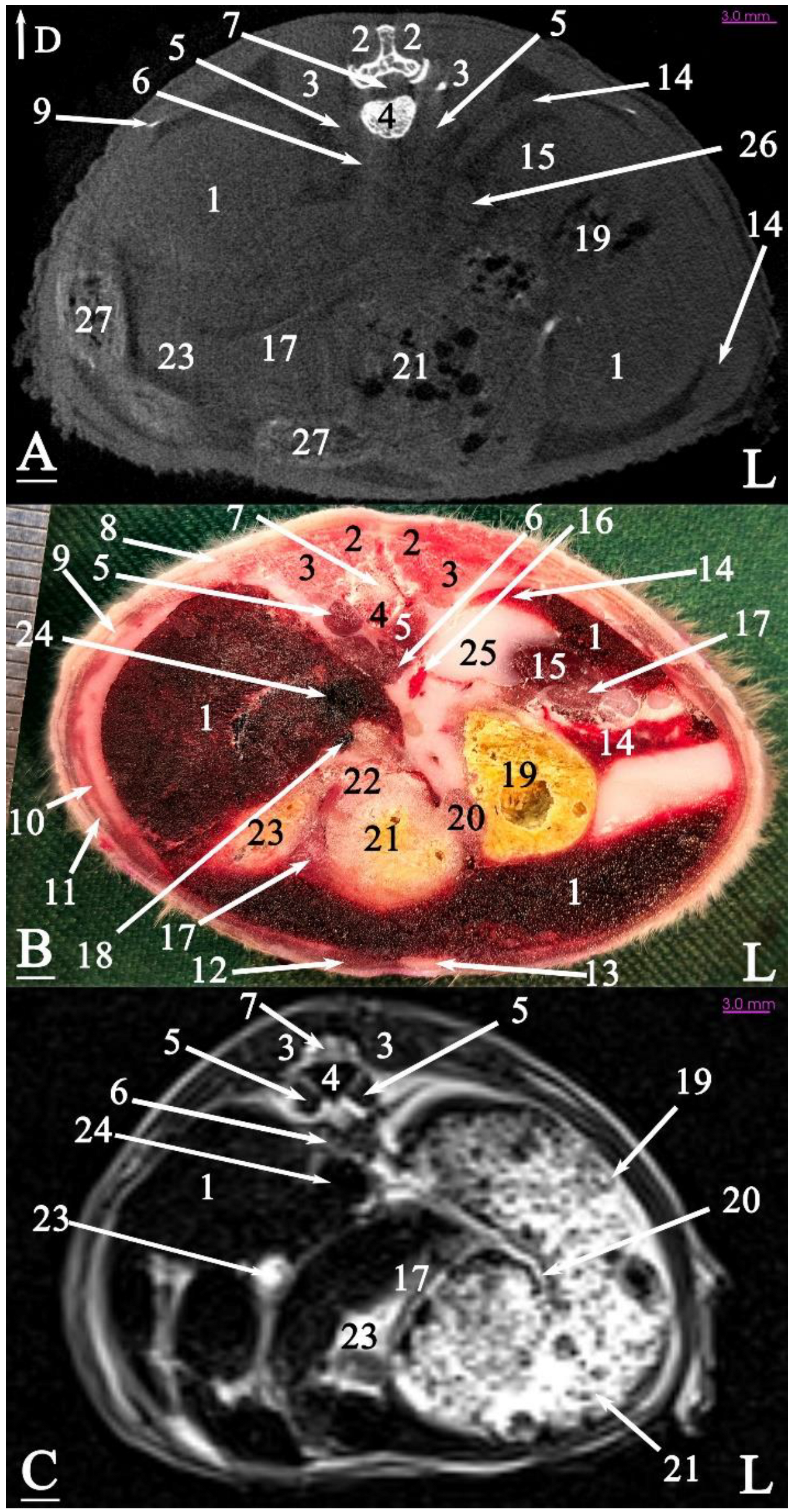
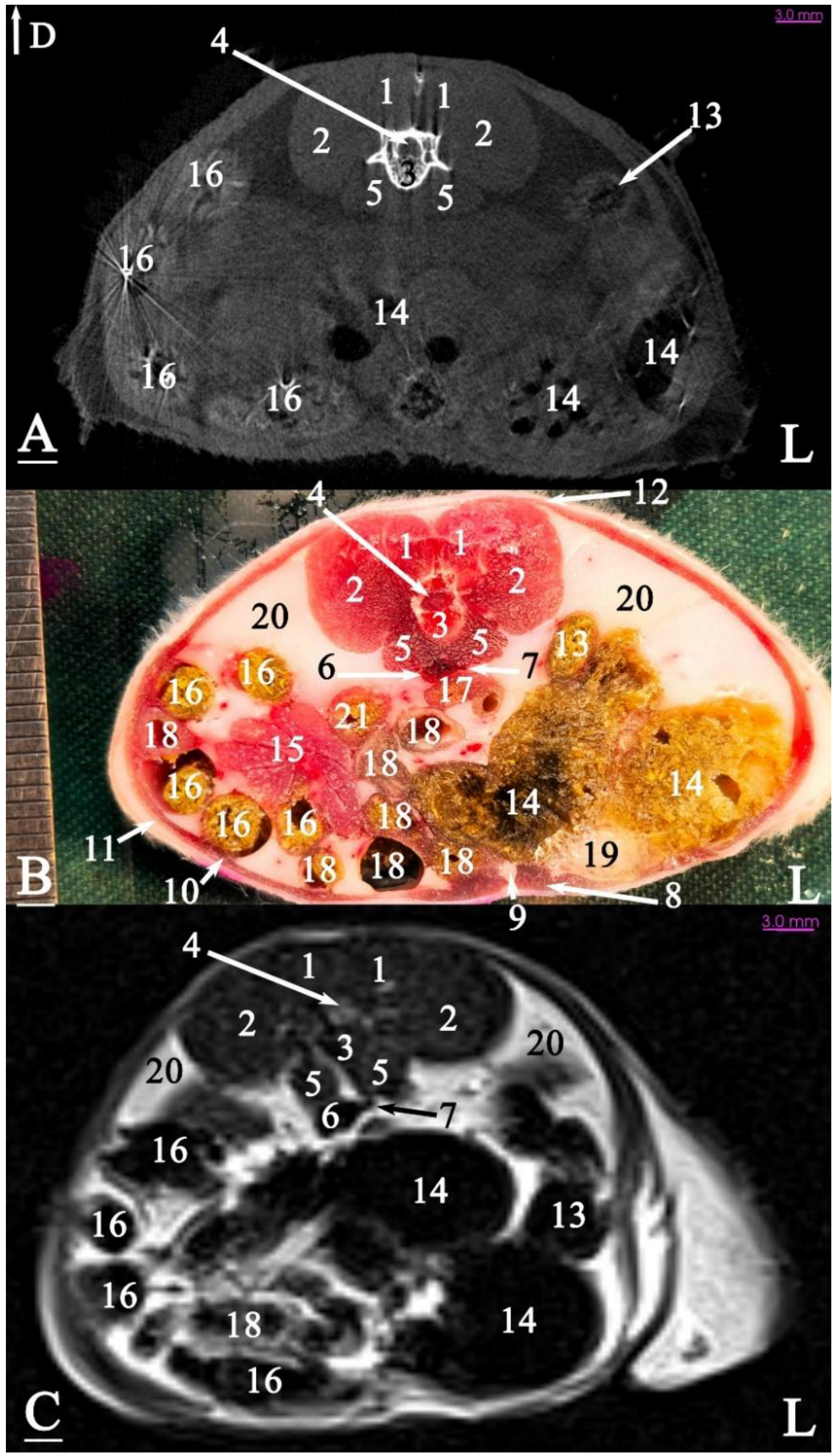
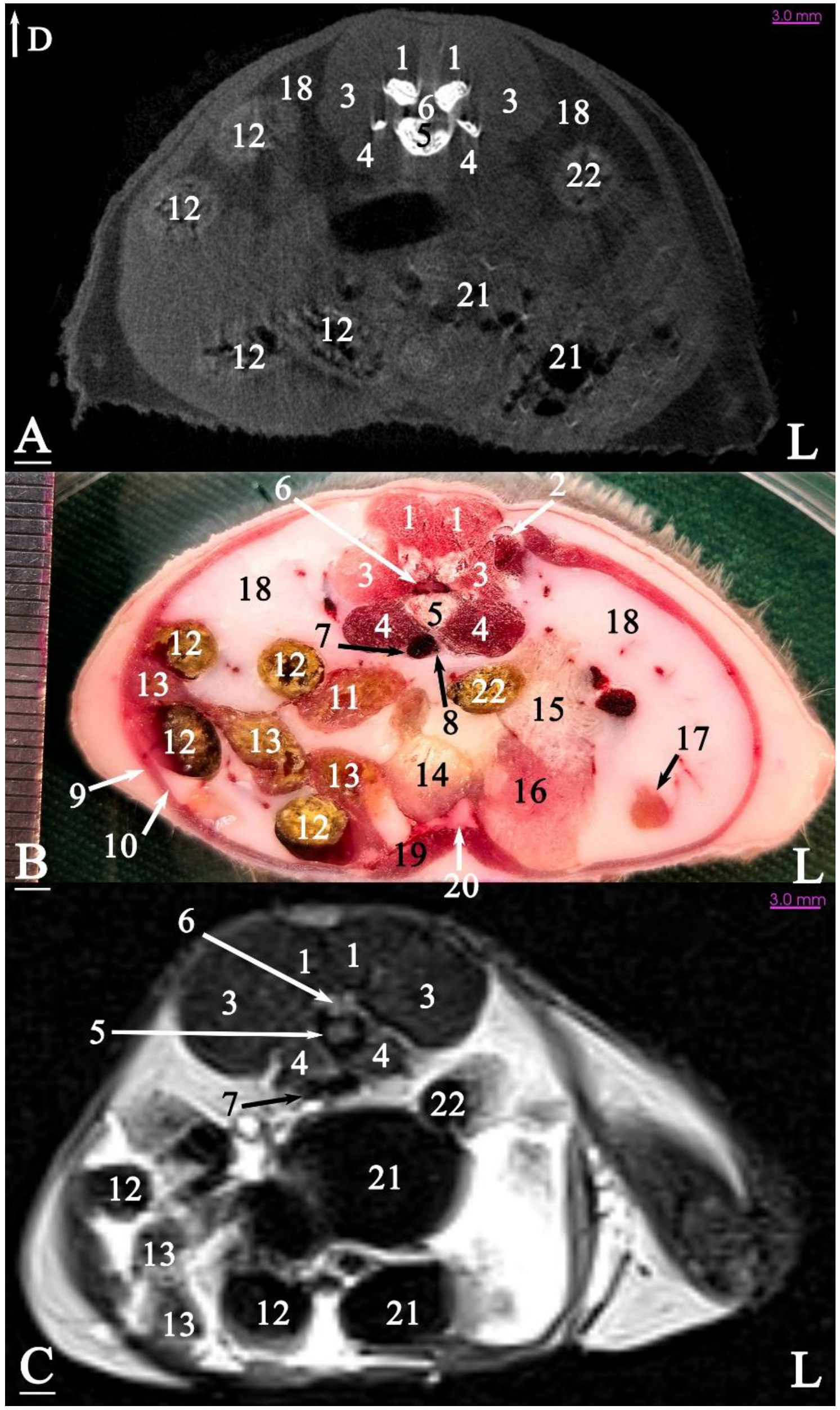
3.2.1. Transverse Cadaver Slices
3.2.2. Dorsal Cadaver Slice
3.2.3. Sagittal Cadaver Slice
3.3. Micro-CT Slices
3.4. MRI Slices
4. Discussion
4.1. Stomach
4.2. Intestines
4.3. Ileum
4.4. Jejunum
4.5. Cecum
4.6. Ascending Colon
4.7. Gall Bladder
4.8. Pancreas
4.9. Spleen
4.10. Kidney
4.11. Adrenal Glands
4.12. Ureter
4.13. Urinary Bladder
4.14. Male Accessory Genital Glands
4.14.1. Vesicular Gland (Seminal Vesicle)
4.14.2. Coagulating Glands
4.14.3. Prostate Gland
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nickel, R.; Schummer, A.; Seiferle, E.; Sack, W.O. The Viscera of the Domestic Mammals; Verlag Paul Parey: Berlin, Germany, 1979. [Google Scholar]
- Huguet, E.E.; Berry, C.R.; Giglio, R. Anatomy, Variants, and Interpretation Paradigm. In Atlas of Small Animal Diagnostic Imaging; Wiley: Hoboken, NJ, USA, 2023; pp. 543–597. [Google Scholar]
- Thrall, D.E. Textbook of Veterinary Diagnostic Radiology; Elsevier Health Sciences: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Kealy, J.K.; McAllister, H.; Graham, J.P. Diagnostic Radiology and Ultrasonography of the Dog and Cat; Elsevier Health Sciences: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Balikci Dorotea, S.; Banzato, T.; Bellini, L.; Contiero, B.; Zotti, A. Radiographic Anatomy of Dwarf Rabbit Abdomen with Normal Measurements. Bulg. J. Vet. Med. 2016, 19, 96–107. [Google Scholar] [CrossRef]
- von Stade, L.; Sadar, M.J. Advanced Imaging of Small Mammals. Adv. Vet. Sci. Comp. Med. 2024, 5, 51–65. [Google Scholar] [CrossRef]
- Gallastegui, A.; Spoldi, E.; Billhymer, A.C.; Stefanou, C.R. Case-based Intensive Veterinary Radiology Clerkship Improves Students’ Radiographic Interpretation Skills and Overall Course Satisfaction Scores. Vet. Radiol. Ultrasound 2022, 63, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Buch, D.; Saldanha, A.; Santos, I.D.A.; Muehlbauer, E.; Gil, E.M.U.; Froes, T.R.; Giglio, R.F. Computed Tomographic Findings of the Gastrointestinal Tract in Rabbits. J. Exot. Pet. Med. 2022, 42, 11–19. [Google Scholar] [CrossRef]
- Schwarz, T.; O’Brien, R. CT Acquisition Principles. In Veterinary Computed Tomography; Wiley: Hoboken, NJ, USA, 2011; pp. 9–27. [Google Scholar]
- Li, H.; Zhang, H.; Tang, Z.; Hu, G. Micro-Computed Tomography for Small Animal Imaging: Technological Details. Prog. Nat. Sci. Mater. Int. 2008, 18, 513–521. [Google Scholar] [CrossRef]
- Rosenhain, S.; Magnuska, Z.A.; Yamoah, G.G.; Rawashdeh, W.A.; Kiessling, F.; Gremse, F. A Preclinical Micro-Computed Tomography Database Including 3D Whole Body Organ Segmentations. Sci. Data 2018, 5, 180294. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.M.; Price, R.E.; Rivera, B.; Cody, D.D. Intraperitoneal Administration of an Iodine-Based Contrast Agent to Improve Abdominal Micro-Computed Tomography Imaging in Mice. Contemp. Top. Lab. Anim. Sci. 2005, 44, 20–27. [Google Scholar]
- Judex, S.; Luu, Y.K.; Ozcivici, E.; Adler, B.; Lublinsky, S.; Rubin, C.T. Quantification of Adiposity in Small Rodents Using Micro-CT. Methods 2010, 50, 14–19. [Google Scholar] [CrossRef]
- Ashton, J.R.; West, J.L.; Badea, C.T. In Vivo Small Animal Micro-CT Using Nanoparticle Contrast Agents. Front. Pharmacol. 2015, 6, 256. [Google Scholar] [CrossRef]
- Głodek, J.; Adamiak, Z.; Przeworski, A. Magnetic Resonance Imaging of Reptiles, Rodents, and Lagomorphs for Clinical Diagnosis and Animal Research. Comp. Med. 2016, 66, 216–219. [Google Scholar]
- Mai, W. Diagnostic MRI in Dogs and Cats; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Elliott, I.; Skerritt, G. Using MRI in Clinical Veterinary Practice; Wiley-Blackwell: Hoboken, NJ, USA, 2010. [Google Scholar]
- Hanpanich, P.; Pinlaor, S.; Charoensuk, L.; Yongvanit, P.; Thomas, C.; Kothan, S.; Mairiang, E. MRI and 1H MRS Evaluation for the Serial Bile Duct Changes in Hamsters after Infection with Opisthorchis Viverrini. Magn. Reson. Imaging 2013, 31, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Gavin, P.R.; Holmes, S.P. Magnetic Resonance Imaging of Abdominal Disease. In Practical Small Animal MRI; Wiley: Hoboken, NJ, USA, 2009; pp. 273–293. [Google Scholar]
- Inderbitzin, D.; Stoupis, C.; Sidler, D.; Gass, M.; Candinas, D. Abdominal Magnetic Resonance Imaging in Small Rodents Using a Clinical 1.5T MR Scanner. Methods 2007, 43, 46–53. [Google Scholar] [CrossRef]
- Samii, V.F.; Biller, D.S.; Koblik, P.D. Normal Cross-Sectional Anatomy of the Feline Thorax and Abdomen: Comparison of Computed Tomography and Cadaver Anatomy. Vet. Radiol. Ultrasound 1998, 39, 504–511. [Google Scholar] [CrossRef]
- Feeney, D.A.; Fletcher, F.T.H.; Hardy, R.M. Atlas of Correlative Imaging Anatomy of the Normal Dog: Ultrasound and Computed Tomography; W B Saunders Co.: Philadelphia, PA, USA, 1991. [Google Scholar]
- Zotti, A.; Banzato, T.; Cozzi, B. Cross-Sectional Anatomy of the Rabbit Neck and Trunk: Comparison of Computed Tomography and Cadaver Anatomy. Res. Vet. Sci. 2009, 87, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Smallwood, J.E.; George, T.F. Anatomic Atlas for Computed Tomography in the Mesaticephalic Dog: Caudal Abdomen and Pelvis. Vet. Radiol. Ultrasound 1993, 34, 143–167. [Google Scholar] [CrossRef]
- Silverman, S.; Tell, L. Radiology of Rodents, Rabbits and Ferrets-An Atlas of Normal Anatomy and Positioning; Elsevier: Missouri, MO, USA, 2005. [Google Scholar]
- Dilek, Ö.G.; Dimitrov, R.; Stamatova-Yovcheva, K.; Ersen, M.; Yovchev, D.; Sabancı, S.S.; Karakurum, E. Computed Tomography and Three—Dimensional Anatomical Study of the Liver in the Chinchilla (Chinchilla lanigera). Anat. Histol. Embryol. 2024, 53, e13025. [Google Scholar] [CrossRef]
- Karakurum, E.; Dimitrov, R.; Stamatova-Yovcheva, K.; Erşen, M.; Dilek, Ö.G. Anatomical Computed Tomography and Magnetic Resonance Imaging Architecture of the Kidneys in the Chinchilla (Chinchilla lanigera). Indian J. Anim. Res. 2022, 58, 785–790. [Google Scholar] [CrossRef]
- Winter, N.; Clauss, M.; Codron, D.; Hummel, J.; Müller, J.; Richter, H.; Kircher, P.; Hatt, J.; Martin, L.F. Sand Accumulation in the Digestive Tract of Rabbits (Oryctolagus Cuniculus) and Guinea Pigs (Cavia porcellus): The Role of the Appendix. J. Morphol. 2022, 283, 5–15. [Google Scholar] [CrossRef]
- Chernev, C.; Procter, T.; Isaac, I.; Koterwas, B.; Eatwell, K.; Keeble, E.; Richardson, J.; Schwarz, T. Computed Tomographic Features of the Normal Spleen in Rabbits (Oryctolagus cuniculus domesticus). Vet. Radiol. Ultrasound 2023, 64, 844–850. [Google Scholar] [CrossRef]
- Buch, D.; Saldanha, A.; Muehlbauer, E.; de Oliveira, W.J.; Gil, E.M.U.; Froes, T.R. Computed Tomographic Findings of the Urinary Tract in Rabbits (Oryctolagus cuniculus). J. Exot. Pet. Med. 2022, 40, 1–7. [Google Scholar] [CrossRef]
- Dimitrov, R. Computed Tomography Imaging of the Prostate Gland in the Rabbit (Oryctolagus cuniculus). Vet. Arh. 2010, 80, 771–778. [Google Scholar]
- Dimitrov, R.; Yonkova, P.; Stamatova, K. Agreement between Sagittal Plane Cross Sectional Anatomy, Sonoanatomy and Computed Tomography of Rabbit Prostate and Bulbourethral Glands. Bulg. J. Vet. Med. 2011, 14, 11–16. [Google Scholar]
- Yuan, Y.-H. MR Diffusion-Weighed Imaging of Rabbit Liver. World J. Gastroenterol. 2005, 11, 5506. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, R. MRI Anatomical Investigation of Rabbit Prostate Gland. Bulg. J. Vet. Med. 2023, 26, 483–495. [Google Scholar] [CrossRef]
- Dimitrov, R.; Stamatova-Yovcheva, K. MRI Anatomical Investigation of Rabbit Bulbourethral Glands. Animals 2023, 13, 1519. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, R.; Stamatova-Yovcheva, K.; Georgiev, G. MRI Investigation of Kidneys, Ureters and Urinary Bladder in Rabbits. Vet. Sci. 2024, 11, 575. [Google Scholar] [CrossRef]
- Keeble, E. Rodents: Biology and Husbandry. In BSAVA Manual of Rodents and Ferrets; British Small Animal Veterinary Association: Gloucester, UK, 2009; pp. 1–17. [Google Scholar]
- Lossi, L.; D’Angelo, L.; De Girolamo, P.; Merighi, A. Anatomical Features for an Adequate Choice of Experimental Animal Model in Biomedicine: II. Small Laboratory Rodents, Rabbit, and Pig. Ann. Anat. 2016, 204, 11–28. [Google Scholar] [CrossRef]
- Kitahashi, T.; Mutoh, M.; Tsurusaki, M.; Iinuma, G.; Suzuki, M.; Moriyama, N.; Yoshimoto, M.; Wakabayashi, K.; Sugimura, T.; Imai, T. Imaging Study of Pancreatic Ductal Adenocarcinomas in Syrian Hamsters Using X-ray Micro-computed Tomography (CT). Cancer Sci. 2010, 101, 1761–1766. [Google Scholar] [CrossRef]
- Baldrey, V. Approaches to Common Conditions of the Gastrointestinal Tract in Pet Hamsters. Companion Anim. 2021, 26, 20–26. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Kim, K.; Brodbelt, D.C.; Church, D.B.; Pegram, C.; Baldrey, V. Demography, Disorders and Mortality of Pet Hamsters under Primary Veterinary Care in the United Kingdom in 2016. J. Small Anim. Pract. 2022, 63, 747–755. [Google Scholar] [CrossRef]
- Brown, C.M.; Donnelly, T. Disease Problems of Small Rodents. In Ferrets, Rabbits, and Rodents: Clinical Medicine and Surgery; Elsevier/Saunders: Missouri, MO, USA, 2012; pp. 354–372. [Google Scholar]
- Suckow, M.A.; Stevens, K.A.; Wilson, R.P. The Laboratory Rabbit, Guinea Pig, Hamster, and Other Rodents; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 9780123809209. [Google Scholar]
- Chen, Y.-M.; Wu, J.-L.; Lin, W.-H. Common Spontaneous Tumors and Tumor-like Lesions in 70 Pet Rodents and Negative MMTV Detection in Mammary Tumors. Animals 2024, 14, 1469. [Google Scholar] [CrossRef]
- Uchida, E.; Matsushita, A.; Yanagi, K.; Hiroi, M.; Aimoto, T.; Nakamura, Y.; Yokoyama, T.; Tajiri, T. Experimental Pancreatic Cancer Model Using PGHAM-1 Cells: Characteristics and Experimental Therapeutic Trials. J. Nippon Med. Sch. 2008, 75, 325–331. [Google Scholar] [CrossRef]
- Rother, N.; Bertram, C.A.; Klopfleisch, R.; Fragoso-Garcia, M.; Bomhard, W.V.; Schandelmaier, C.; Müller, K. Tumours in 177 Pet Hamsters. Vet. Rec. 2021, 188, e14. [Google Scholar] [CrossRef] [PubMed]
- Hallak, A.; Ranjbar, R.; Nourinezhad, J. Anatomical Study of Arterial Arrangement of the Spinal Cord in Syrian Hamsters (Mesocricetus auratus). Anat. Sci. Int. 2023, 98, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Nourinezhad, J.; Ranjbar, R.; Rostamizadeh, V.; Tabrizinejad, M.N.; Hallak, A.; Janeczek, M. Morphology of the Pattern of Branching of the Aortic Arch (Arcus aortae) in Syrian Hamsters (Mesocricetus auratus). Vet. Res. Commun. 2023, 47, 51–60. [Google Scholar] [CrossRef]
- Nourinezhad, J.; Tabrizinejad, M.N.; Janeczek, M. Detailed Gross Anatomy and Topography of the Sympathetic Cardiac Nerves and Related Ganglia in Syrian Hamsters (Mesocricetus auratus). Ann. Anat. 2022, 239, 151842. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Nourinezhad, J.; Moarabi, A.; Hanafi, M.G.; Janeczek, M. Sectional Anatomy, Micro-Computed Tomography, and Magnetic Resonance Imaging of the Syrian Hamsters (Mesocricetus auratus) Head. In Proceedings of the 34rd Congress of the European Association of Veterinary Anatomists, Oslo, Norway, 26–29 July 2023; Norwegian University of Life Sciences: Oslo, Norway, 2023. [Google Scholar]
- Nourinezhad, J.; Homayonnezhad, Z.; Moarabi, A.; Hanafi, M.G.; Janeczek, M. Evaluation of Sectional Anatomic, Micro-Computed Tomographic, and Magnetic Resonance Imaging Features of the Thorax in Syrian hamsters (Mesocricetus auratus). Vet. Res. Commun. 2025, 49, 81. [Google Scholar] [CrossRef]
- Gad, S.C. Rodents Model for Toxicity Testing and Biomarkers. In Biomarkers in Toxicology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 7–69. [Google Scholar]
- Hallman, R.M.; Brandão, J. Diagnostic Imaging of the Renal System in Exotic Companion Mammals. Vet. Clin. N. Am. Exot. Anim. Pract. 2020, 23, 195–214. [Google Scholar] [CrossRef]
- Capello, V.; Lennox, A.M. Clinical Radiology of Exotic Companion Mammals; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Brown, R.W.; Cheng, Y.N.; Haacke, E.M.; Thompson, M.R.; Venkatesan, R. Magnetic Resonance Imaging; Wiley: Hoboken, NJ, USA, 2014; ISBN 9780471720850. [Google Scholar]
- Hanpanich, P.; Laha, T.; Sripa, B.; Mairiang, E.; Sereerak, P.; Upontain, S.; Tangkawattana, P.; Brindley, P.J.; Tangkawattana, S. Decreased Risk of Cholangiocarcinogenesis Following Repeated Cycles of Opisthorchis Viverrini Infection-Praziquantel Treatment: Magnetic Resonance Imaging (MRI) and Histopathological Study in a Hamster Model. Parasitol. Int. 2017, 66, 464–470. [Google Scholar] [CrossRef]
- Müllhaupt, D.; Augsburger, H.; Schwarz, A.; Fischer, G.; Kircher, P.; Hatt, J.-M.; Ohlerth, S. Magnetic Resonance Imaging Anatomy of the Rabbit Brain at 3 T. Acta Vet. Scand. 2015, 57, 47. [Google Scholar] [CrossRef]
- Leary, S.L.; Underwood, W.; Anthony, R.; Cartner, S.; Corey, D.; Grandin, T.; Greenacre, C.; Gwaltney-Brant, S.; McCrackin, M.A.; Meyer, R.; et al. AVMA Guidelines for the Euthanasia of Animals; American Veterinary Medical Association: Schaumburg, IL, USA, 2013. [Google Scholar]
- Popesko, P.; Rajtová, V.; Horák, J. A Colour Atlas of the Anatomy of Small Laboratory Animals; Wolfe Pub Ltd.: London, UK, 1992. [Google Scholar]
- Smallwood, J.E. A Guided Tour of Veterinary Anatomy: Domestic Ungulates and Laboratory Mammals; WB Saunders Co.: Philadelphia, PA, USA, 1992. [Google Scholar]
- Nomina Anatomica Veterinaria. International Committee on Veterinary Gross Anatomical Nomenclature (I.C.V.G.A.N), 5th ed.; Published by the Editorial Committee: Hanover, Germany; Columbia, MO, USA; Ghent, Belgium; Sapporo, Japan, 2017. [Google Scholar]
- De Rycke, L.M.; Boone, M.N.; Van Caelenberg, A.I.; Dierick, M.; Van Hoorebeke, L.; van Bree, H.; Gielen, I.M. Micro-Computed Tomography of the Head and Dentition in Cadavers of Clinically Normal Rabbits. Am. J. Vet. Res. 2012, 73, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Sether, L.A.; Nguyen, C.; Yu, S.N.; Haughton, V.M.; Ho, K.C.; Biller, D.S.; Strandt, J.A.; Eurell, J.C. Canine Intervertebral Disks: Correlation of Anatomy and MR Imaging. Radiology 1990, 175, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Quesenberry, K.; Mans, C.; Orcutt, C. Ferrets, Rabbits and Rodents Clinical Medicine and Surgery; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Sasai, H.; Fujita, D.; Tagami, Y.; Seto, E.; Denda, Y.; Hamakita, H.; Ichihashi, T.; Okamura, K.; Furuya, M.; Tani, H.; et al. Characteristics of Bone Fractures and Usefulness of Micro–Computed Tomography for Fracture Detection in Rabbits: 210 Cases (2007–2013). J. Am. Vet. Med. Assoc. 2015, 246, 1339–1344. [Google Scholar] [CrossRef]
- Schwarz, T. Artifacts in CT. In Veterinary Computed Tomography; Wiley: Hoboken, NJ, USA, 2011; pp. 35–55. [Google Scholar]
- Martiniova, L.; Schimel, D.; Lai, E.W.; Limpuangthip, A.; Kvetnansky, R.; Pacak, K. In Vivo Micro-CT Imaging of Liver Lesions in Small Animal Models. Methods 2010, 50, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Choi, B.; Lee, S.; Choi, J. 3.0-Tesla MRI of Normal Canine Adrenal Glands. Vet. Radiol. Ultrasound 2022, 63, 206–215. [Google Scholar] [CrossRef]
- Bilreiro, C.; Fernandes, F.F.; Andrade, L.; Chavarrías, C.; Simões, R.V.; Matos, C.; Shemesh, N. Effective Bowel Motion Reduction in Mouse Abdominal MRI Using Hyoscine Butylbromide. Magn. Reson. Med. 2021, 86, 2146–2155. [Google Scholar] [CrossRef]
- Manley, R.; Matthews, A.R.; Morandi, F.; Henry, G.A.; DeAnna, K.H.; Conklin, G.; Reed, A. Magnetic Resonance Imaging of the Canine Abdomen: Effect of Pulse Sequence on Diagnostic Quality. Vet. Radiol. Ultrasound 2013, 54, 253–262. [Google Scholar] [CrossRef]
- Newell, S.M.; Graham, J.P.; Roberts, G.D.; Ginn, P.E.; Chewning, C.L.; Harrison, J.M.; Andrzejewski, C. Quantitative Magnetic Resonance Imaging of the Normal Feline Cranial Abdomen. Vet. Radiol. Ultrasound 2000, 41, 27–34. [Google Scholar] [CrossRef]
- Samii, V.F.; Biller, D.S.; Koblik, P.D. Magnetic Resonance Imaging of the Normal Feline Abdomen: An Anatomic Reference. Vet. Radiol. Ultrasound 1999, 40, 486–490. [Google Scholar] [CrossRef]
- McConnell, M.V.; Aikawa, M.; Maier, S.E.; Ganz, P.; Libby, P.; Lee, R.T. MRI of Rabbit Atherosclerosis in Response to Dietary Cholesterol Lowering. Arter. Thromb. Vasc. Biol. 1999, 19, 1956–1959. [Google Scholar] [CrossRef]
- Shin, M.; Brott, B.C.; Lloyd, S.G.; Evanochko, W.T.; Kiss, P.; Baker, R.A.; Anayiotos, A.S. MRI Evaluation of a Stented Abdominal Aorta of a Rabbit. In Proceedings of the ASME 2007 Summer Bioengineering Conference, Keystone, CO, USA, 20–24 June 2007; American Society of Mechanical Engineers: New York, NY, USA, 2007; pp. 727–728. [Google Scholar]
- Rojo, D.; Vázquez, J.M.; Sánchez, C.; Arencibia, A.; García, M.I.; Soler, M.; Kilroy, D.; Ramírez, G. Sectional Anatomic and Tomographic Study of the Feline Abdominal Cavity for Obtaining a Three-Dimensional Vascular Model. Iran J. Vet. Res. 2020, 21, 279–286. [Google Scholar] [PubMed]
- Rojo Ríos, D.; Ramírez Zarzosa, G.; Soler Laguía, M.; Kilroy, D.; Martínez Gomariz, F.; Sánchez Collado, C.; Gil Cano, F.; García García, M.I.; Ayala Florenciano, M.D.; Arencibia Espinosa, A. Anatomical and Three-Dimensional Study of the Female Feline Abdominal and Pelvic Vascular System Using Dissections, Computed Tomography Angiography and Magnetic Resonance Angiography. Vet. Sci. 2023, 10, 704. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.; Gil, F.; Vazquez, J.M.; Cardoso, L.; Arencibia, A.; Ramirez-Zarzosa, G.; Agut, A. Helical Computed Tomographic Anatomy of the Canine Abdomen. Vet. J. 2007, 174, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Daggett, A.; Loeber, S.; Le Roux, A.B.; Beaufrere, H.; Doss, G. Computed Tomography with Hounsfield Unit Assessment Is Useful in the Diagnosis of Liver Lobe Torsion in Pet Rabbits (Oryctolagus cuniculus). Vet. Radiol. Ultrasound 2021, 62, 210–217. [Google Scholar] [CrossRef]
- Di Girolamo, N.; Tollefson, C. Computed Tomographic Diagnosis and Clinical Outcomes of Small Intestinal Obstruction Caused by Trichobezoars in Client-owned Rabbits. Vet. Radiol. Ultrasound 2024, 65, 264–274. [Google Scholar] [CrossRef]
- Clark, D.P.; Badea, C.T. Advances in micro-CT imaging of small animals. Phys. Medica 2021, 88, 175–192. [Google Scholar] [CrossRef]
- Grant, K. Rodent Nutrition. Vet. Clin. N. Am. Exot. Anim. Pract. 2014, 17, 471–483. [Google Scholar] [CrossRef]
- Nagy, T.R.; Pistole, D.H. The Effects of Fasting on Some Physiological Parameters in the Meadow Vole, Microtus Pennsylvanicus. Comp. Biochem. Physiol. A Physiol. 1988, 91, 679–684. [Google Scholar] [CrossRef]
- Barone, R.; Pavaux, C.; Bline, P.C.; Cuq, P. Anatomie Comparée des Mammifères Domestiques. Splanchnologie I. Appareil Digestif, Appareil Respiratoir; Tome 4, Vigot Frére: Paris, France, 2001. [Google Scholar]
- Ritzman, T.K. Diagnosis and Clinical Management of Gastrointestinal Conditions in Exotic Companion Mammals (Rabbits, Guinea Pigs, and Chinchillas). Vet. Clin. N. Am. Exot. Anim. Pract. 2014, 17, 179–194. [Google Scholar] [CrossRef]
- Barone, R. Atlas d’anatomie Du Lapin; Masson & C Editeurs: Paris, France, 1973. [Google Scholar]
- Hebel, R.; Stromberg, M.W. Anatomy of the Laboratory Rat; The Williams and Wilkins Company: Baltimore, MD, USA, 1976. [Google Scholar]
- Cooper, G.; Schiller, A.L. Anatomy of the Guinea Pig; Harvard University Press: Cambridge, MA, USA, 1975. [Google Scholar]
- Stan, F.G. Anatomical Particularities of Male Reproductive System of Guinea Pigs (Cavia porcellus). Bull. Univ. Agric. Sci. Vet. Med. 2015, 72, 288–295. [Google Scholar] [CrossRef][Green Version]
- Capalbo, A.C.; Cruz, C.; Oliveira, F.S.D.; Sasahara, T.H.C.; Leal, L.M.; Simões, L.S.; Miglino, M.A.; Machado, M.R.F. Análise Histoquímica Das Glândulas Anexas Do Aparelho Reprodutor Do Macho de Paca (Cuniculus paca). Pesq. Vet. Bras. 2016, 36, 1009–1013. [Google Scholar] [CrossRef]
- Stan, F.G. Comparative Study of the Stomach Morphology in Rabbit and Chinchilla. AgroLife Sci. J. 2013, 2, 73–78. [Google Scholar]
- Stan, F.G. Macroscopic Anatomy of Pancreas in Rats, Guinea Pigs, Chinchillas and Rabbits. Sci. Works. Ser. C Vet. Med. 2018, 64, 15–20. [Google Scholar]
- Stan, F.; Damian, A.; Gudea, A.; Dezdrobitu, C.; Bob, D.; Martonoș, C.; Bochis, I.; Pogana, B. Comparative Anatomical Study of the Large Intestine in Rabbit and Chinchilla. Bull. UASVM Vet. Med. 2014, 71, 208–212. [Google Scholar]
- Snipes, R.L.; Clauss, W.; Weber, A.; Hrnicke, H. Structural and Functional Differences in Various Divisions of the Rabbit Colon. Cell Tissue Res. 1982, 225, 331–346. [Google Scholar] [CrossRef] [PubMed]
- Snipes, R.L. Anatomy of the Rabbit Cecum. Anat. Embryol. (Berl.) 1979, 155, 57–80. [Google Scholar] [CrossRef]
- Schwarz, T.; Saunders, J. Veterinary Computed Tomography; Wiley: Hoboken, NJ, USA, 2011; ISBN 9780813817477. [Google Scholar]
- Pérez, W.; Vazquez, N.; Jerbi, H. Gross Anatomy of the Intestine and Their Peritoneal Folds in the Chinchilla (Chinchilla lanigera). J. Morphol. Sci. 2017, 28, 180–183. [Google Scholar]
- Snipes, R.L. Anatomy of the Cecum of the Laboratory Mouse and Rat. Anat. Embryol. (Berl.) 1981, 162, 455–474. [Google Scholar] [CrossRef]
- du Plessis, W.M.; Groenewald, H.B.; Elliott, D. Computed Tomography of the Abdomen in Eight Clinically Normal Common Marmosets (Callithrix jacchus). Anat. Histol. Embryol. 2017, 46, 365–372. [Google Scholar] [CrossRef]
- Longo, M.; Thierry, F.; Eatwell, K.; Schwarz, T.; del Pozo, J.; Richardson, J. Ultrasound and Computed Tomography of Sacculitis and Appendicitis in a Rabbit. Vet. Radiol. Ultrasound 2018, 59, E56–E60. [Google Scholar] [CrossRef]
- Snipes, R.L. Anatomy of the Cecum of the Dwarf Hamster (Phodopus sungorus). Anat. Embryol. (Berl.) 1979, 157, 329–346. [Google Scholar] [CrossRef]
- Head, L.L.; Daniel, G.B.; Tobias, K.; Morandi, F.; Denovo, R.C.; Donnell, R. Evaluation of the Feline Pancreas Using Computed Tomography and Radiolabeled Leukocyte. Vet. Radiol. Ultrasound 2003, 44, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Dilek, O.G.; Dimitrov, R.S.; Stamatova-Yovcheva, K.D.; Yovchev, D.G.; Mihaylov, R. Importance for Experiments in Human Medicine of Imaging Modalities for Macroanatomical and Histological Study of Rabbit Suprarenal Glands. Med. Weter 2019, 75, 684–692. [Google Scholar] [CrossRef]
- Dimitrov, R.; Toneva, Y.; Vladova, D.; Stamatova, K.; Stefanov, M. Computed Tomographic Imaging of Vesicular Glands in Rabbits. J. Anim. Vet. Adv. 2011, 10, 55–59. [Google Scholar] [CrossRef][Green Version]
- Dimitrov, R.; Stamatova, K.; Dimitar, K. Comparative Imaging of the Vesicular Glands in New Zealand White Rabbits (Oryctolagus cuniculus). Turk. J. Vet. Anim. Sci. 2013, 37, 97–101. [Google Scholar] [CrossRef]
- Banzato, T.; Bellini, L.; Contiero, B.; Selleri, P.; Zotti, A. Abdominal Ultrasound Features and Reference Values in 21 Healthy Rabbits. Vet. Rec. 2015, 176, 101. [Google Scholar] [CrossRef]
- Banzato, T.; Bellini, L.; Contiero, B.; Martin, A.; Balikçi, S.; Zotti, A. Abdominal Anatomic Features and Reference Values Determined by Use of Ultrasonography in Healthy Common Rats (Rattus norvegicus). Am. J. Vet. Res. 2014, 75, 67–76. [Google Scholar] [CrossRef]
- Chan, S.C.; Kiupel, M. Seminal Vesiculitis in Golden Hamsters (Mesocricetus auratus): Two Cases (2022). J. Exot. Pet. Med. 2024, 49, 58–63. [Google Scholar] [CrossRef]
- Gómez Martin, N.; Domínguez Miño, E.; García de Carellán, A.; Vilalta Solé, L. Abdominal Ultrasound Features and Reference Values in Healthy Guinea Pigs (Cavia porcellus). Vet. Rec. 2024, 194, e3668. [Google Scholar] [CrossRef]
- Nourinezhad, J.; Rostamizadeh, V.; Ranjbar, R. Morphotopographic Characteristics of the Extrinsic Innervation of the Heart in Guinea Pigs (Cavia porcellus). Ann. Anat. 2022, 242, 151911. [Google Scholar] [CrossRef]
- Shinya, K.; Makino, A.; Tanaka, H.; Hatta, M.; Watanabe, T.; Le, M.Q.; Imai, H.; Kawaoka, Y. Systemic Dissemination of H5N1 Influenza A Viruses in Ferrets and Hamsters after Direct Intragastric Inoculation. J. Virol. 2011, 85, 4673–4678. [Google Scholar] [CrossRef] [PubMed]
- Petrini, D.; Di Giuseppe, M.; Deli, G.; De Caro Carella, C. Cystolithiasis in a Syrian Hamster: A Different Outcome. Open Vet. J. 2016, 6, 135. [Google Scholar] [CrossRef] [PubMed]
- Capello, V. Surgical Techniques in Pet Hamsters. Exot. DVM 2003, 5, 32–37. [Google Scholar]
- Parasuraman, S.; Raveendran, R.; Kesavan, R. Blood Sample Collection in Small Laboratory Animals. J. Pharmacol. Pharmacother. 2010, 1, 87–93. [Google Scholar] [CrossRef]
- Orcutt, C.J. Common Hamster Diseases and Treatment. In Proceedings of the North American Veterinary Conference, Orlando, FL, USA, 8–12 January 2005; North American Veterinary Conference: Orlando, FL, USA, 2005; pp. 1358–1360. [Google Scholar]
- Orr, H. Rodents: Neoplastic and Endocrine Disease. In BSAVA Manual of Rodents and Ferrets; British Small Animal Veterinary Association: Gloucester, UK, 2009; pp. 181–192. [Google Scholar]
- Kaup, F.-J.; Konstýř, I.; Drommer, W. Characteristic of Spontaneous Intraperitoneal Cysts in Golden Hamsters and European Hamsters. Exp. Pathol. 1990, 40, 205–212. [Google Scholar] [CrossRef]
- Greenacre, C.B. Spontaneous Tumors of Small Mammals. Vet. Clin. N. Am. Exot. Anim. Pract. 2004, 7, 627–651. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammadzadeh, N.; Nourinezhad, J.; Moarabi, A.; Janeczek, M. Sectional Anatomy with Micro-Computed Tomography and Magnetic Resonance Imaging Correlation of the Middle and Caudal Abdominal Regions in the Syrian Hamster (Mesocricetus auratus). Animals 2025, 15, 1315. https://doi.org/10.3390/ani15091315
Mohammadzadeh N, Nourinezhad J, Moarabi A, Janeczek M. Sectional Anatomy with Micro-Computed Tomography and Magnetic Resonance Imaging Correlation of the Middle and Caudal Abdominal Regions in the Syrian Hamster (Mesocricetus auratus). Animals. 2025; 15(9):1315. https://doi.org/10.3390/ani15091315
Chicago/Turabian StyleMohammadzadeh, Nima, Jamal Nourinezhad, Abdolvahed Moarabi, and Maciej Janeczek. 2025. "Sectional Anatomy with Micro-Computed Tomography and Magnetic Resonance Imaging Correlation of the Middle and Caudal Abdominal Regions in the Syrian Hamster (Mesocricetus auratus)" Animals 15, no. 9: 1315. https://doi.org/10.3390/ani15091315
APA StyleMohammadzadeh, N., Nourinezhad, J., Moarabi, A., & Janeczek, M. (2025). Sectional Anatomy with Micro-Computed Tomography and Magnetic Resonance Imaging Correlation of the Middle and Caudal Abdominal Regions in the Syrian Hamster (Mesocricetus auratus). Animals, 15(9), 1315. https://doi.org/10.3390/ani15091315






