Estradiol Alleviates Elevated Temperature-Induced Damage in Yak Oviductal Epithelial Cells by Maintaining Endoplasmic Reticulum Calcium Homeostasis
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Cell Isolation and Culture
2.2. Western Blotting Analyses
2.3. Immunofluorescence
2.4. TUNEL Assay
2.5. Fluo-4AM Staining
2.6. Endoplasmic Reticulum Tracker Staining
2.7. Data Analysis
3. Result
3.1. Identification of Yak Oviduct Epithelial Cells
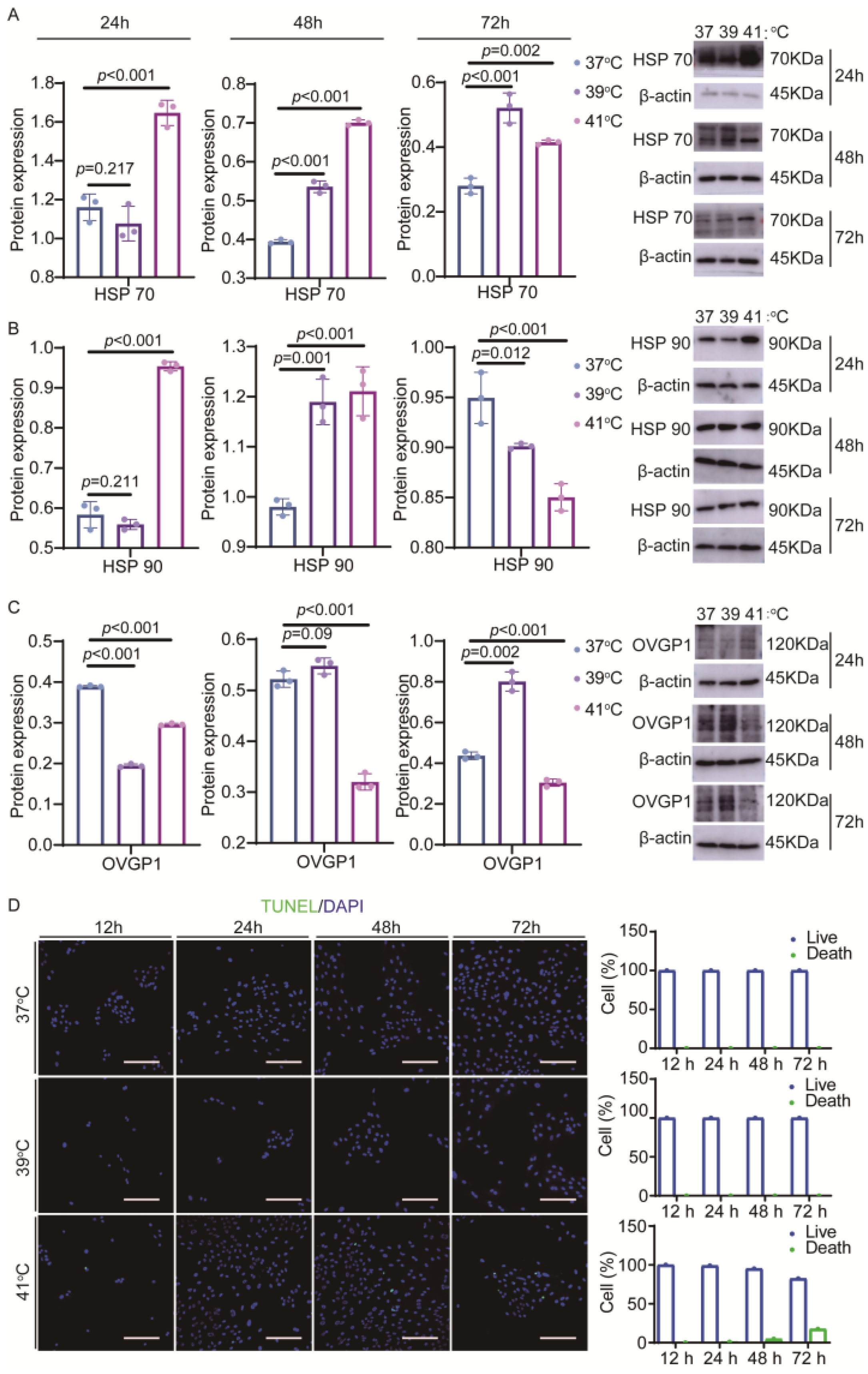
3.2. Impact of Elevated Temperature on Yak Oviduct Epithelial Cells
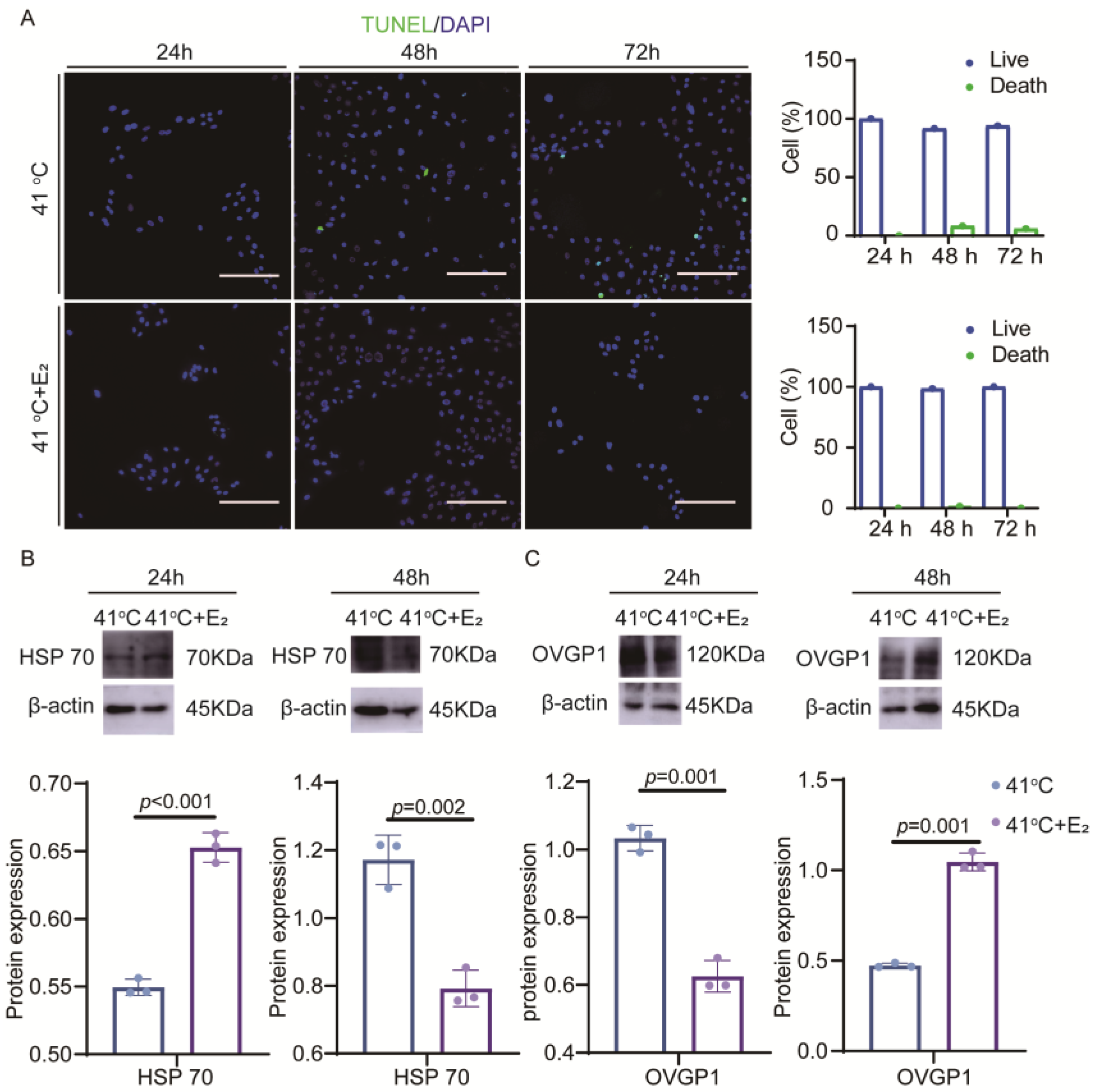
3.3. Effect of Estradiol on Elevated Temperature Injury in Yak Oviduct Epithelial Cells
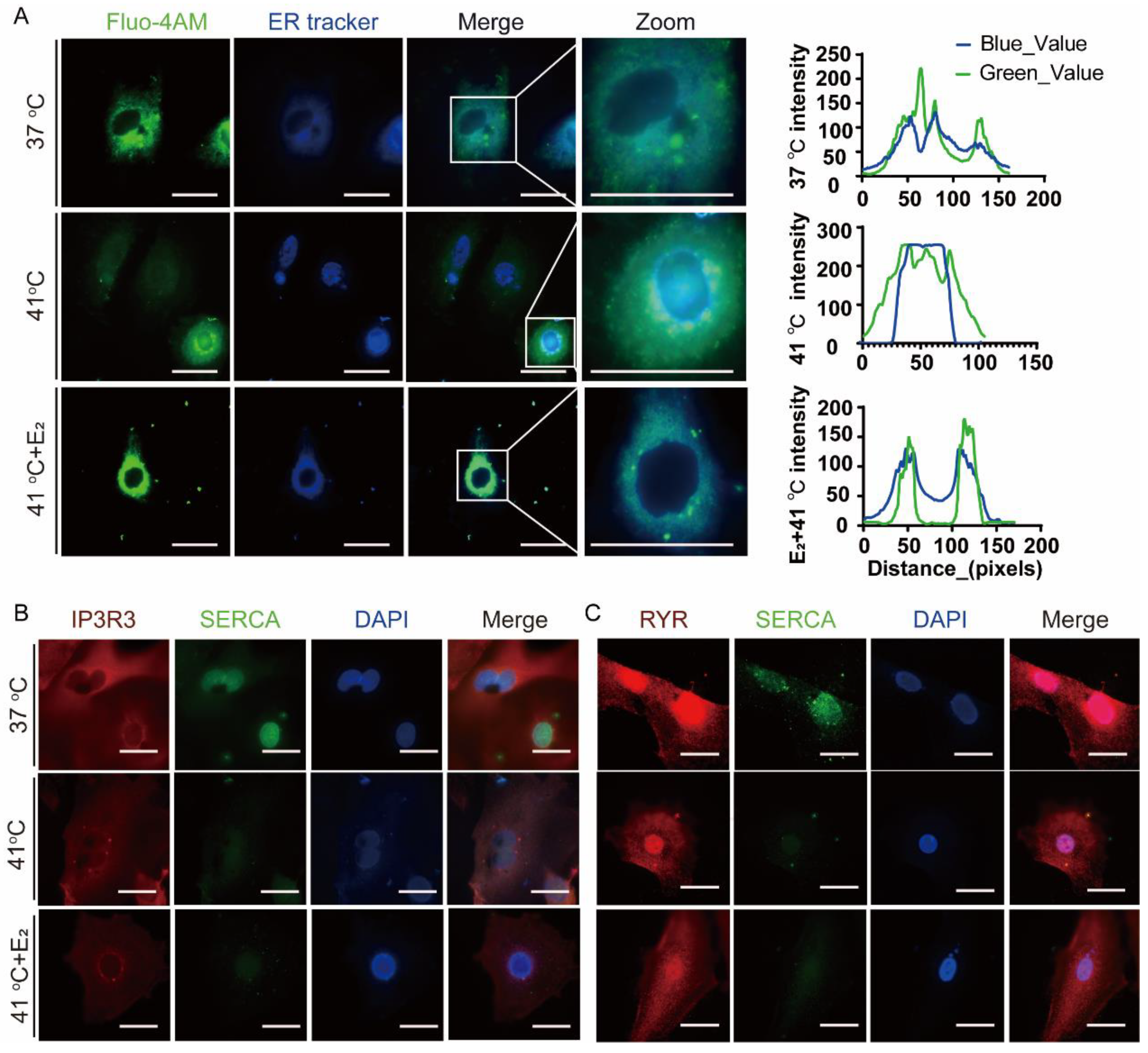
3.4. Effects of Elevated Temperature on Endoplasmic Reticulum Ca2+ Distribution and Related Protein Expression of Yak Oviductal Epithelial Cells
4. Discussion
4.1. Estradiol Can Alleviate Damage in Elevated Temperature Yak Oviduct Epithelial Cell
4.2. E2 Restores Elevated-Temperature-Induced Endoplasmic Reticulum Ca2+ Dysregulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCA | bicinchoninic acid |
| Ctrl | control |
| CK 18 | cytokeratin 18 |
| DAPI | 4’,6–diamidino–2–phenylindole |
| ER | endoplasmic reticulum |
| ELISA | enzyme–linked immunosorbent assay |
| E2 | estradiol |
| FBS | fetal bovine serum |
| HSP70 | heat shock protein 70 |
| OECs | oviduct epithelial cells |
| OVGP1 | oviductal glycoprotein 1 |
| RYR | ryanodine receptor |
| SERCA | sarco/endoplasmic reticulum calcium ATPase |
| treat | treatment |
| IP3R3 | type 3 inositol–1,4,5–trisphosphate receptor |
| YOECs | yak oviduct epithelial cells |
References
- Gupta, M.; Vaidya, M.; Kumar, S.; Singh, G.; Osei-Amponsah, R.; Chauhan, S.S. Heat stress: A major threat to ruminant reproduction and mitigating strategies. Int. J. Biometeorol. 2025, 69, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guzmán, J.A.; Parra-Bracamonte, G.M.; Velazquez, M.A. Impact of Heat Stress on Oocyte Developmental Competence and Pre-Implantation Embryo Viability in Cattle. Animals 2024, 14, 2280. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miętkiewska, K.; Kordowitzki, P.; Pareek, C.S. Effects of Heat Stress on Bovine Oocytes and Early Embryonic Development—An Update. Cells 2022, 11, 4073. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, S.; Winuthayanon, W. Oviduct: Roles in fertilization and early embryo development. J. Endocrinol. 2017, 232, R1–R26. [Google Scholar] [CrossRef] [PubMed]
- Menjivar, N.G.; Gad, A.; Thompson, R.E.; Meyers, M.A.; Hollinshead, F.K.; Tesfaye, D. Bovine oviductal organoids: A multi-omics approach to capture the cellular and extracellular molecular response of the oviduct to heat stress. BMC Genom. 2023, 24, 646. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Han, J.H.; Karki, R.; Malireddi, R.K.S.; Mall, R.; Sarkar, R.; Sharma, B.R.; Klein, J.; Berns, H.; Pisharath, H.; Pruett-Miller, S.M.; et al. NINJ1 mediates inflammatory cell death, PANoptosis, and lethality during infection conditions and heat stress. Nat. Commun. 2024, 15, 1739. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amaral, C.S.; Koch, J.; Correa Júnior, E.E.; Bertolin, K.; Mujica, L.K.S.; Fiorenza, M.F.; Rosa, S.G.; Nogueira, C.W.; Comim, F.V.; Portela, V.V.M.; et al. Heat stress on oocyte or zygote compromises embryo development, impairs interferon tau production and increases reactive oxygen species and oxidative stress in bovine embryos produced in vitro. Mol. Reprod. Dev. 2020, 87, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Oghbaei, H.; Hosseini, L.; Farajdokht, F.; Rahigh Aghsan, S.; Majdi, A.; Sadigh-Eteghad, S.; Sandoghchian Shotorbani, S.; Mahmoudi, J. Heat stress aggravates oxidative stress, apoptosis, and endoplasmic reticulum stress in the cerebellum of male C57 mice. Mol. Biol. Rep. 2021, 48, 5881–5887. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Li, B.; Wang, K.; Li, J.; He, S. Betaine ameliorates heat stress-induced apoptosis by affecting oxidative and endoplasmic reticulum stress in mouse Leydig cells. Biosci. Biotechnol. Biochem. 2023, 88, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, D.S.; Blower, M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell. Mol. Life Sci. 2016, 73, 79–94. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, S.; Wang, X.; Zhao, D.; Liu, H.; Hu, Y. Calcium homeostasis and cancer: Insights from endoplasmic reticulum-centered organelle communications. Trends Cell Biol. 2023, 33, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; Gauglitz, G.G.; Song, J.; Kulp, G.A.; Finnerty, C.C.; Cox, R.A.; Barral, J.M.; Herndon, D.N.; Boehning, D. Calcium and ER stress mediate hepatic apoptosis after burn injury. J. Cell. Mol. Med. 2009, 13, 1857–1865. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Saint-Dizier, M.; Marnier, C.; Tahir, M.Z.; Grimard, B.; Thoumire, S.; Chastant-Maillard, S.; Reynaud, K. OVGP1 is expressed in the canine oviduct at the time and place of oocyte maturation and fertilization. Mol. Reprod. Dev. 2014, 81, 972–982. [Google Scholar] [CrossRef] [PubMed]
- An, S.Y.; Gao, X.X.; Wang, Z.B.; Liang, Y.X.; Wang, S.T.; Xiao, S.H.; Xia, J.T.; You, P.H.; Wang, F.; Zhang, G.M. Estradiol-17β regulates proliferation and apoptosis of sheep endometrial epithelial cells by regulating the relative abundance of YAP1. Anim. Reprod. Sci. 2020, 215, 106328. [Google Scholar] [CrossRef] [PubMed]
- Chaube, S.K.; Prasad, P.V.; Thakur, S.C.; Shrivastav, T.G. Estradiol protects clomiphene citrate-induced apoptosis in ovarian follicular cells and ovulated cumulus-oocyte complexes. Fertil. Steril. 2005, 84 (Suppl. S2), 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Goff, A.; Rudzinskas, S.A.; Jung, Y.; Dubey, N.; Hoffman, J.; Hipolito, D.; Mazzu, M.; Rubinow, D.R.; Schmidt, P.J.; et al. Altered estradiol-dependent cellular Ca2+ homeostasis and endoplasmic reticulum stress response in Premenstrual Dysphoric Disorder. Mol. Psychiatry 2021, 26, 6963–6974. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boni, R. Heat stress, a serious threat to reproductive function in animals and humans. Mol. Reprod. Dev. 2019, 86, 1307–1323. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Pan, W.; Li, C.; Cai, M.; Shi, W.; Ren, Z.; Lu, H.; Zhou, Q.; Shen, H. Heat stress induces calcium dyshomeostasis to subsequent cognitive impairment through ERS-mediated apoptosis via SERCA/PERK/eIF2α pathway. Cell Death Discov. 2024, 10, 280. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ayalew, W.; Chu, M.; Liang, C.; Wu, X.; Yan, P. Adaptation Mechanisms of Yak (Bos grunniens) to High-Altitude Environmental Stress. Animals 2021, 11, 2344. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sapkota, S.; Acharya, K.P.; Laven, R.; Acharya, N. Possible Consequences of Climate Change on Survival, Productivity and Reproductive Performance, and Welfare of Himalayan Yak (Bos grunniens). Vet. Sci. 2022, 9, 449. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krishnan, G.; Paul, V.; Biswas, T.K.; Chouhan, V.S.; Das, P.J.; Sejian, V. Adaptation strategies of yak to seasonally driven environmental temperatures in its natural habitat. Int. J. Biometeorol. 2018, 62, 1497–1506. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pan, Y.; Wang, M.; Xu, R.; Han, X.; Ma, R.; Zhao, L.; Zhang, T.; Wang, Y.; Zhao, T.; et al. Follicular fluid exosomes regulate OVGP1 secretion in yak oviduct epithelial cells via autophagy in vitro. J. Cell. Physiol. 2023, 238, 1020–1035. [Google Scholar] [CrossRef] [PubMed]
- Terrell, K.; Choi, S.; Choi, S. Calcium’s Role and Signaling in Aging Muscle, Cellular Senescence, and Mineral Interactions. Int. J. Mol. Sci. 2023, 24, 17034. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kobayashi, Y.; Wakamiya, K.; Kohka, M.; Yamamoto, Y.; Okuda, K. Summer heat stress affects prostaglandin synthesis in the bovine oviduct. Reproduction 2013, 146, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, C.; Elsheikh, N.A.H.; Li, C.; Yang, F.; Wang, G.; Li, L. HO-1 reduces heat stress-induced apoptosis in bovine granulosa cells by suppressing oxidative stress. Aging 2019, 11, 5535–5547. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, Y.S.; Sun, Z.; Ma, J.; Cui, W.; Gao, B.; Zhang, H.Y.; Han, Y.H.; Hu, H.M.; Wang, L.; Fan, J.; et al. 17β-Estradiol inhibits ER stress-induced apoptosis through promotion of TFII-I-dependent Grp78 induction in osteoblasts. Lab. Investig. 2014, 94, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Krebs, J.; Agellon, L.B.; Michalak, M. Ca2+ homeostasis and endoplasmic reticulum (ER) stress: An integrated view of calcium signaling. Biochem. Biophys. Res. Commun. 2015, 460, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Reddish, F.N.; Miller, C.L.; Gorkhali, R.; Yang, J.J. Monitoring ER/SR Calcium Release with the Targeted Ca2+ Sensor CatchER. J. Vis. Exp. 2017, 55822. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, Y.G.; Chen, Y.; Miao, G.; Zhao, H.; Qu, W.; Li, D.; Wang, Z.; Liu, N.; Li, L.; Chen, S.; et al. The ER-Localized Transmembrane Protein EPG-3/VMP1 Regulates SERCA Activity to Control ER-Isolation Membrane Contacts for Autophagosome Formation. Mol. Cell. 2017, 67, 974–989.e6. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, C.; Paula-Lima, A. RyR-mediated calcium release in hippocampal health and disease. Trends Mol. Med. 2024, 30, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Filadi, R.; Leal, N.S.; Schreiner, B.; Rossi, A.; Dentoni, G.; Pinho, C.M.; Wiehager, B.; Cieri, D.; Calì, T.; Pizzo, P.; et al. TOM70 Sustains Cell Bioenergetics by Promoting IP3R3-Mediated ER to Mitochondria Ca2+ Transfer. Curr. Biol. 2018, 28, 369–382.e6. [Google Scholar] [CrossRef] [PubMed]
- Kuchay, S.; Saeed, M.; Giorgi, C.; Li, J.; Hoffmann, H.H.; Pinton, P.; Rice, C.M.; Pagano, M. NS5A Promotes Constitutive Degradation of IP3R3 to Counteract Apoptosis Induced by Hepatitis C Virus. Cell Rep. 2018, 25, 833–840.e3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dreier, R.; Ising, T.; Ramroth, M.; Rellmann, Y. Estradiol Inhibits ER Stress-Induced Apoptosis in Chondrocytes and Contributes to a Reduced Osteoarthritic Cartilage Degeneration in Female Mice. Front. Cell Dev. Biol. 2022, 10, 913118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhihao, L.; Jingyu, N.; Lan, L.; Michael, S.; Rui, G.; Xiyun, B.; Xiaozhi, L.; Guanwei, F. SERCA2a: A key protein in the Ca2+ cycle of the heart failure. Heart Fail. Rev. 2020, 25, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Machuki, J.O.; Wu, Q.; Shi, M.; Fu, L.; Adekunle, A.O.; Tao, X.; Xu, C.; Hu, X.; Yin, Z.; et al. Estrogen and calcium handling proteins: New discoveries and mechanisms in cardiovascular diseases. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H820–H829. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharan, S.; Kandasamy, K.K.; Dayalan, P.; Ramamurthy, V. Estrogen induced concentration dependent differential gene expression in human breast cancer (MCF7) cells: Role of transcription factors. Biochem. Biophys. Res. Commun. 2013, 437, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Schmidhauser, M.; Hankele, A.K.; Ulbrich, S.E. Reconsidering “low-dose”-Impacts of oral estrogen exposure during preimplantation embryo development. Mol. Reprod. Dev. 2023, 90, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Kiyama, R.; Wada-Kiyama, Y. Estrogenic endocrine disruptors: Molecular mechanisms of action. Environ. Int. 2015, 83, 11–40. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, X.; Wang, M.; Qiu, S.; Pan, Y.; Cui, Y.; Yu, S. Estradiol Alleviates Elevated Temperature-Induced Damage in Yak Oviductal Epithelial Cells by Maintaining Endoplasmic Reticulum Calcium Homeostasis. Animals 2025, 15, 1305. https://doi.org/10.3390/ani15091305
Ye X, Wang M, Qiu S, Pan Y, Cui Y, Yu S. Estradiol Alleviates Elevated Temperature-Induced Damage in Yak Oviductal Epithelial Cells by Maintaining Endoplasmic Reticulum Calcium Homeostasis. Animals. 2025; 15(9):1305. https://doi.org/10.3390/ani15091305
Chicago/Turabian StyleYe, Xiaolin, Meng Wang, Shantong Qiu, Yangyang Pan, Yan Cui, and Sijiu Yu. 2025. "Estradiol Alleviates Elevated Temperature-Induced Damage in Yak Oviductal Epithelial Cells by Maintaining Endoplasmic Reticulum Calcium Homeostasis" Animals 15, no. 9: 1305. https://doi.org/10.3390/ani15091305
APA StyleYe, X., Wang, M., Qiu, S., Pan, Y., Cui, Y., & Yu, S. (2025). Estradiol Alleviates Elevated Temperature-Induced Damage in Yak Oviductal Epithelial Cells by Maintaining Endoplasmic Reticulum Calcium Homeostasis. Animals, 15(9), 1305. https://doi.org/10.3390/ani15091305





