Simple Summary
The emergence of antimicrobial resistance and drug residues caused by antibiotic overuse has become a critical constraint on the sustainable development of animal husbandry. Probiotics have attracted significant attention due to their potential to enhance gut health and improve growth performance in livestock. However, their efficacy is highly dependent on strain specificity, dosage, and origin. Consequently, screening and validating safe and high-efficiency functional probiotics as antibiotic substitutes to optimize the feed conversion ratio (FCR) in broilers remains a key focus in animal nutrition research. In this study, B. coagulans BCH0 (B. coagulans BCH0, 1 × 109 CFU/kg) was added to broiler diets to evaluate its potential as an antibiotic replacement. The results demonstrated that B. coagulans BCH0 significantly improved growth performance (p < 0.05) by modulating intestinal morphology, optimizing gut microbiota composition, and upregulating the transcription of nutrient digestion- and absorption-related genes. These findings underscored the substantial potential of B. coagulans BCH0 as a sustainable, antibiotic-alternative feed additive.
Abstract
Studies demonstrated that Bacillus coagulans (B. coagulans) as a dietary additive enhanced broiler growth performance, yet its mechanisms of action modulation remained unclear. Therefore, this study investigated effects of dietary B. coagulans BCH0 (1 × 109 CFU/kg) on growth performance, intestinal morphology, gut microbiota, and ileal transcriptomics in Arbor Acres broilers using a completely randomized design. A total of 200 one-day-old broilers were allocated to control (Con, basal diet) and experimental (BCH0, basal diet + 1 × 109 CFU/kg B. coagulans BCH0) groups (10 replicates/group, 10 birds/replicate) over a 42-day trial. The results revealed that BCH0 significantly increased body weights (BW) at 21 and 42 days (p < 0.05), improved the average daily gain (ADG) during the starter (1–21 days) and overall phases (1–42 days), and reduced the ratio of feed intake to body weight gain (F/G) across all phases (p < 0.05). Duodenal morphology analysis indicated a BCH0 elevated villus height (+16.9%, p < 0.01) and villus height/crypt depth (V/C) (p < 0.01) and no significant differences in crypt depth (p = 0.46). In the ileum, the BCH0 group exhibited a significantly greater villus height (p < 0.01), crypt depth (p < 0.05), and V/C (p < 0.05) than the Con group. Microbiota analysis revealed no significant differences in α-diversity or β-diversity, but phylum-level shifts involved an increase in Firmicutes and a reduction in Actinobacteriota in the BCH0 group. At the genus level, dominance shifted from Romboutsia (Control group) to Lactobacillus (BCH0 group), accompanied by marked reductions in Turicibacter, Ldatus_arthromitus, and Rothia. Ileal transcriptomics identified 605 differentially expressed genes, with KEGG enrichment highlighting activated nutrient assimilation pathways (p < 0.05), including carbohydrate, mineral, fat, and protein digestion/absorption. These findings collectively demonstrated that B. coagulans BCH0 enhanced broiler growth through the synergistic modulation of beneficial microbiota, the upregulation of nutrient metabolism genes, and intestinal architectural optimization, supporting its role as a sustainable microbial additive for enhancing poultry productivity and gut health.
1. Introduction
Numerous studies have demonstrated that probiotics intake effectively maintains gut health [1,2]. As fundamental bioactive components, these microorganisms are strategically added to functional food matrices, including fermented dairy products and dietary additives [3]. The intake of food products enriched with probiotics can enhance intestinal immunity and improve lactose tolerance [4]. Probiotics also promote intestinal digestion and nutrient absorption, inhibit the colonization of pathogenic bacteria, prevent gastroenteritis, and improve gut microbiota [5,6,7]. Studies indicated that a daily intake of viable probiotics could modulate gut microbiota balance [8]. Studies have shown that a daily intake of at least 1 × 109 CFU/kg of probiotics was effective in improving intestinal health [9].
B. coagulans, a significant edible probiotic, is a gram-positive, parthenogenetic anaerobic bacterium with non-pathogenic endospore-forming characteristics [10]. B. coagulans is highly resistant to environmental stresses and exhibits strong tolerance to high temperatures, gastric acid, and bile salts [11]. It is also widely recognized for its probiotic properties [12,13]. In addition, B. coagulans is not resistant to antibiotics and can be used as an substitute to antibiotics in medicine and animal husbandry [14,15]. B. coagulans improves digestibility by secreting enzymes such as protease, α-amylase, xylanase, and lipase, as well as producing amino acids and vitamins [16,17]. B. coagulans can effectively improve gastrointestinal discomfort in humans [18,19] and attenuate intestinal mucosal damage in immunosuppressed mice [20] by modulating the intestinal barrier, inflammatory response, and intestinal microbiota. Studies have demonstrated its capacity to increase the height of ileocecal villus while reducing crypt depth in weaned piglets, thereby improving nutrient absorption [21]. Adding B. coagulans to the feed can positively influence improvements in feed consumption and weight gain in heat-stressed broiler chickens and enhance the development effect in broiler chickens after infection with Clostridium perfringens and Salmonella [22].Compared to traditional B. coagulans, B. coagulans BCH0 exhibits high-temperature resistance, acid–bile salt tolerance, and other characteristics. Moreover, its solid-state fermentation process reduces production costs while achieving high yields. Additionally, it poses no risk of antibiotic resistance and possesses unique advantages due to its special acid-producing metabolic pathway. Although previous studies have shown that B. coagulans could enhance intestinal health and growth performance in broilers, the cascade mechanisms underlying this repair process remain unclear. Therefore, this study explored effects of adding B. coagulans BCH0 to diet on growth performance, tissue structures, and gut microbiota in broilers, which aims to investigate the safety and mechanism of B. coagulans BCH0.
2. Materials and Methods
2.1. Preparation of B. coagulans BCH0 Additive
The additive used in this study was a dried preparation of the B. coagulans BCH0 strain produced via solid-state fermentation and provided by the College of Animal Science and Technology, China Agricultural University (Beijing, China). The product contains B. coagulans spores and solid-state fermentation substrates, with a viable bacterial concentration of 2.52 × 1011 CFU/kg. The mixture was homogenized by pulverization through a 20-mesh sieve. When added to broiler feed at a 0.5% inclusion rate, this additive amount ensured a final microbial concentration of 1 × 109 CFU/kg. This inclusion level (1 × 109 CFU/kg) was based on evidence that B. coagulans enhanced the intestinal mucosal barrier function and immune responses in broilers [23,24].
2.2. Animals and Experimental Location
Animals were housed in metal cages (1.8 mL × 0.8 mW × 0.5 mH), each equipped with automated water-line systems (replacing conventional drinkers), troughs, and natural ventilation. The lighting regime was artificially controlled to provide 16 h of light daily, with 8 h of darkness at night. The house temperature was maintained at 33–35 °C during days 1–7 post-hatching, then gradually decreased by 2–3 °C weekly until reaching an ambient temperature (20–25 °C), which was sustained thereafter. Temperature adjustments were made based on the behavioral responses of the broilers. Relative humidity was regulated as follows: 60–70% during days 1–7, progressively reduced to 50–60% during days 8–21, and stabilized at 45–55% during days 22–42. All the broilers were housed in a single-tier cage system to ensure uniform environmental conditions across experimental groups. The broilers had ad libitum feed access, with routine vaccination and daily sanitation protocols maintained throughout the trial.
2.3. Animals and Trial Design
This experiment was managed and designed according to the Chinese Guidelines for Animal Welfare upon approval by the Institutional Animal Care and Application Ethics Committee of China Agricultural University (Approval Number: AW72205202-1-02).
This research was carried out at the experimental farm of the College of Animal Science and Technology, China Agricultural University. A completely randomized design was adopted, with a total of 200 one-day-old Arbor Acres (AA) broilers allotted to two groups, each containing 10 replicates of 10 broilers. The broilers in the control group were fed a basal diet (Con group), while the experimental group received diets containing 1 × 109 CFU/kg of B. coagulans BCH0 through a 0.5% product inclusion rate (BCH0 group). The experiment lasted for 42 days, divided into two phases (1–21 and 22–42 days). The trial employed a corn–soybean meal-based diet, with the basal diet composition and nutritional profile detailed in Table 1.

Table 1.
The ingredients and nutritional components of the diet (DM basis).
2.4. Sample Collection and Assay
BW were recorded per replicate at days 1, 21, and 42. Feed intake was quantified for three phases: days 1–21, days 22–42, and days 1–42. The key metrics were calculated as follows:
Average daily gain (ADG) = (Final body weight − Initial body weight) (g)/Duration (day)
The ratio of feed intake to body weight gain (F/G) = Total feed intake (g)/Total body weight gain (g).
The broilers were fasted for 12 h with free access to water before slaughter, placed in a 40 L transparent polycarbonate chamber, and exposed to high-purity CO2 (>99%) through a flow meter and pressure regulator. After loss of consciousness, carotid artery cannulation was performed for exsanguination, followed by the aseptic collection of intestinal samples. Through midline celiotomy, 2 cm duodenal segments (immediately distal to the pylorus) and ileal segments (proximal to the ileocecal junction) were excised with sterile iris scissors. Tissues were immersion-fixed in 4% neutral-buffered formalin (NBF, pH 7.2) for 24–48 h at 4 °C before paraffin embedding.
Fixed tissues were dehydrated with gradient ethanol and xylene, and then paraffin was embedded. The embedded blocks were sectioned into 4 µm sections using a pathology microtome (Leica, Wetzlar, Germany). The sections were dewaxed, rehydrated using xylene and graded alcohol concentrations, and stained with hematoxylin and eosin solution (Solarbio, Beijing, China).
Tissue morphology, villus height, and crypt depth were obtained by optical microscope (Olympus BX63, Tokyo, Japan). Three sections were selected from each intestinal segment. For each tissue section, 10 morphologically intact villi and their associated crypts were randomly chosen.
Ileal chyme and mucosal specimens were aseptically collected into 2 mL cryovials, immediately flash-frozen in liquid nitrogen during transport, and archived at −80 °C. For multi-omics profiling, 1 g aliquots of ileal chyme and mucosa were submitted to Majorbio Bio-pharm Technology Co., Ltd. (Shanghai, China) for parallel 16S rRNA amplicon sequencing and transcriptomic analysis.
2.5. Statistical Analysis
The data on growth performance (BW, ADG, F/G) and intestinal morphological structure (villus heigh, crypt depth, V/C) were processed using SPSS 26.0 statistical software (IBM Corporation, Chicago, IL USA) for Windows, with the independent samples t-test for analysis. All data are presented as mean values, with statistical significance set at p < 0.05.
The 16S rRNA gene sequencing was performed using the MajorBio Cloud Platform https://cloud.majorbio.com (accessed on 26 February 2025). The alpha diversity of the gut microbiota was evaluated using indices including ACE, Chao1, Simpson, and Shannon. The beta diversity of the microbial communities was assessed via principal coordinate analysis. The relative abundances of microbial taxa at the genus level were calculated to characterize the compositional features of the gut microbiota. Differential microbial communities were further identified through LEfSe analysis. Raw transcriptomic sequencing data were analyzed for differentially expressed genes (DEGs) on the MajorBio Cloud Platform, followed by gene ontology (GO) functional annotation and KEGG pathway enrichment analysis.
3. Results
3.1. Growth Performance
As presented in Table 2, adding B. coagulans BCH0 to the diet significantly enhanced growth performance in AA broilers. Specifically, the BCH0 group exhibited significantly higher BW than the Con group at both 21 and 42 days of age (p < 0.05). Notably, the ADG was markedly increased in the B. coagulans-added group during the 1–21 day and 1–42 day periods (p < 0.05). Furthermore, the F/G demonstrated significant improvements in the treatment group across all measured intervals (1–21 days and 1–42 days) compared to the Con group (p < 0.05).

Table 2.
Effects of B. coagulans BCH0 on the growth performance of AA broilers.
3.2. Intestinal Morphology
As illustrated in Figure 1, histomorphology features of the intestinal villi in broiler chickens are presented. As presented in Table 3, duodenal morphological analysis demonstrated that the BCH0 group exhibited significantly greater villus height than the Con group (p < 0.01), while no significant difference in crypt depth was observed between the groups (p = 0.4641). Notably, the ratio of V/C was markedly elevated in the BCH0-treated animals (p < 0.01), indicating enhanced intestinal absorptive capacity. In the ileum, the Con group had a greater villus height (p < 0.01), crypt depth (p < 0.05), and V/C (p < 0.05) than the experimental group.
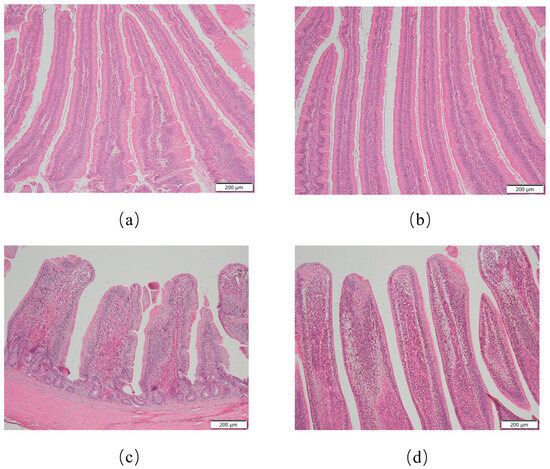
Figure 1.
Histological morphology of intestinal villi in broilers. (a) Duodenal villus architecture in the control group (Con); (b) Duodenal villus in the B. coagulans BCH0-added group; (c) Ileal villus morphology in the Con group; (d) Ileal villus in the B. coagulans BCH0-added group. Scale bar: 200 μm.

Table 3.
Effects of B. coagulans BCH0 on tissue structures of AA broilers.
3.3. Gut Microbiota
As presented in Figure 2, the experimental group showed no significant differences in Chao1, Shannon, Simpson, and Ace indices compared to the control group, indicating no significant differences in α diversity (p > 0.05).
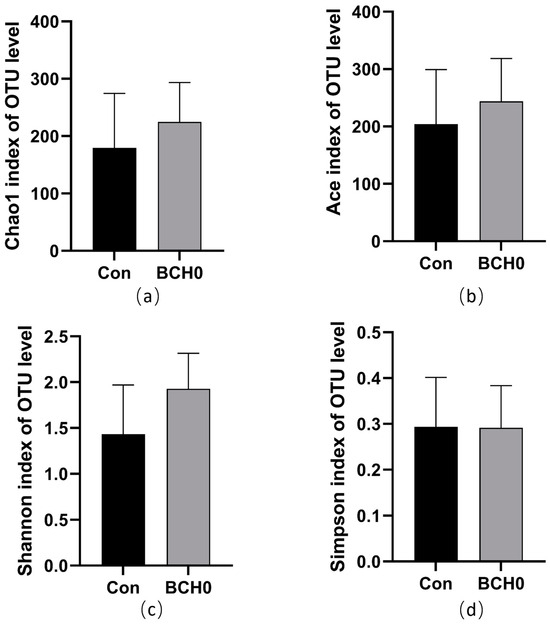
Figure 2.
Comparative analysis of α-diversity indices between experimental groups. (a) Chao1 index at OTU level; (b) Ace index at OTU level; (c) Shannon index at OTU level; (d) Simpson index at OTU level.
Comparative analysis of the beta-diversity indices between the experimental groups (Figure 3a) revealed no statistically significant differences (p > 0.05). Beta-diversity analysis of ileal bacterial communities, as demonstrated by principal coordinate analysis (PCoA) (R2 = 0.1904, p = 0.067), revealed no significant difference in composition between the experimental and Con groups. The proximity of data points in analyses indicated a high degree of similarity in the microbial community structure, suggesting minimal intervention-driven alterations in ileal bacterial beta diversity. At the phylum level, Firmicutes remained the dominant and most stable taxon in both the Con and experimental groups. The relative abundance of Firmicutes in the experimental group showed a marginal increase, while Actinobacteriota displayed a slight reduction. Hierarchical clustering analysis of the dominant microbiota (Figure 3c) revealed partial segregation between the Con and B. coagulans-added groups, with subsets of samples clustered by treatment. This pattern suggested moderate intra-group homogeneity in genus-level microbial composition, although overlapping clusters highlight shared taxonomic features between groups. Bar plot visualization further demonstrated genus-specific shifts-Romboutsia was predominant in the Con group, whereas Lactobacillus emerged as the dominant genus in the experimental group.
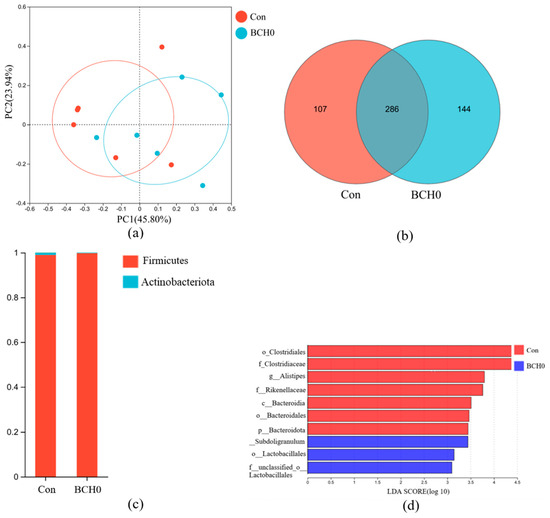
Figure 3.
Microbial diversity analysis. (a) The analysis of β-diversity contrasts was made by PCoA (R2 = 0.1904, p = 0.067); (b) Venn diagram of OTU; (c) phylum-level abundance; (d) genus-level heatmap with dendrogram.
3.4. Intestinal Tissue Gene Transcription
As shown in Figure 4, in the transcriptional analysis of ileum tissues, the Venn diagram (Figure 4a) reveals that 12,066 genes (93.13%) were commonly expressed in both the Con and BCH0 groups, while 319 unique genes (2.46%) are specific to the BCH0 group and 571 genes (4.41%) are exclusive to the Con group. Furthermore, the differential expression bar chart (Figure 4b) and volcano plot (Figure 4c) demonstrate 358 significantly upregulated genes and 247 downregulated genes in the BCH0 group compared to the Con group. This distinct imbalance toward upregulated genes suggested that BCH0 treatment predominantly enhanced transcriptional activity in ileum tissues.
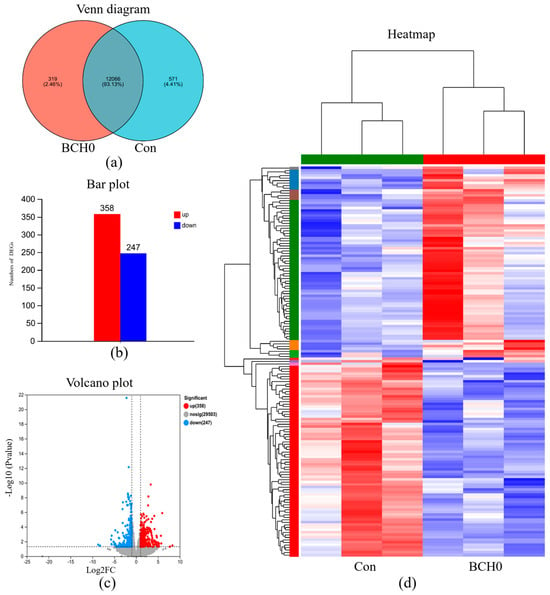
Figure 4.
Transcriptome analysis of the B. coagulans BCH0 group. (a) Venn diagram: overlap of differentially expressed genes (DEGs); (b) Bar plot: quantitative comparison of regulated differentially expressed genes (DEGs); (c) Volcano plot: visualization of DEG significance versus magnitude. (d) Heatmap: hierarchical clustering of DEG expression profiles.
KEGG and GO enrichment analyses are presented in Figure 5a and Figure 5b, respectively. GO enrichment analysis on the differentially expressed genes was performed and shows that the enrichment of differentially expressed genes in biological processes (BP) mostly includes the cellular process, biological regulation, and the metabolic process. To further examine the role of differentially expressed genes, KEGG enrichment analysis was performed. It displayed the top 11 differentially expressed pathways. These included carbohydrate digestion and absorption, mineral absorption, fat digestion and absorption, an intestinal immune network for IgA generation, a B-cell receptor signaling pathway, vitamin digestion and absorption, protein digestion and absorption, starch and sucrose metabolism, and galactose metabolism.
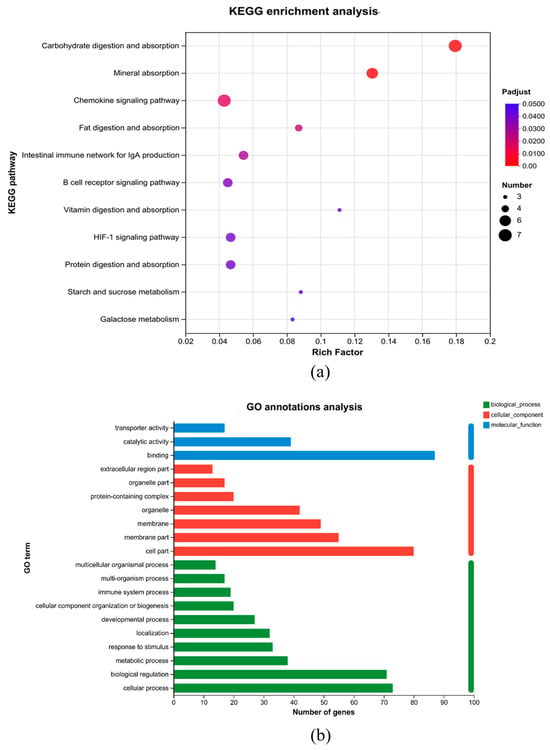
Figure 5.
Transcriptome analysis of the B. coagulans BCH0 group. (a) Gene ontology (GO) term enrichment analysis of the DEGs. (b) KEGG enrichment analysis. The criteria for filtering KEGG pathways with diversities is p < 0.05.
4. Discussion
Experimental evidence and meta-analytical reviews consistently demonstrated that probiotics represent a viable antibiotic-alternative strategy for enhancing growth performance in livestock [25,26]. The current investigation revealed that adding B. coagulans BCH0 to the diet significantly improved key growth parameters in AA broilers, as evidenced by an increased final BW, a higher ADG, and an optimized feed conversion ratio [27,28]. It is noteworthy that the substantial ADG improvements during both the starter phase (1–21 days) and the entire trial period (1–42 days), indicating probiotic efficacy during early developmental stages. These findings align with previous reports documenting a 4.6–5.3% ADG increase when B. coagulans was added to broiler diets [29,30]. The BCH0 group maintained consistent F/G improvements across all growth phases, underscoring this strain’s capacity to enhance nutrient utilization efficiency throughout the production cycle. The mechanistic basis for these improvements appears multifactorial. B. coagulans exhibits exceptional gastrointestinal survival capabilities due to its acid–bile resistance, facilitating intestinal colonization and the subsequent production of bioactive metabolites. A study demonstrated that intestinal colonization by B. coagulans stimulates endogenous lipase and protease secretion, enhancing the hydrolysis of complex dietary substrates [31]. From an applied perspective, the demonstrated growth enhancement carries dual implications: economic benefits through improved feed efficiency and environmental advantages via a reduced reliance on growth-promoting antibiotics. These findings substantiate B. coagulans BCH0 as a sustainable growth promoter in modern poultry production systems.
The small intestine serves as the primary site for nutrient absorption, with its functional efficiency being directly influenced by intestinal structural integrity. The development of intestinal villi plays a decisive role in determining digestive capacity. Morphological parameters such as villus height and crypt depth in the intestinal mucosa represent the most direct indicators for evaluating intestinal morphology [32]. A well-developed villus architecture characterized by intact, elongated villi and appropriate crypt depth facilitates optimal nutrient assimilation. Conversely, epithelial cell damage in the intestinal mucosa leads to significant villus shortening and crypt depth enlargement. This morphological alteration reflects the compensatory proliferation of crypt stem cells, which actively regenerate to repair the damaged villus epithelium [33,34]. Previous studies have found that dietary B. coagulans contributed to the preservation of normal intestinal morphology by antagonizing pathogenic bacterial colonization and counteracting toxin invasion [35]. Morphological analysis revealed that adding B. coagulans BCH0 to the diet significantly improved the intestinal architecture compared to the control group. In the duodenum, the BCH0 group demonstrated a markedly increased villus height (p < 0.01) and a reduced crypt depth, resulting in the elevated V/C. This enhancement was particularly pronounced in the ileum, where both the villus height and V/C showed a significant increase. These structural improvements align with established probiotic mechanisms, as prior studies confirm that adding B. coagulans BCH0 to the diet promotes villus elongation while reducing crypt depth, ultimately optimizing mucosal morphology for enhanced nutrient absorption [36,37,38]. The observed intestinal improvements might be attributed to the unique sporulation capacity of B. coagulans, enabling effective intestinal colonization. Upon reaching the gastrointestinal tract, BCH0 modulated microbial homeostasis through the biosynthesis of antimicrobial compounds, digestive enzymes, and bioactive metabolites. This multipronged action enhances luminal nutrient bioavailability, mitigates epithelial damage via anti-inflammatory mediators, and stimulates trophic factors for villus development [39,40]. Collectively, our findings substantiated that the dietary addition of B. coagulans BCH0 effectively preserved the intestinal mucosal architecture by synergistically reinforcing digestive competence, barrier function, and epithelial regeneration capacity.
Studies have demonstrated that gut microbiota played a pivotal role in nutrient digestion and absorption, the regulation of host fat deposition, the promotion of intestinal epithelial cell renewal, and immune system enhancement [20,41,42]. Probiotic addition has been shown to influence gut flora characteristics, particularly in microbial diversity, community composition, and relative abundance, with dietary probiotics serving as an effective modulator of microbial diversity in animals [43]. Our analysis of ileal microbiota revealed subtle but meaningful compositional changes following the addition of B. coagulans BCH0 to the diet. The experimental group showed no significant differences in the Chao1, Shannon, Simpson, and Ace indices compared to the control (Con) group, indicating no significant differences in α diversity (p > 0.05). The beta-diversity analysis via principal coordinate analysis (PCoA, R2 = 0.1904, p = 0.067) demonstrated no statistically significant differences in microbial composition between the experimental and control groups. At the phylum level, Firmicutes maintained dominance in both groups, while genus-level analysis revealed a notable ecological shift, with Lactobacillus replacing Romboutsia as the predominant genus in added subjects. This selective enrichment of Lactobacillus, a genus well-documented for its beneficial effects on intestinal health regulation, immune enhancement, and growth promotion in broiler chickens, suggests that B. coagulans BCH0 exerts targeted modulatory effects on ileal microbial communities [44]. The mechanism might be that B. coagulans enhances the growth of Lactobacillus by consuming oxygen to create an anaerobic environment while producing lactic acid and short-chain fatty acids that modulate the gut pH and supply metabolic substrates, collectively supporting the colonization of lactobacilli [45,46]. The efficacy of B. coagulans BCH0 may vary between cage and litter rearing systems due to differences in microbial competition and colonization resistance [47,48]. In cage systems, where gut microbiota diversity is typically lower, B. coagulans BCH0 may establish more effectively, potentially exerting stronger probiotic effects on growth performance and immune modulation. Conversely, in litter systems, the richer microbial environment could enhance competition, limiting B. coagulans BCH0 colonization but possibly synergizing with native Actinobacteria. Thus, B. coagulans BCH0 may require higher doses or complementary strategies in litter systems to overcome microbial interference, whereas cage-reared birds might benefit more from direct probiotic addition [49]. The observed microbial modifications align with established probiotic mechanisms, including pathogen inhibition, the structural optimization of gut microbial communities, and subsequent improvements in intestinal health parameters [50]. These findings collectively indicate that adding B. coagulans BCH0 to the diet induces specific, genus-level microbial adaptations while maintaining the overall community architecture, demonstrating the potential for the precision modulation of gut microbiota through probiotic interventions.
Through analysis of the transcriptome of the ileal tissues of AA broiler chickens, the differential pathways indicated that B. coagulans BCH0 improved the absorption and utilization of nutrients in AA broilers, promoted the improvement of broiler production performance, and had an impact on the intestinal immune function of AA broilers. These benefits included disrupting latent pathogens, enhancing barrier function and producing neurotransmitters [51]. We analyzed the transcriptome data by KEGG enrichment. After analysis, we found that carbohydrate digestion and absorption, mineral absorption, fat digestion and absorption, the intestinal immune system for IgA generation, the B-cell receptor signaling pathway, vitamin digestion and absorption, protein digestion and absorption, starch and sucrose metabolism, and galactose metabolism were upregulated. Previous studies have demonstrated that adding B. coagulans BCH0 to the diet alleviated inflammation, inhibited tumorigenesis, and enhanced immune function [52].
5. Conclusions
In this study, we demonstrated that the dietary addition of B. coagulans BCH0 activated the transcriptional expression of nutrient digestion-related genes, restored the intestinal morphology and V/C, and enhanced the colonization of beneficial microbial taxa such as Lactobacillus. These synergistic effects collectively increased the ADG of broilers and optimized the feed conversion ratio, highlighting its potential as a sustainable microbial additive for enhancing poultry productivity and gut health.
Author Contributions
Conceptualization, Z.N. and X.M.; methodology, L.J. and C.Z.; investigation, J.S., Z.C. and C.Z.; writing—original draft, Z.N. and P.T.; data curation, Z.N., Y.Z. and L.J.; resources, X.M.; validation, J.S. and Z.M.; visualization, Z.N.; writing—review and editing, P.T. and X.M.; funding acquisition, X.M.; project administration, X.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key R&D Program of China (2022YFD1300404), the National Natural Science Foundation of China (U22A20514, U23A20232), the Pinduoduo–China Agricultural University Research Fund (PC2023A01001), the 2115 Talent Development Program of China Agricultural University (1041-00109019).
Institutional Review Board Statement
The animal experimental protocol employed in this study was reviewed and approved by the Laboratory Animal Welfare and Animal Experimental Ethical Committee of China Agricultural University, Beijing, China (Approval number: AW72205202-1-02). All procedures involving animals were conducted in strict accordance with the ethical guidelines and regulations for the care and use of laboratory animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets supporting the conclusions of this article are available in the NCBI Sequence Read Archive (SRA) repository under accession number PRJNA1255734 and PRJNA1255727 (available on 26 April 2025).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gupta, V.K.; Rajendraprasad, S.; Ozkan, M.; Ramachandran, D.; Ahmad, S.; Bakken, J.S.; Laudanski, K.; Gajic, O.; Bauer, B.; Zec, S.; et al. Safety, Feasibility, and Impact on the Gut Microbiome of Kefir Administration in Critically Ill Adults. BMC Med. 2024, 22, 80. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ma, X.K.; Tu, C.K.; Teng, H.; Shao, X.; Chen, J.; Hu, M.X. Lactobacillus reuteri Biofilms Formed on Porous Zein/Cellulose Scaffolds: Synbiotics to Regulate Intestinal Microbiota. Int. J. Biol. Macromol. 2024, 262 Pt 2, 130152. [Google Scholar] [CrossRef] [PubMed]
- Muhammed, M.; Shaheen, M.; Sivakumar, A.; Furqan, A.; Kirankumar, B. Comparative Evaluation for Thermostability and Gastrointestinal Survival of Probiotic Bacillus coagulans MTCC 5856. Biosci. Biotechnol. Biochem. 2021, 85, 962–971. [Google Scholar]
- Galvão, A.M.; Rodrigues, S.; Fernandes, F.A. Probiotic Dried Apple Snacks: Development of Probiotic Coating and Shelf-Life Studies. J. Food Process. Preserv. 2020, 44, e14974. [Google Scholar] [CrossRef]
- Ma, N.; Chen, X.; Johnston, L.J.; Ma, X. Gut Microbiota-Stem Cell Niche Crosstalk: A New Territory for Maintaining Intestinal Homeostasis. iMeta 2022, 1, e54. [Google Scholar] [CrossRef]
- Song, B.; Li, P.; Xu, H.; Wang, Z.; Yuan, J.; Zhang, B.; Lv, Z.; Song, Z.; Guo, Y. Effects of Rearing System and Antibiotic Treatment on Immune Function, Gut Microbiota and Metabolites of Broiler Chickens. J. Anim. Sci. Biotechnol. 2023, 14, 827–852. [Google Scholar] [CrossRef]
- Lavrentev, F.V.; Ashikhmina, M.S.; Ulasevich, S.A.; Morozova, O.V.; Orlova, O.Y.; Skorb, E.V.; Iakovchenko, N.V. Perspectives of Bacillus coagulans Mtcc 5856 in the Production of Fermented Dairy Products. LWT 2021, 148, 111623. [Google Scholar] [CrossRef]
- Zhuang, X.; Stephanie, C.; Nuria, A. Bigels-Oleocolloid Matrices-as Probiotic Protective Systems in Yogurt. J. Food Sci. 2021, 86, 4892–4900. [Google Scholar] [CrossRef]
- Roobab, U.; Batool, Z.; Manzoor, M.F.; Shabbir, M.A.; Khan, M.R.; Aadil, R.M. Sources, Formulations, Advanced Delivery and Health Benefits of Probiotics. Curr. Opin. Food Sci. 2020, 32, 17–28. [Google Scholar] [CrossRef]
- Gözde, K.A.; Zerrin, E. Identification and Characterization of Bacillus coagulans Strains for Probiotic Activity and Safety. LWT 2021, 151, 112233. [Google Scholar]
- Sui, L.; Zhu, X.; Wu, D.; Ma, T.; Tuo, Y.; Jiang, S.; Qian, F.; Mu, G. In Vitro Assessment of Probiotic and Functional Properties of Bacillus coagulans T242. Food Biosci. 2020, 36, 100675. [Google Scholar] [CrossRef]
- Aulitto, M.; Alfano, A.; Maresca, E.; Avolio, R.; Errico, M.E.; Gentile, G.; Cozzolino, F.; Monti, M.; Pirozzi, A.; Donsì, F.; et al. Thermophilic Biocatalysts for One-Step Conversion of Citrus Waste into Lactic Acid. Appl. Microbiol. Biotechnol. 2024, 108, 155. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.P.; Liu, D.M.; Zhao, S.; Huang, Y.Y.; Yu, J.J.; Zhou, Q.Y. Assessing the Safety and Probiotic Characteristics of Bacillus coagulans 13002 Based on Complete Genome and Phenotype Analysis. LWT 2022, 155, 112847. [Google Scholar] [CrossRef]
- Tan, P.; Wu, C.; Tang, Q.; Wang, T.; Zhou, C.; Ding, Y.; Fu, H.; Xu, S.; Feng, Y.; Zhang, Y.; et al. pH-Triggered Size-Transformable and Bioactivity-Switchable Self-Assembling Chimeric Peptide Nano-Assemblies for Combating Drug-Resistant Bacteria and Biofilms. Adv. Mater. 2023, 35, e2210766. [Google Scholar] [CrossRef]
- Tan, P.; Sun, Z.; Tang, Q.; Xu, S.; Wang, T.; Ding, Y.; Fu, H.; Zhou, C.; Zhang, Y.; Yue, Z.; et al. Manipulation of Hydrophobic Motifs and Optimization of Sequence Patterns to Design High Stability Peptides against Piglet Bacterial Infections. Nano Today 2023, 49, 101793. [Google Scholar] [CrossRef]
- Liu, C.; Ma, N.; Feng, Y.; Zhou, M.; Li, H.; Zhang, X.; Ma, X. From Probiotics to Postbiotics: Concepts and Applications. Anim. Res. One Health 2023, 1, 92–114. [Google Scholar] [CrossRef]
- Li, S.; Ke, W.; Zhang, Q.; Undersander, D.; Zhang, G. Effects of Bacillus coagulans and Lactobacillus plantarum on the Fermentation Quality, Aerobic Stability and Microbial Community of Triticale Silage. Chem. Biol. Technol. Agric. 2023, 10, 79. [Google Scholar] [CrossRef]
- Sudha, M.R.; Jayanthi, N.; Aasin, M.; Dhanashri, R.D.; Anirudh, T. Efficacy of Bacillus coagulans Unique Is2 in Treatment of Irritable Bowel Syndrome in Children: A Double Blind, Randomised Placebo Controlled Study. Benef. Microbes 2018, 9, 563–572. [Google Scholar] [CrossRef]
- Madempudi, R.S.; Ahire, J.J.; Neelamraju, J.; Tripathi, A.; Nanal, S. Randomized Clinical Trial: The Effect of Probiotic Bacillus coagulans Unique Is2 vs. Placebo on the Symptoms Management of Irritable Bowel Syndrome in Adults. Sci. Rep. 2019, 9, 12210. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, M.; Cui, X.; Chen, J.; Liu, C.; Zhang, X. Bacillus coagulans MZY531 Alleviates Intestinal Mucosal Injury in Immunosuppressive Mice Via Modulating Intestinal Barrier, Inflammatory Response, and Gut Microbiota. Sci. Rep. 2023, 13, 11181. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, Z.; Yang, Y.; Ding, L.; Yao, W. The Synbiotic Mixture of Lactulose and Bacillus coagulans Protects Intestinal Barrier Dysfunction and Apoptosis in Weaned Piglets Challenged with Lipopolysaccharide. J. Anim. Sci. Biotechnol. 2023, 14, 80. [Google Scholar] [CrossRef] [PubMed]
- Laura, F.; Wilfried, V.; Jürgen, Z.; Ronald, G.; EvaMaria, S. The Impact of Pre- and Probiotic Product Combinations on Ex Vivo Growth of Avian Pathogenic Escherichia coli and Salmonella Enteritidis. Microorganisms 2022, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Radebe, S.M.; Zhang, H.; Jia, J.; Xie, S.; Shi, M.; Yu, Q. Effect of Bacillus coagulans on Maintaining the Integrity Intestinal Mucosal Barrier in Broilers. Vet. Microbiol. 2022, 266, 109357. [Google Scholar] [CrossRef]
- Guo, S.; Xi, Y.; Xia, Y.; Wu, T.; Zhao, D.; Zhang, Z.; Ding, B. Dietary Lactobacillus fermentum and Bacillus coagulans Supplementation Modulates Intestinal Immunity and Microbiota of Broiler Chickens Challenged by Clostridium perfringens. Front. Vet. Sci. 2021, 8, 680742. [Google Scholar] [CrossRef]
- Sjofjan, O.; Adli, D.N.; Harahap, R.P.; Jayanegara, A.; Utama, D.T.; Seruni, A.P. The Effects of Lactic Acid Bacteria and Yeasts as Probiotics on the Growth Performance, Relative Organ Weight, Blood Parameters, and Immune Responses of Broiler: A Meta-Analysis. F1000Research 2021, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Uzabaci, E.; Yibar, A. Effects of Probiotic Supplementation on Broiler Growth Performance: A Meta-Analysis of Randomised Controlled Trials. Anim. Prod. Sci. 2023, 63, 645–651. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, L.; Liu, H.; Shen, J.; Zhang, Y.; Lu, L.; Zhang, X.; Ma, X. Bacillus subtilis M6 Improves Intestinal Barrier, Antioxidant Capacity and Gut Microbial Composition in Aa Broiler. Front. Nutr. 2022, 9, 965310. [Google Scholar] [CrossRef]
- Zhang, L.; Laila, B.S.; Nadège, H.; Séverine, Z.; Sory, D.M.; Ismail, F. Effects of Drinking Water Supplementation with Lactobacillus reuteri, and a Mixture of Reuterin and Microcin J25 on the Growth Performance, Caecal Microbiota and Selected Metabolites of Broiler Chickens. J. Anim. Sci. Biotechnol. 2022, 13, 34. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, H.; Yu, Y.; Zhang, R.; Wu, Y.; Yue, M.; Yang, C. Effects of Bacillus coagulans on Growth Performance, Antioxidant Capacity, Immunity Function, and Gut Health in Broilers. Poult. Sci. 2021, 100, 101168. [Google Scholar] [CrossRef]
- Wu, Y.; Shao, Y.; Song, B.; Zhen, W.; Wang, Z.; Guo, Y.; Shahid, M.S.; Nie, W. Effects of Bacillus coagulans Supplementation on the Growth Performance and Gut Health of Broiler Chickens with Clostridium perfringens-Induced Necrotic Enteritis. J. Anim. Sci. Biotechnol. 2018, 9, 9. [Google Scholar] [CrossRef]
- Criado-Mesas, L.; Abdelli, N.; Noce, A.; Farré, M.; Pérez, J.F.; Solà-Oriol, D.; Martin-Venegas, R.; Forouzandeh, A.; González-Solé, F.; Folch, J.M. Transversal Gene Expression Panel to Evaluate Intestinal Health in Broiler Chickens in Different Challenging Conditions. Sci. Rep. 2021, 11, 6315. [Google Scholar] [CrossRef]
- Mikako, S.; Tatsuyuki, T.; Takaharu, K.; Junji, S.; Yohei, K. Development of Active Jejunal Glucose Absorption in Broiler Chickens. Poult. Sci. 2023, 102, 102804. [Google Scholar]
- Wang, X.; Jian, H.; Zhao, W.; Li, J.; Zou, X.; Dong, X. Effects of Dietary Bacillus coagulans on the Productive Performance, Egg Quality, Serum Parameters, and Intestinal Morphology of Laying Hens During the Late Laying Period. Ital. J. Anim. Sci. 2023, 22, 95–105. [Google Scholar] [CrossRef]
- Zi, Q.; Zhu, S.; Li, P.; Liao, Y.; Chen, D.; He, C.; Guo, S.; Zou, X. Effects of Combined Bacillus coagulans and Yeast Fermentation Culture on Growth Performance, Plasma Biochemical Indices, Intestinal Morphology and Microbial of Broilers. J. Anim. Sci. 2025, skae325. [Google Scholar] [CrossRef]
- Pan, L.; Zhao, P.F.; Ma, X.K.; Shang, Q.H.; Xu, Y.T.; Long, S.F.; Wu, Y.; Yuan, F.M.; Piao, X.S. Probiotic Supplementation Protects Weaned Pigs Against Enterotoxigenic Escherichia coli K88 Challenge and Improves Performance Similar to Antibiotics. J. Anim. Sci. 2017, 95, 2627–2639. [Google Scholar]
- Fu, R.; Liang, C.; Chen, D.; Yan, H.; Tian, G.; Zheng, P.; He, J.; Yu, J.; Mao, X.; Huang, Z.; et al. Effects of Dietary Bacillus coagulans and Yeast Hydrolysate Supplementation on Growth Performance, Immune Response and Intestinal Barrier Function in Weaned Piglets. J. Anim. Physiol. Anim. Nutr. 2021, 105, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Sokale, A.O.; Menconi, A.; Mathis, G.F.; Lumpkins, B.; Sims, M.D.; Whelan, R.A.; Doranalli, K. Effect of Bacillus subtilis Dsm 32315 on the Intestinal Structural Integrity and Growth Performance of Broiler Chickens under Necrotic Enteritis Challenge. Poult. Sci. 2019, 98, 5392–5400. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, Y.; Lv, Y.; Li, P.; Yi, D.; Wang, L.; Zhao, D.; Chen, H.; Gong, J.; Hou, Y. Beneficial Impact and Molecular Mechanism of Bacillus coagulans on Piglets’ Intestine. Int. J. Mol. Sci. 2018, 19, 2084. [Google Scholar] [CrossRef]
- Fu, T.; Kalbacher, H.; Hoffmann, W. TFF1 is Differentially Expressed in Stationary and Migratory Rat Gastric Epithelial Cells (Rgm-1) after in Vitro Wounding: Influence of Tff1 Rna Interference on Cell Migration. Cell. Physiol. Biochem. 2013, 32, 997–1010. [Google Scholar] [CrossRef]
- Hung, A.T.; Lin, S.-Y.; Yang, T.-Y.; Chou, C.-K.; Liu, H.-C.; Lu, J.-J.; Wang, B.; Chen, S.-Y.; Lien, T.-F. Effects of Bacillus coagulans Atcc 7050 on Growth Performance, Intestinal Morphology, and Microflora Composition in Broiler Chickens. Anim. Prod. Sci. 2012, 52, 874–879. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.; Yue, Z.; Tan, P.; Sun, M.; Ji, L.; Bai, Y.; Ma, X. Wickerhamomyces Anomalus Relieves Weaning Diarrhea Via Improving Gut Microbiota and Redox Homeostasis Using a Piglet Model. Food Funct. 2022, 13, 11223–11235. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Li, M.; Qian, M.; Yang, Z.; Han, X. Co-Cultures of Lactobacillus acidophilus and Bacillus subtilis Enhance Mucosal Barrier by Modulating Gut Microbiota-Derived Short-Chain Fatty Acids. Nutrients 2022, 14, 4475. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, B.; Hao, Z.; Li, H.; Cong, W.; Kang, Y. Dietary Supplementation with Weissella Cibaria C-10 and Bacillus amyloliquefaciens T-5 Enhance Immunity against Aeromonas veronii Infection in Crucian Carp (Carassiu auratus). Microb. Pathog. 2022, 167, 105559. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, S.; Chen, X.; Yang, S.; Deng, X.; Tu, M.; Tao, Y.; Xiang, W.; Rao, Y. Metabolic Profiles of Lactobacillus paraplantarum in Biofilm and Planktonic States and Investigation of Its Intestinal Modulation and Immunoregulation in Dogs. Food Funct. 2021, 12, 5317–5332. [Google Scholar] [CrossRef]
- Li, F.; Lv, B.; Zuo, J.; Nawaz, S.; Wang, Z.; Lian, L.; Yin, H.; Chen, S.; Han, X.; Wang, H. Effect of Solid-State Fermentation Products of Lactobacillus plantarum, Candida utilis, and Bacillus coagulans on Growth Performance of Broilers and Prevention of Avian Colibacillosis. Vet. Sci. 2024, 11, 468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tian, X.; Dong, Y.; Li, R.; Shen, M.; Yi, D.; Wu, T.; Wang, L.; Zhao, D.; Hou, Y. Bacillus coagulans Prevents the Decline in Average Daily Feed Intake in Young Piglets Infected with Enterotoxigenic Escherichia coli K88 by Reducing Intestinal Injury and Regulating the Gut Microbiota. Front. Cell. Infect. Microbiol. 2023, 13, 1284166. [Google Scholar] [CrossRef]
- Stanley, D.; Hughes Robert, J.; Moore Robert, J. Microbiota of the Chicken Gastrointestinal Tract: Influence on Health, Productivity and Disease. Appl. Microbiol. Biotechnol. 2014, 98, 4301–4310. [Google Scholar] [CrossRef]
- Roto, S.M.; Rubinelli, P.M.; Ricke, S.C. An Introduction to the Avian Gut Microbiota and the Effects of Yeast-Based Prebiotic-Type Compounds as Potential Feed Additives. Front. Vet. Sci. 2015, 2, 28. [Google Scholar] [CrossRef]
- Yan, L.; Lv, Z.Z.; An, S.; Xing, K.; Wang, Z.G.; Lv, M.B.; Choct, M.; Guo, Y.M.; Zhou, G.L. Effects of Rearing System and Narasin on Growth Performance, Gastro-Intestinal Development and Gut Microbiota of Broilers. Poult. Sci. 2020, 100, 100840. [Google Scholar] [CrossRef]
- Chang, X.; Kang, M.; Shen, Y.; Yun, L.; Yang, G.; Zhu, L.; Meng, X.; Zhang, J.; Su, X. Bacillus coagulans Scc-19 Maintains Intestinal Health in Cadmium-Exposed Common Carp (Cyprinus carpio L.) by Strengthening the Gut Barriers, Relieving Oxidative Stress and Modulating the Intestinal Microflora. Ecotoxicol. Environ. Saf. 2021, 228, 112977. [Google Scholar] [CrossRef]
- Borja, S.; Susana, D.; Aitor, B.-M.; Anália, L.; Miguel, G.; Abelardo, M. Probiotics, Gut Microbiota, and Their Influence on Host Health and Disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar]
- Michelangelo, C.; Hsien, T.C.; Valentina, P.; Chih, H.P.; Claudio, M. Lactate Modulation of Immune Responses in Inflammatory Versus Tumour Microenvironments. Nat. Rev. Immunol. 2020, 21, 151–161. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).