Dietary Supplementation with Complex Enzymes and Tea Residue Improved the Production Efficiency of Xiangling Pigs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design
2.3. Feeding and Management
2.4. Determination Indicators and Methods
2.4.1. Growth Performance
2.4.2. Serum Biochemical Indices
2.4.3. Meat Quality and Flavor Indices
2.4.4. Collection of Cecal Chyme and Determination of Intestinal Microbiota
2.5. Data Analysis
3. Results
3.1. Growth Performance
3.2. Serum Biochemical and Antioxidant Capacity
3.3. Meat Quality and Flavor Substances
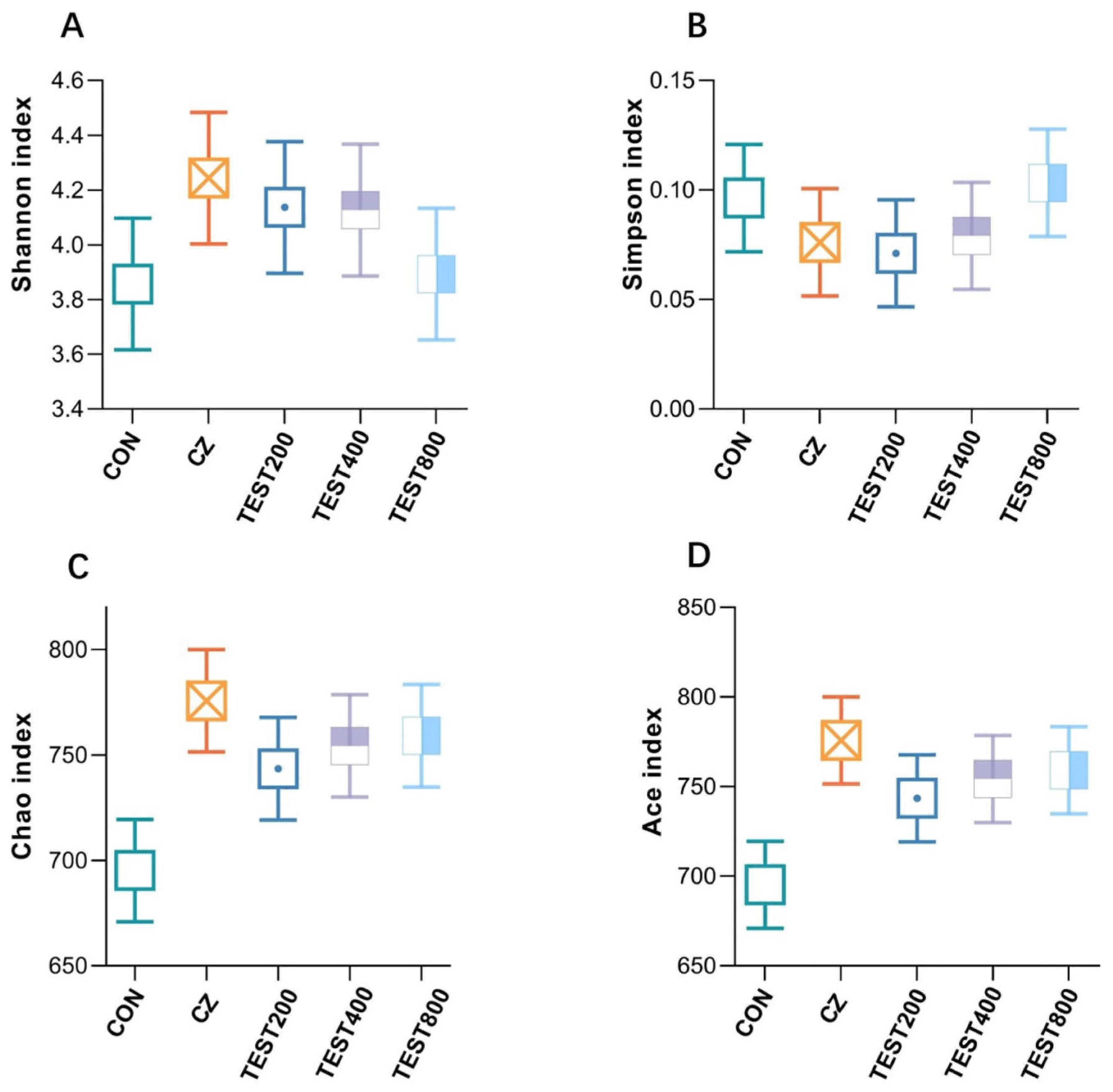
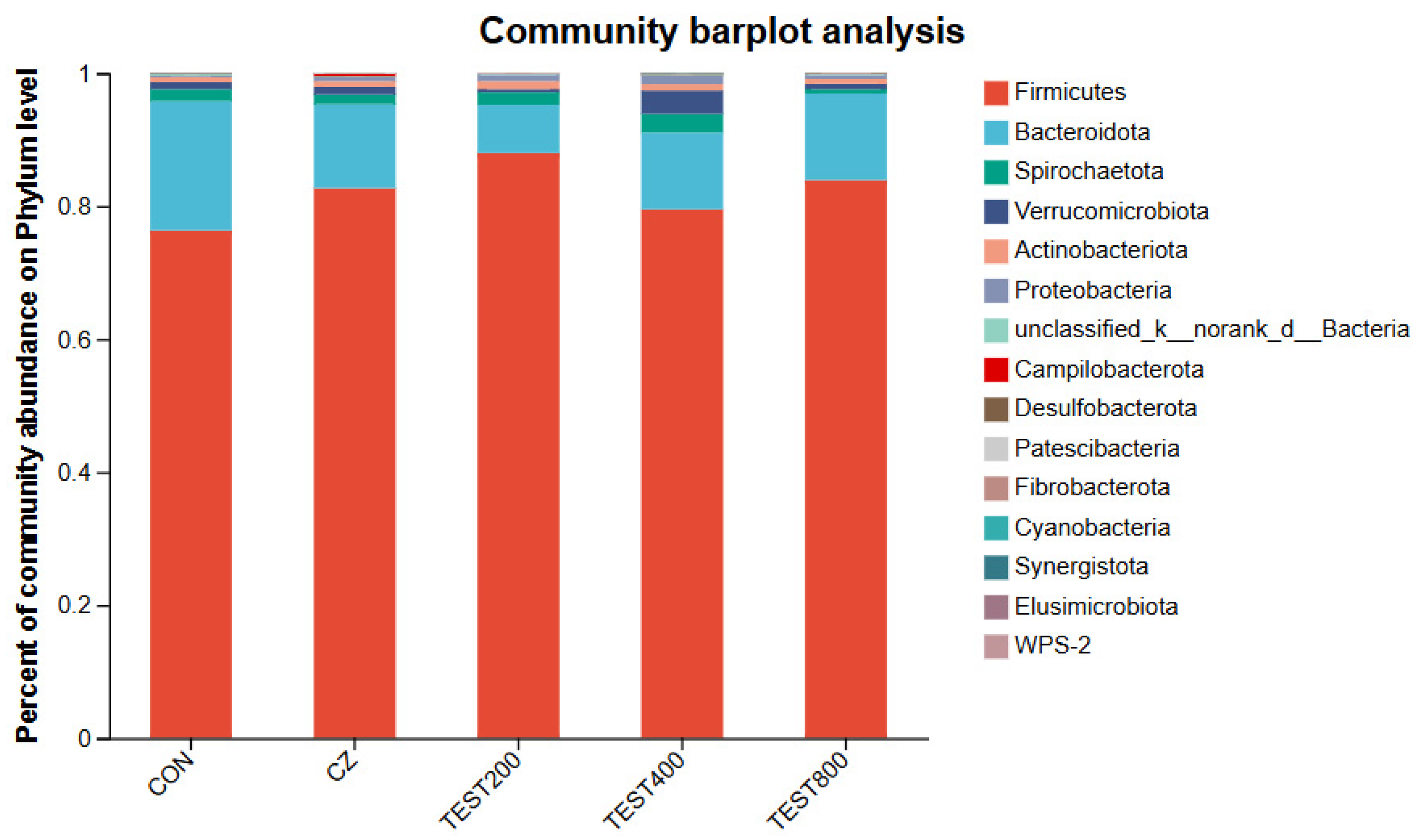
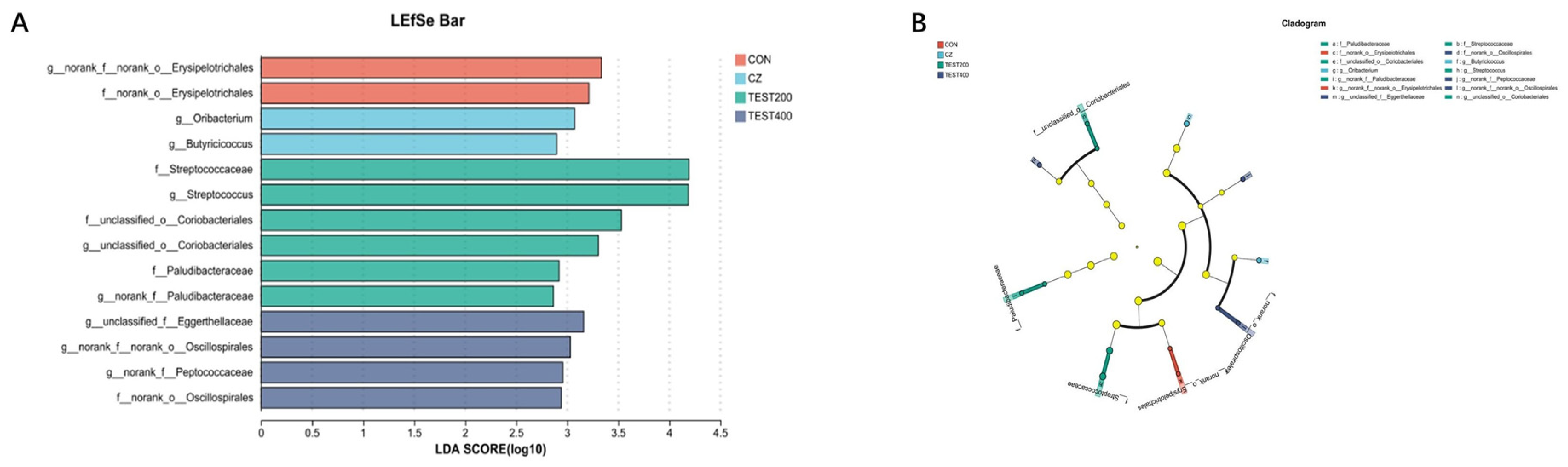
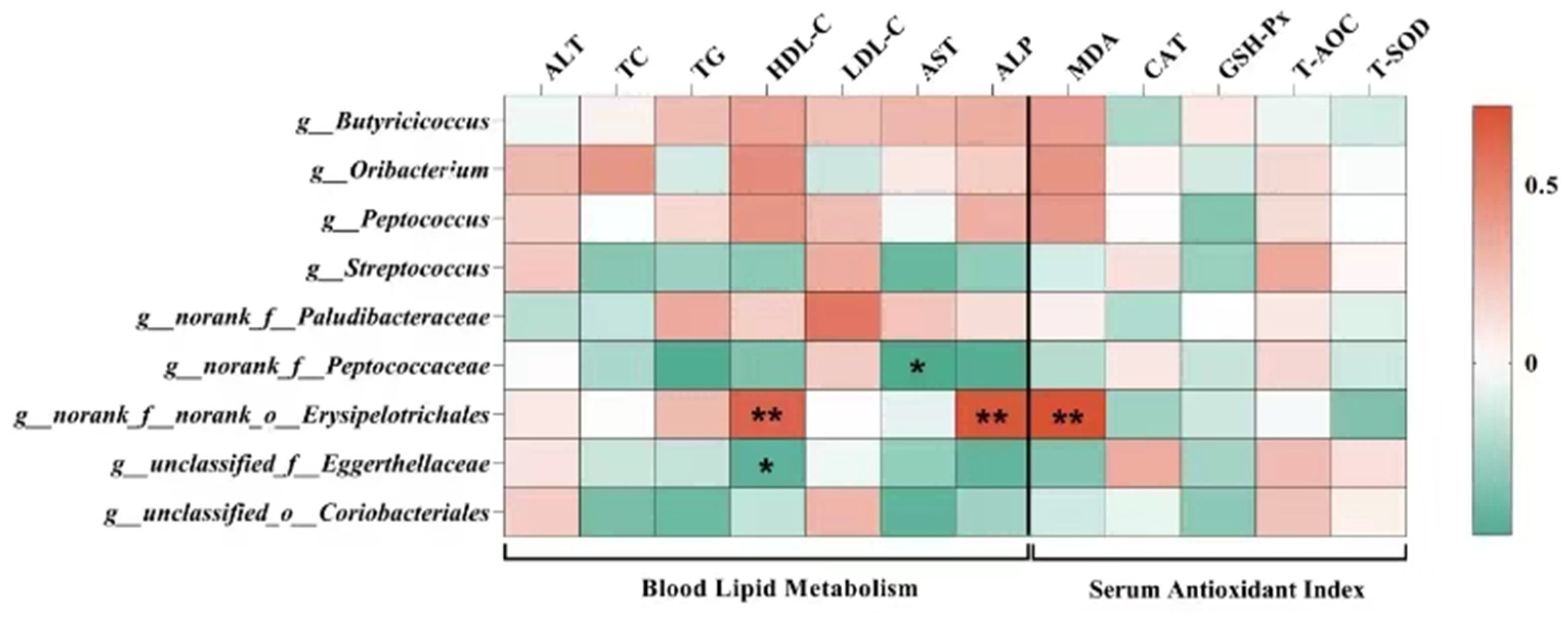
3.4. Intestinal Microbiota
4. Discussion
4.1. Effects of Tea Residue and Complex Enzymes on the Growth Performance of Xiangling Fattening Pigs
4.2. Effects of Tea Residue and Complex Enzyme Addition on Serum Biochemical and Antioxidant Indicators of Xiangling Fattening Pigs
4.3. Effects of Tea Residue and Complex Enzyme Addition on the Quality and Flavor Substances of Xiangling Fattening Pork
4.4. Effects of Tea Residue and Complex Enzyme Addition on the Intestinal Microbiota of Xiangling Fattening Pigs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Item | Unit |
| IBW | Initial body weight |
| FBW | Final body weight |
| TWG | Total weight gain |
| ADFI | Average daily feed intake |
| FCR | Feed conversion ratio |
| AA | Amino acid |
| TC | Total cholesterol |
| TG | Triglyceride |
| HDL-C | High-density lipoprotein cholesterol |
| LDL-C | Low-density lipoprotein cholesterol |
| ALP | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| T-AOC | Total antioxidant capacity |
| MDA | Malondialdehyde |
| GSH-PX | Glutathione peroxidase |
| CAT | Catalase |
| SOD | Superoxide dismutase |
| Imp | Inosine monophosphate |
References
- Yan, Y. Research on the Competitiveness Evaluation of Animal Husbandry Enterprises. Master’s Thesis, Jilin University, Changchun, China, 2023. [Google Scholar]
- Yin, J.; Liu, H.; Li, T.; Yin, Y. Current Situation and Developmental Suggestions on Shortage of Feeding Protein Resources in Chinese Pig Industry. Bull. Chin. Acad. Sci. 2019, 34, 89–93. [Google Scholar]
- Li, F. How to leapfrog the billion-level Hunan tea industry. Xiang Sheng News, 28 June 2024. [Google Scholar]
- Yin, X.; Li, Y.; Yang, H.; Teng, D.; Hong, H.; Pan, H.; Su, Z.; Ying, H. Effect of Pu-Erh Tea Residue on The Meat Quality of Chahua Chicken No. 2. Feed. Ind. Mag. 2024, 45, 43–49. [Google Scholar]
- Sun, L.; Guan, L. Research progress on active components of tea by-products and its application in animal production. Feed Res. 2024, 47, 166–171. [Google Scholar]
- Su, B.; Chen, X. Current status and potential of Moringa oleifera leaf as an alternative protein source for animal feeds. Front. Vet. Sci. 2020, 7, 53. [Google Scholar] [CrossRef]
- Li, Z.; Tang, L.; Liu, N.; Zhang, F.; Liu, X.; Jiang, Q.; Chen, J.; Ma, X. Comparative Effects of Compound Enzyme and Antibiotics on Growth Performance, Nutrient Digestibility, Blood Biochemical Index, and Intestinal Health in Weaned Pigs. Front Microbiol. 2021, 12, 768767. [Google Scholar] [CrossRef]
- Bai, Y. Determination of Productive Performance and Establishment of Comprehensive Selection Index in Xiangling Swine Breeding Herd. Master’s Thesis, Hunan Agricultural University, Changsha, China, 2022. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–500. [Google Scholar] [CrossRef]
- Gou, A. Effect of Diet with Tea Powder on Meat Quality and Carcass Traits in Pork. Master’s Thesis, China Sichuan Agricultural University, Ya’an, China, 2008. [Google Scholar]
- Wang, Y.F.; Mao, F.F.; Wei, X.L. Characterization and antioxidant activities of polysaccharides from leaves, flowers and seeds of green tea. J. Carbohydr. Polym. 2012, 55, 146–153. [Google Scholar] [CrossRef]
- Piccione, G.; Casella, S.; Lutri, L.; Vazzana, I. Reference values for some haematological, haematochemical, and electrophoretic parameters in the Girgentana goat. J. Vet. Anim. Sci. 2010, 34, 197–204. [Google Scholar] [CrossRef]
- Nzeyimana, J.B. The Effects of Green Tea Waste on Performance, Rumen Fermentation, Nutrient Digestion and Blood Parameters in Sheep. Master’s Thesis, Anhui Agricultural University, Hefei, China, 2019. (In Chinese). [Google Scholar]
- Ahmed, S.T.; Lee, J.W.; Mun, H.S.; Yang, C.J. Effects of supplementation with green tea by-products on growth performance, meat quality, blood metabolites and immune cell proliferation in goats. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1127–1137. [Google Scholar] [CrossRef]
- Cai, X.; Hayashi, S.; Fang, C.; Hao, S.; Wang, X.; Nishiguchi, S.; Tsutsui, H.; Sheng, J. Puerh tea extract-mediated protection against hepatosteatosis and insulin resistance in mice with diet-induced obesity is associated with the induction of de novo lipogenesis in visceral adipose tissue. Gastroenterology 2017, 52, 1240–1251. [Google Scholar]
- Zhang, M. Study on the Effect of Reducing Dietary Nutrition Level and Adding Compound Enzyme Preparation on Broilers. Master’s Thesis, Shandong Agricultural University, Tai’an, China, 2023. [Google Scholar]
- Li, Q.D.; Shan, A.S.; Ma, D.Y.; Du, J. Effect of Ligustrum lucidum, Schisandra chinensis and MOS on antioxidant status and blood biochemical parameters of broilers. Acta Zoonutrimenta Sin. 2005, 17, 45–48. [Google Scholar]
- Yang, C.S.; Wang, H.; Li, G.X.; Yang, Z.; Guan, F.; Jin, H. Cancer prevention by tea: Evidence from laboratory studies. Pharmacol. Res. 2011, 64, 113–122. [Google Scholar] [CrossRef]
- Han, X.; Yan, F.; Nie, X.; Xia, W.; Chen, S.; Zhang, X.-X.; Qian, L.-C. Effect of replacing antibiotics using multi-enzyme preparations on growth performance and antioxidant levels in piglets. J. Integr. Agric. 2017, 16, 640–647. [Google Scholar] [CrossRef]
- Ding, X.; Li, H.; Qian, L. Effects of Fermented Tea Residue on Fattening Performance, Meat Quality, Digestive Performance, Serum Antioxidant Capacity, and Intestinal Morphology in Fatteners. Animals 2020, 10, 185. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, U.K.; Sharma, A.K.; Pandey, A.K. Protective efficacy of Solanum xanthocarpum root extracts against free radical damage: Phytochemical analysis and antioxidant effect. Cell Mol. Biol. 2012, 58, 174–181. [Google Scholar]
- Li, X.H.; Zhang, Y.; Guo, J.Q.; Wu, J.Y. Effects of tomato pomace fermentation feed on anti-oxidation and immunity of serum and milk for Xinjiang brown cows. China Anim. Husb. Vet. Med. 2011, 38, 9–12. [Google Scholar]
- Sun, Y.; Zhang, M.; Li, Y.; Mao, Z.; Hong, L.; Wei, X.; Qi, H.; Chuan, Z.; Bo, F. Effects of dietary supplementation of tea seed cake meal on growth performance, immune function and antioxidant function of broilers. Feed Res. 2016, 39, 32–37. [Google Scholar]
- Yang, Z.; Yin, Y.; Xu, W.; Chen, G.; Zheng, M.; Yan, Z.; Liu, Y.; Zhang, G.; Peng, J.; Jiang, H.; et al. Effects of Organic Acid Combined with Essential Oil, Tannic Acid and Compound Enzyme Preparation on Growth Performance, Serum Biochemical Indexes and Intestinal Health of Weaned Piglets. China Anim. Husb. 2024, 51, 978–989. [Google Scholar]
- Mehta, N.; Ahlawat, S.; Sharma, D.; Dabur, R. Novel trends in development of dietary fiber rich meat products—A critical review. J. Food Sci. Technol. 2015, 52, 633–647. [Google Scholar] [CrossRef]
- Hamm, R. Functional properties of the myofibrillar system and their measurements. In Muscleas Food; Bechtel, P.J., Ed.; Academic Press: Orlando, FL, USA, 1986. [Google Scholar]
- Miller, K.D.; Ellis, M.; Bidner, B.; McKeith, F.K. Porcine longissimus glycolytic potential level effects on growth performance, carcass, and meat quality characteristics. J. Muscle Foods 2000, 11, 169–181. [Google Scholar] [CrossRef]
- Xie, Y.; Sun, X.; Wang, D.; Guo, C.; Jun, L.; Ting, C.; Qian, X.; Yong, Z.; Jia, S. Effects of Herbal Tea Residue on Growth Performance, Carcass Traits and Meat Quality of Finishing Pigs. Chin. J. Anim. Nutr. 2019, 31, 4776–4783. [Google Scholar]
- Joo, S.T.; Kauffman, R.G.; Kim, B.C.; Park, G.B. The relationship of sarcoplasmic and myofibrillar protein solubility to colour and water holding capacity in porcine longissimus muscle. Meat Sci. 1999, 52, 291–297. [Google Scholar] [CrossRef]
- Chen, G.H.; Liu, M.Z. Analysis of muscle nutrition characteristics of wild pig hybrids. Pig Breed. 2004, 1, 24–27. [Google Scholar]
- Xu, X.; Chen, X.; Chen, D.; Yu, B.; Yin, J.; Huang, Z. Effects of dietary apple polyphenol supplementation on carcass traits, meat quality, muscle amino acid and fatty acid composition in finishing pigs. Food Funct. 2019, 10, 7426–7434. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F. The Flavor of Meat and Aquatic Products; China Light Industry Press: Beijing, China, 2001; Version 2; Volume 1–10, pp. 54–69. [Google Scholar]
- Lee, C.W.; Lee, J.R.; Kim, M.K.; Jo, C.; Lee, K.H.; You, I.; Jung, S. Quality improvement of pork loin by dry aging. Korean Food Sci. Anim. Resour. 2016, 36, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Huang, Z.; Yu, S.; Fei, L.; Jian, Y. Research Progress of Nutritional Regulation for Meat Quality of Pigs. Chin. J. Anim. Nutr. 2020, 32, 4555–4564. [Google Scholar]
- Jung, S.; Bae, Y.S.; Kim, H.J.; Jayasena, D.D.; Lee, J.H.; Park, H.B.; Heo, K.N.; Jo, C. Carnosine, anserine, creatine, and inosine 5′-monophosphate contents in breast and thigh meats from 5 lines of Korean native chicken. Pig Sci. 2013, 92, 3275–3282. [Google Scholar] [CrossRef]
- Gong, J.X.; Cao, C.C.; Hou, L.; Du, R.Q.; Zhang, L.; Xie, J.C.; Sun, B.G. The initial-Maillard pathway to meat flavor during the preparation of thermal reaction meat flavorings. Chin. Inst. Food Sci. Technol. 2016, 16, 68–75. [Google Scholar]
- Jalbout, A.F.; Shipar, M.A.H.; Navarro, J.L. Density functional computational studies on ribose and glycine Maillard reaction: Formation of the Amadori rearrangement products in aqueous solution. Food Chem. 2007, 103, 919–926. [Google Scholar] [CrossRef]
- Normah, I.; JamUan, B.; Saarin, N.; Che, M.Y. Chemical and taste characteristics of threadfin bream (Nemipterus japonicus) hydrolysate. J. Sci. Food Agric. 2010, 84, 1290–1298. [Google Scholar] [CrossRef]
- Wu, M.; Chen, X.; Li, S.; Song, B.; Xin, Z.; Li, M.; Li, J.; Zheng, L. Effect of Dietary Fermented Feed with Tea Residue, Fungus and Enzyme on Growth Performance, Slaughter Performance and Muscle Flavor of Cyan-shank Partridge Chicken. China Poultry 2022, 44, 43–50. [Google Scholar]
- Liu, W.; Rouzembhr, F.; Seidavi, A. Effect of amount and duration of waste green tea powder on the growth performance, carcass characteristics, blood parameters, and lipid metabolites of growing broilers. Environ. Sci. Pollut. Res. Int. 2018, 25, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.C.; Wei, R.X.; Deng, D.H.; Luo, Z.; Abdulai, M.; Liu, H.; Kang, B.; Hu, S.; Li, L.; Xu, H.; et al. Effect of different types of sugar on gut physiology and microbiota in overfed goose. Poult. Sci. 2021, 100, 101208. [Google Scholar] [CrossRef]
- Li, R. Effects of Tea Residue Feed on Growth Performance, Slaughter Performance and Intestinal Develpoment of Zhedong White. Master’s Thesis, China Xinjiang Agricultural University, Urumqi, China, 2022. [Google Scholar]
- Wang, M.; Lkhagva, E.; Kim, S.; Zhai, C.; Islam, M.; Kim, H.J.; Hong, S.-T. The gut microbe pair of Oribacterium sp. GMB0313 and Ruminococcus sp. GMB0270 confers complete protection against SARS-CoV-2 infection by activating CD8+ T cell-mediated immunity. Gut Microbes 2024, 16, 2342497. [Google Scholar] [CrossRef]
- Long, S.; Hu, J.; Mahfus, S.; Ma, H.; Piao, X. Effects of dietary supplementation of compound enzymes on performance, nutrient digestibility, serum antioxidant status, immunoglobulins, intestinal morphology and microbiota community in weaned pigs. Arch. Anim. Nutr. 2021, 75, 31–47. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, J. Effects of Exogenous Complex Enzymes on Growth Performance, Nutrient Digestion and Intestinal Health of Weaned Piglets. China Feed 2020, 10, 43–46. [Google Scholar]
- Spencer, M.D.; Hamp, T.J.; Reid, R.W.; Fischer, L.M.; Zeisel, S.H.; Fodor, A.A. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology 2011, 140, 976–986. [Google Scholar] [CrossRef]
- Kamagata, Y. Oscillospira. Bergey’s Manual of Systematics of Archaea and Bacteria; Wiley: Hoboken, NJ, USA, 2015; pp. 1–6. Available online: https://onlinelibrary.wiley.com/doi/book/10.1002/9781118960608 (accessed on 22 April 2025).
- Yanagita, K.; Manome, A.; Meng, X.Y.; Hanada, S.; Kanagawa, T.; Tsuchida, T.; Mackie, R.I.; Kamagata, Y. Flow cytometric sorting, phylogenetic analysis and in situ detection of Oscillospira guillermondii, a large, morphologically conspicuous but uncultured ruminal bacterium. Int. J. Syst. Evol. Microbiol. 2003, 53, 1609–1614. [Google Scholar] [CrossRef]
- Gophna, U.; Konikoff, T.; Nielsen, H.B. Oscillospira and related bacteria—From metagenomic species to metabolic features. Environ. Microbiol. 2017, 19, 835–841. [Google Scholar] [CrossRef]
- Konikoff, T.; Gophna, U. Oscillospira: A central, enigmatic component of the human gut microbiota. Trends Microbiol. 2016, 24, 523–524. [Google Scholar] [CrossRef]

| Items | Tea Residue |
|---|---|
| Gross energy (MJ/kg) | 21.43 |
| Dry matter % | 92.32 |
| Crude protein % | 28.12 |
| Crude fiber % | 17.56 |
| Neutral detergent fiber % | 58.74 |
| Acid detergent fiber % | 32.51 |
| Crude ash % | 4.30 |
| Calcium % | 0.55 |
| Total phosphorus % | 0.22 |
| Tannin % | 0.08 |
| Caffeine % | 0.50 |
| Lysine % | 1.89 |
| Methionine % | 1.53 |
| Threonine % | 0.05 |
| Tryptophan % | 0.32 |
| Ingredient % | Basal Diet | Tea Residue Feed |
|---|---|---|
| Tea residue | 5.80 | |
| Paddy | 49.04 | 52.00 |
| Corn | 7.00 | |
| Wheat bran | 6.00 | 11.70 |
| Rice bran | 15.00 | 8.50 |
| Defatted rice bran | 3.00 | |
| Sugar cane molasses | 1.50 | 1.50 |
| Broken rice | 10.00 | 14.00 |
| 43 soybean meal | 5.50 | 3.70 |
| Salt | 0.40 | 0.41 |
| Limestone | 1.05 | 0.96 |
| Dicalcium phosphate | 0.16 | 0.20 |
| 70% lysine | 0.54 | 0.46 |
| 98% threonine | 0.05 | 0.01 |
| Choline chloride | 0.05 | 0.05 |
| Antioxidant | 0.01 | 0.01 |
| Antifungal agents | 0.20 | 0.20 |
| Premix | 0.50 | 0.50 |
| Total | 100.00 | 100.00 |
| Calculated Nutritional Value | ||
| Digestive energy MJ/Kg | 11.97 | 11.98 |
| Crude protein % | 11.26 | 11.49 |
| Calcium % | 0.54 | 0.54 |
| Total phosphorus % | 0.60 | 0.57 |
| Energy-to-nitrogen ratio | 1.06 | 1.05 |
| Lysine % | 0.79 | 0.79 |
| Methionine % | 0.22 | 0.22 |
| Threonine % | 0.44 | 0.44 |
| Tryptophan % | 0.12 | 0.11 |
| Items | CON | CZ | M200 | M400 | M800 | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| IBW (kg) | 48.00 | 48.01 | 47.86 | 47.87 | 47.94 | 0.62 | 0.86 |
| FBW (kg) | 86.43 | 83.51 | 85.32 | 83.15 | 81.99 | 0.89 | 0.27 |
| TWG (kg) | 37.9 | 35.5 | 36.9 | 35.3 | 34.1 | 0.66 | 0.4 |
| ADFI (g/d) | 1777.29 | 1784.06 | 1675.82 | 1785.15 | 1779.94 | 0.83 | 0.41 |
| FCR | 4.01 | 4.23 | 3.91 | 4.23 | 4.48 | 0.64 | 0.4 |
| Items | CON | CZ | M200 | M400 | M800 | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| TC/(mmol/L) | 2.70 ab | 2.74 a | 2.69 ab | 2.61 b | 2.71 ab | 0.020 | 0.03 |
| TG/(mmol/L) | 0.56 | 0.54 | 0.55 | 0.54 | 0.55 | 0.004 | 0.93 |
| HDL-C/(mmol/L) | 0.40 a | 0.39 ab | 0.38 ab | 0.37 b | 0.39 ab | 0.003 | 0.01 |
| LDL-C/(mmol/L) | 1.67 ab | 1.68 a | 1.69 a | 1.65 ab | 1.60 b | 0.010 | 0.02 |
| ALP/(U/L) | 190.79 a | 182.12 b | 184.11 b | 180.56 b | 182.27 b | 1.130 | <0.01 |
| ALT/(U/L) | 20.27 a | 19.68 ab | 20.14 ab | 19.10 b | 19.38 b | 1.390 | <0.01 |
| AST/(U/L) | 41.40 a | 39.01 b | 38.87 b | 38.33 b | 40.38 ab | 0.350 | 0.02 |
| T-AOC/(U/mL) | 7.33 b | 7.75 a | 7.45 b | 7.86 a | 7.50 b | 0.060 | 0.01 |
| MDA/(nmol/L) | 10.18 a | 10.03 a | 9.75 b | 9.64 b | 9.83 b | 0.050 | <0.01 |
| GSH-PX/(U/mL) | 189.81 ab | 178.76 b | 186.98 b | 186.55 b | 195.74 a | 1.460 | <0.01 |
| CAT/(U/mL) | 1.19 b | 1.30 a | 1.16 b | 1.26 a | 1.21 b | 0.011 | <0.01 |
| SOD/(U/mL) | 1.88 b | 1.96 a | 1.95 ab | 2.00 a | 1.92 ab | 0.013 | <0.01 |
| Items | CON | CZ | M200 | M400 | M800 | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| pH1h | 6.42 | 6.45 | 6.26 | 6.35 | 6.26 | 0.059 | 0.81 |
| pH24h | 6.04 a | 5.77 b | 5.72 b | 5.62 b | 5.72 b | 0.046 | 0.02 |
| L*1h | 39.07 | 38.4 | 40.5 | 37.23 | 39.13 | 0.473 | 0.3 |
| L*24h | 41.12 b | 43.18 ab | 43.15 ab | 45.33 a | 42.73 ab | 0.630 | 0.04 |
| a*1h | 7.6 | 6.32 | 6.61 | 6.29 | 6.76 | 0.311 | 0.72 |
| a*24h | 7.81 | 7.58 | 8.63 | 9.11 | 8.48 | 0.313 | 0.57 |
| b*1h | 2.72 a | 1.84 b | 2.43 ab | 1.98 ab | 2.08 ab | 0.126 | 0.02 |
| b*24h | 3.98 | 4.81 | 5.75 | 5.91 | 4.85 | 0.251 | 0.26 |
| Marbling score | 3.375 | 3.375 | 3.25 | 3.125 | 3.25 | 0.057 | 0.66 |
| Shear force (N) | 12.97 a | 12.46 a | 10.98 ab | 9.29 b | 11.21 ab | 0.469 | < 0.01 |
| Cooking loss (%) | 0.72 | 0.7 | 0.73 | 0.71 | 0.65 | 0.114 | 0.08 |
| Drip loss (%) | 0.018 | 0.015 | 0.015 | 0.016 | 0.015 | 0.001 | 0.08 |
| Water binding capacity (%) | 0.868 | 0.863 | 0.851 | 0.837 | 0.847 | 0.015 | 0.26 |
| Items | CON | CZ | M200 | M400 | M800 | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| Aspartic acid | 2.05 b | 2.07 a | 2.13 a | 2.07 a | 2.07 a | 0.011 | 0.02 |
| Threonine | 1.02 | 1.03 | 1.05 | 1.03 | 1.03 | 0.005 | 0.32 |
| Serine | 0.87 | 0.87 | 0.9 | 0.87 | 0.88 | 0.005 | 0.29 |
| Glutamic acid | 3.25 b | 3.24 b | 3.36 a | 3.26 b | 3.26 b | 0.016 | 0.03 |
| Glycine | 0.94 ab | 0.95 ab | 0.96 a | 0.93 b | 0.95 ab | 0.004 | 0.02 |
| Alanine | 1.27 b | 1.28 ab | 1.31 a | 1.28 ab | 1.29 ab | 0.006 | 0.02 |
| Methionine | 0.34 | 0.35 | 0.36 | 0.36 | 0.34 | 0.005 | 0.73 |
| Valine | 1.15 b | 1.16 b | 1.20 a | 1.17 ab | 1.17 ab | 0.007 | 0.02 |
| Isoleucine | 1.00 b | 1.01 b | 1.07 a | 1.02 b | 1.02 b | 0.007 | <0.01 |
| Leucine | 1.77 b | 1.78 b | 1.86 a | 1.79 b | 1.79 b | 0.010 | <0.01 |
| Tyrosine | 0.60 b | 0.61 ab | 0.63 a | 0.60 b | 0.60 b | 0.004 | 0.02 |
| Lysine | 1.96 b | 1.98 b | 2.04 a | 1.99 ab | 1.98 b | 0.010 | 0.01 |
| Phenylalanine | 0.89 b | 0.90 b | 0.94 a | 0.91 ab | 0.91 ab | 0.023 | <0.01 |
| Histidine | 1.01 | 1.04 | 1.07 | 1.01 | 1.06 | 0.012 | 0.29 |
| Arginine | 1.37 | 1.38 | 1.41 | 1.37 | 1.37 | 0.006 | 0.26 |
| Praline | 0.85 ab | 0.84 b | 0.88 a | 0.83 b | 0.83 b | 0.006 | <0.01 |
| Items | CON | CZ | M200 | M400 | M800 | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| Capric acid (C10:0) | 0.06 b | 0.07 ab | 0.06 b | 0.08 ab | 0.09 a | 0.004 | 0.03 |
| Lauric acid (C12:0) | 0.06 | 0.07 | 0.07 | 0.06 | 0.07 | 0.004 | 0.92 |
| Myristic acid (C14:0) | 1.11 | 1.22 | 1.09 | 1.13 | 1.1 | 0.034 | 0.84 |
| Pentadecanoic acid (C15:0) | 0.04 b | 0.05 ab | 0.05 ab | 0.05 ab | 0.07 a | 0.009 | 0.02 |
| Palmitic acid (C16:0) | 22.95 ab | 23.48 a | 22.68 ab | 22.98 ab | 21.85 b | 0.229 | 0.03 |
| Palmitoleic acid (C16:1) | 3.27 | 3.41 | 3.36 | 3.83 | 3.78 | 0.125 | 0.54 |
| Margaric acid (C17:0) | 0.12 b | 0.15 ab | 0.14 ab | 0.14 ab | 0.17 a | 0.007 | 0.02 |
| Heptadecenoic acid (C17:1) | 0.11 | 0.12 | 0.12 | 0.12 | 0.14 | 0.005 | 0.43 |
| Stearic acid (C18:0) | 11.63 | 11.45 | 11.58 | 11.6 | 11.23 | 0.188 | 0.37 |
| Oleic acid (C18:1n-9c) | 41.3 | 40.85 | 41.7 | 40.68 | 38.1 | 0.837 | 0.73 |
| Linoleic acid (C18:2n-6c) | 12.82 | 11.69 | 12.06 | 12.49 | 14.8 | 0.668 | 0.67 |
| α-Linolenic acid (C18:3n-3) | 0.29 | 0.31 | 0.3 | 0.32 | 0.35 | 0.013 | 0.59 |
| Arachidic acid (C20:0) | 0.25 | 0.26 | 0.26 | 0.24 | 0.25 | 0.005 | 0.48 |
| Eicosenoic acid (C20:1) | 0.84 ab | 0.82 ab | 0.88 a | 0.76 ab | 0.68 b | 0.026 | 0.04 |
| Eicosadienoic acid (C20:2) | 0.52 | 0.53 | 0.56 | 0.55 | 0.64 | 0.028 | 0.72 |
| Eicosenoic acid (C20:3n-6) | 0.38 | 0.4 | 0.41 | 0.41 | 0.54 | 0.029 | 0.43 |
| Eicosenoic acid (C20:3n-3) | 0.06 | 0.08 | 0.09 | 0.1 | 0.11 | 0.007 | 0.32 |
| Eicosenoic acid (C20:4n-6) | 3.56 | 3.29 | 3.84 | 3.79 | 4.89 | 0.297 | 0.54 |
| Heneicosanoic acid (C21:0) | 0.11 | 0.12 | 0.12 | 0.14 | 0.17 | 0.010 | 0.47 |
| Behenic acid (C22:0) | 0.18 | 0.23 | 0.25 | 0.19 | 0.36 | 0.026 | 0.72 |
| Tetracosanoic acid (C24:0) | 0.14 | 0.12 | 0.11 | 0.12 | 0.17 | 0.010 | 0.42 |
| Nervonic acid (C24:1) | 0.23 | 0.25 | 0.29 | 0.21 | 0.47 | 0.039 | 0.19 |
| Items | CON | CZ | M200 | M400 | M800 | SEM | p-Value |
|---|---|---|---|---|---|---|---|
| Inosine monophosphate | 1.75 b | 2.10 a | 2.15 a | 2.18 a | 2.14 a | 0.065 | <0.01 |
| Aromatic ingredients | 0.994 b | 0.997 ab | 0.999 a | 1.001 a | 1.000 a | 0.014 | 0.02 |
| Nitrogen oxides | 1.349 a | 1.245 b | 1.368 a | 1.249 b | 1.256 b | 0.003 | <0.01 |
| Ammonia | 0.954 b | 0.959 a | 0.956 b | 0.961 a | 0.959 a | 0.001 | <0.01 |
| Hydrogen | 1.001 a | 1.002 a | 0.999 b | 1.003 a | 1.002 a | 0.001 | <0.01 |
| Alkane aromatic ingredients | 0.974 b | 0.973 b | 0.975 a | 0.975 a | 0.972 b | 0.001 | 0.03 |
| Short-chain alkanes | 2.834 a | 2.303 b | 2.745 a | 2.274 b | 2.312 b | 0.027 | <0.01 |
| Sulfides | 1.093 b | 1.104 b | 1.177 a | 1.136 a | 1.112 b | 0.007 | <0.01 |
| Alcohols | 1.379 a | 1.249 b | 1.363 a | 1.238 b | 1.255 b | 0.007 | <0.01 |
| Organic sulfides | 1.511 b | 1.416 b | 1.601 a | 1.435 b | 1.442 b | 0.007 | <0.01 |
| Alkanes | 1.044 b | 1.046 a | 1.043 b | 1.043 b | 1.045 a | 0.001 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, R.; Li, Y.; Lei, Z.; Wu, M.; Tan, H.; Liu, F.; Gong, Y.; Zhong, W.; He, J.; Zeng, S.; et al. Dietary Supplementation with Complex Enzymes and Tea Residue Improved the Production Efficiency of Xiangling Pigs. Animals 2025, 15, 1229. https://doi.org/10.3390/ani15091229
Yang R, Li Y, Lei Z, Wu M, Tan H, Liu F, Gong Y, Zhong W, He J, Zeng S, et al. Dietary Supplementation with Complex Enzymes and Tea Residue Improved the Production Efficiency of Xiangling Pigs. Animals. 2025; 15(9):1229. https://doi.org/10.3390/ani15091229
Chicago/Turabian StyleYang, Runhua, Yulian Li, Zhenyu Lei, Maisheng Wu, Hong Tan, Fang Liu, Yanmei Gong, Weijian Zhong, Jiayan He, Shujuan Zeng, and et al. 2025. "Dietary Supplementation with Complex Enzymes and Tea Residue Improved the Production Efficiency of Xiangling Pigs" Animals 15, no. 9: 1229. https://doi.org/10.3390/ani15091229
APA StyleYang, R., Li, Y., Lei, Z., Wu, M., Tan, H., Liu, F., Gong, Y., Zhong, W., He, J., Zeng, S., Fan, Z., & Wu, S. (2025). Dietary Supplementation with Complex Enzymes and Tea Residue Improved the Production Efficiency of Xiangling Pigs. Animals, 15(9), 1229. https://doi.org/10.3390/ani15091229






