Guinea Pig (Cavia porcellus) Welfare: Associations Between Husbandry Practices, Human–Animal Interactions, and Animal Behaviour
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Questionnaire Creation and Content
2.2. Survey and Requirements for Participation
2.3. Recruitment of Participants
2.4. Data Analysis
3. Results
3.1. Participants Characteristics
3.2. Characteristics of Focus Guinea Pigs
3.3. Husbandry
3.3.1. Social Environment
3.3.2. Housing Type and Free Roaming
3.3.3. Furnishings and Enrichment
3.3.4. Human–Animal Interactions
3.3.5. Feeding
3.4. Health Status, Care Measures, and Cleaning of Enclosures and Equipment
3.5. Guinea Pig Behaviour
3.5.1. Social Behaviour
Differences in Social Behaviour in Relation to Housing Types
Associations of Social Behaviour with Husbandry, Human–Animal Interactions, and Focus Animal Characteristics
3.5.2. Behaviours Observed in the Main Living Area and During Roaming
Differences in General Behaviour in the Main Living Area and During Roaming in Relation to Housing Types
Associations of General Behaviour in the Main Living Area with Husbandry, Human–Animal Interactions, and Focus Animal Characteristics
4. Discussion
4.1. Insight into Current Husbandry, Including Human–Animal Interactions, Health Status, and the Behaviour of Guinea Pigs Kept as Companion Animals
4.1.1. Social Environment
4.1.2. Housing Type and Free Roaming
4.1.3. Furnishings and Enrichment
4.1.4. Human–Animal Interactions
4.1.5. Feeding
4.1.6. Health Status, Health Care, and Maintenance Measures
4.1.7. Guinea Pig Behaviour
4.2. Associations Between Husbandry, Human–Animal Relationship, and Behaviours of Guinea Pigs
4.2.1. Differences in Behaviour in Relation to Housing Types
4.2.2. Associations of Guinea Pig Behaviour with Husbandry Conditions, Human–Animal Interactions, and Focus Animal Characteristics
Associations with Social Behaviours
Associations with General Behaviour in the Main Living Area and During Roaming
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lord, E.; Collins, C.; Defrance, S.; LeFebvre, M.J.; Pigière, F.; Eeckhout, P.; Erauw, C.; Fitzpatrick, S.M.; Healy, P.F.; Martínez-Polanco, M.F.; et al. Ancient DNA of Guinea Pigs (Cavia Spp.) Indicates a Probable New Center of Domestication and Pathways of Global Distribution. Sci. Rep. 2020, 10, 8901. [Google Scholar] [CrossRef]
- Witkowska, A.; Price, J.; Hughes, C.; Smith, D.; White, K.; Alibhai, A.; Rutland, C. The Effects of Diet on Anatomy, Physiology and Health in the Guinea Pig. J. Anim. Health Behav. Sci. 2017, 1, 103. [Google Scholar]
- Langenecker, M.; Clauss, M.; Hässig, M.; Hatt, J.-M. Vergleichende Untersuchung zur Krankheits-verteilung bei Kaninchen, Meerschweinchen, Ratten und Frettchen. Tieraerztliche Prax. Ausg. Kleintiere Heimtiere 2009, 37, 326–333. [Google Scholar] [CrossRef]
- Drescher, B.; Hamel, I. Heimtier und Patient Meerschweinchen, 3rd ed.; Enke: Stuttgart, Germany, 2012; ISBN 978-3-8304-1157-4. [Google Scholar]
- Schneider, B. Meerschweinchen. In Verhaltensberatung bei Kleinen Heimtieren, 1st ed.; Schneider, B., Döring, D., Eds.; Schattauer GmbH: Stuttgart, Germany, 2017; p. 103. ISBN 978-3-7945-3112-7. [Google Scholar]
- McBride, E.A. Small Prey Species’ Behaviour and Welfare: Implications for Veterinary Professionals. J. Small Anim. Pr. 2017, 58, 423–436. [Google Scholar] [CrossRef]
- Baumans, V.; Van Loo, P. How to Improve Housing Conditions of Laboratory Animals: The Possibilities of Environmental Refinement. Veter. J. 2013, 195, 24–32. [Google Scholar] [CrossRef]
- Asher, M.; Lippmann, T.; Epplen, J.T.; Kraus, C.; Trillmich, F.; Sachser, N. Large Males Dominate: Ecology, Social Organization, and Mating System of Wild Cavies, the Ancestors of the Guinea Pig. Behav. Ecol. Sociobiol. 2008, 62, 1509–1521. [Google Scholar] [CrossRef]
- Bläske, A.; Hofmann, N.; Schwarzer, A.; Ebner, M.V.; Bergmann, S.; Reese, S.; Erhard, M.; Wöhr, A.-C. Haltungsbedingungen und Herkunft von als Heimtiere gehaltenen (exotischen) Säugetieren in Deutschland (EXOPET). Berl. Münch. Tierärztl. Wochenschr. 2018, 132, 112–124. [Google Scholar] [CrossRef]
- Harrup, A.J.; Rooney, N. Current Welfare State of Pet Guinea Pigs in the UK. Veter. Rec. 2020, 186, 282. [Google Scholar] [CrossRef]
- Norman, R.; Wills, A.P. An Investigation into the Relationship between Owner Knowledge, Diet, and Dental Disease in Guinea Pigs (Cavia porcellus). Animals 2016, 6, 73. [Google Scholar] [CrossRef]
- Cameron, K.; Holder, H.; Connor, R. Cross-Sectional Survey of Housing for Pet Guinea Pigs (Cavia porcellus) in New Zealand. N. Z. Veter. J. 2022, 70, 228–232. [Google Scholar] [CrossRef]
- Cameron, K.; Holder, H.; Connor, R.; Gear, R. Cross-Sectional Survey of Husbandry for Pet Guinea Pigs (Cavia porcellus) in New Zealand. N. Z. Veter. J. 2022, 71, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E. Health Survey of Pet Guinea Pigs in Norway. TDK Thesis, Clinical Dietetics University of Veterinary Medicine Budapest, Budapest, Hungary, 2023. Available online: https://huveta.hu/bitstream/handle/10832/3841/521471468.pdf?sequence=1&isAllowed=y (accessed on 1 April 2024).
- Sachser, N.; Lick, C.; Stanzel, K. The Environment, Hormones, and Aggressive Behaviour: A 5-Year-Study in Guinea Pigs. Psychoneuroendocrinology 1994, 19, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Nevalainen, T. Research, Animals and Welfare. Regulations, Alternatives and Guidelines. In The Welfare of Laboratory Animals; Kaliste, E., Ed.; Animal Welfare; Springer: Dordrecht, The Netherlands, 2007; Volume 2, pp. 15–22. ISBN 978-1-4020-2270-8. [Google Scholar]
- Mancinelli, E. Guinea Pig Husbandry—Housing, Diet and Handling. Vet Times 2016, 46, 31. [Google Scholar]
- Verordnung der Bundesministerin für Gesundheit Über die Haltung von Wirbeltieren, die Nicht Unter die 1. Tierhal Tungsverordnung Fallen, Über Wildtiere, die Besondere Anforderungen an die Haltung Stellen und Über Wildtierarten, Deren Haltung aus Gründen des Tierschutzes Verboten Ist (2. Tierhaltungsverordnung). Available online: https://www.ris.bka.gv.at/GeltendeFassung.Wxe?Abfrage=Bundesnormen&Gesetzesnummer=20003860 (accessed on 20 January 2025).
- Tierschutzverordnung Vom 23. April 2008 des Schweizerischen Bundesrates. Available online: https://www.Fedlex.Admin.Ch/Eli/Cc/2008/416/de (accessed on 20 January 2025).
- Donnelly, T.M.; Brown, C.J. Guinea Pig and Chinchilla Care and Husbandry. Veter. Clin. N. Am. Exot. Anim. Pr. 2004, 7, 351–373. [Google Scholar] [CrossRef]
- Lürzel, S. Lecture: Behaviour, Husbandry and Welfare of Companion Animals: Guinea Pigs; Veterinärmedizinische Universität Wien: Vienna, Austria, 2020. [Google Scholar]
- Sachser, N. Sozialphysiologische Untersuchungen an Hausmeerschweinchen; Merkenschlager, M., Gärtner, K., Militzer, K., Eds.; Schriftenreihe Versuchstierkunde, H. 16; Paul Parey: Berlin, Germany; Hamburg, Germany, 1994; ISBN 3-489-58316-7. [Google Scholar]
- Kaiser, S.; Kruijver, F.P.; Swaab, D.F.; Sachser, N. Early Social Stress in Female Guinea Pigs Induces a Masculinization of Adult Behavior and Corresponding Changes in Brain and Neuroendocrine Function. Behav. Brain Res. 2003, 144, 199–210. [Google Scholar] [CrossRef]
- Brewer, J.S.; A Bellinger, S.; Joshi, P.; A Kleven, G. Enriched Open Field Facilitates Exercise and Social Interaction in 2 Strains of Guinea Pigs (Cavia porcellus). J. Am. Assoc. Lab. Anim. Sci. 2014, 53, 344–355. [Google Scholar]
- Minarikova, A.; Hauptman, K.; Jeklova, E.; Knotek, Z.; Jekl, V. Diseases in Pet Guinea Pigs: A Retrospective Study in 1000 Animals. Veter. Rec. 2015, 177, 200. [Google Scholar] [CrossRef]
- Waiblinger, S. Agricultural Animals. In Anthrozoology: Human–Animal Interactions in Domesticated and Wild Animals; Oxford University Press: Oxford, UK, 2019; pp. 32–58. ISBN 978-0-19-875363-6. [Google Scholar]
- Windschnurer, I.; Häusler, A.; Waiblinger, S.; Coleman, G.J. Relationships between Owner and Household Characteristics and Enrichment and Cat Behaviour. Appl. Anim. Behav. Sci. 2022, 247, 105562. [Google Scholar] [CrossRef]
- Gut, W.; Crump, L.; Zinsstag, J.; Hattendorf, J.; Hediger, K. The Effect of Human Interaction on Guinea Pig Behavior in Animal-Assisted Therapy. J. Veter. Behav. 2018, 25, 56–64. [Google Scholar] [CrossRef]
- Wirth, S.; Gebhardt-Henrich, S.; Riemer, S.; Hattendorf, J.; Zinsstag, J.; Hediger, K. The Influence of Human Interaction on Guinea Pigs: Behavioral and Thermographic Changes during Animal-Assisted Therapy. Physiol. Behav. 2020, 225, 113076. [Google Scholar] [CrossRef]
- Köbrunner, D.; Waiblinger, S.; Stetina, B.U.; Künzel, F.; Windschnurer, I. Insight into Husbandry Conditions, Health, and Behavior of Pet Ferrets (Mustela Putorius Furo) among Ger-man-Speaking Ferret Owners. J. Veter. Behav. 2020, 37, 8–19. [Google Scholar] [CrossRef]
- Gilhofer, E.M.; Hebesberger, D.V.; Waiblinger, S.; Künzel, F.; Rouha-Mülleder, C.; Mariti, C.; Windschnurer, I. Husbandry Conditions and Welfare State of Pet Chinchillas (Chinchilla lanigera) and Caretakers’ Perceptions of Stress and Emotional Closeness to Their Animals. Animals 2024, 14, 3155. [Google Scholar] [CrossRef] [PubMed]
- Décieux, J.P.; Mergener, A.; Neufang, K.M.; Sischka, P. Implementation of the Forced Answering Option within Online Surveys: Do Higher Item Response Rates Come at the Expense of Participation and Answer Quality? Psihologija 2015, 48, 311–326. [Google Scholar] [CrossRef]
- Rooney, N.J.; Blackwell, E.J.; Mullan, S.M.; Saunders, R.; E Baker, P.; Hill, J.M.; E Sealey, C.; Turner, M.J.; Held, S.D.E. The Current State of Welfare, Housing and Husbandry of the English Pet Rabbit Population. BMC Res. Notes 2014, 7, 942. [Google Scholar] [CrossRef]
- Schneidewind, S.; Lesch, R.; Heizmann, V.; Windschnurer, I. A Comprehensive Survey of Husbandry, Health, Behavior, and the Associations between Caretaker Attitudes, Attachment, and Husbandry Practices. J. Veter. Behav. 2024, 75, 1–19. [Google Scholar] [CrossRef]
- Pallant, J. SPSS Survival Manual: A Step by Step Guide to Data Analysis Using IBM SPSS, 7th ed.; Routledge: Abingdon, NY, USA, 2020; ISBN 978-1-00-311745-2. [Google Scholar]
- Morgenegg, R. Artgerechte Haltung Ist Ein Grundrecht—Auch für Meerschweinchen, 5th ed.; TB: Obfelden, Switzerland, 2012; ISBN 978-3-9522661-0-6. [Google Scholar]
- Magnus, E. Behaviour of the Pet Rabbit: What Is Normal and Why Do Problems Develop? Pract. 2005, 27, 531–535. [Google Scholar] [CrossRef]
- Mullan, S.M.; Main, D.C.J. Behaviour and Personality of Pet Rabbits and Their Interactions with Their Owners. Veter. Rec. 2007, 160, 516–520. [Google Scholar] [CrossRef]
- Fawcett, A. Management of Husbandry-related Problems in Guinea Pigs. Practice 2011, 33, 163–171. [Google Scholar] [CrossRef]
- Kameyama, H.; Fujimoto, Y.; Tomioka, Y.; Yamamoto, S.; Suyama, H.; Inoue, H.; Takahashi, E.; Ono, E. Pathogenicity of Bordetella Bronchiseptica Isolated from Apparently Healthy Rabbits in Guinea Pig, Rat, and Mouse. J. Veter. Med Sci. 2022, 84, 574–581. [Google Scholar] [CrossRef]
- Tierärztliche Vereinigung für Tierschutz e.V. Merkblatt Nr. 159—Heimtiere: Meerschweinchen (Stand: Oktober 2020); Tierärztliche Vereinigung für Tierschutz e.V.: Belm, Germany, 2020. [Google Scholar]
- Forbes, D.; Federation of European Laboratory Animal Science Associations (Eds.) Euroguide: On the Accommodation and Care of Animals Used for Experimental and Other Scientific Purposes; Based of the Revised Appendix A of the European Copnvention ETS 123; Felasa: London, UK, 2007; ISBN 978-1-85315-751-6. [Google Scholar]
- Přibylová, L.; Součková, M.; Kolářová, M.F.; Vostrá-Vydrová, H.; Chaloupková, H. Does a Stronger Bond with Pet Rabbits Equate to Better Husbandry Conditions for Them? Appl. Anim. Behav. Sci. 2023, 270, 106143. [Google Scholar] [CrossRef]
- Brust, V.; Guenther, A. Domestication Effects on Behavioural Traits and Learning Performance: Comparing Wild Cavies to Guinea Pigs. Anim. Cogn. 2014, 18, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Brust, V.; Guenther, A. Stability of the Guinea Pigs Personality—Cognition—Linkage over Time. Behav. Process. 2017, 134, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Riggs, S.M. Guinea Pigs. In Manual of Exotic Pet Practice, 1st ed.; Mitchell, M.A., Tully, T.N., Eds.; Saunders Elsevier: St. Louis, MO, USA, 2009; Volume 18, pp. 456–473. ISBN 9781-4160-0119-5. [Google Scholar]
- Castelhano-Carlos, M.J.; Baumans, V. The Impact of Light, Noise, Cage Cleaning and In-House Transport on Welfare and Stress of Laboratory Rats. Lab. Anim. 2009, 43, 311–327. [Google Scholar] [CrossRef]
- Ellis, S.L.H.; Rodan, I.; Carney, H.C.; Heath, S.; Rochlitz, I.; Shearburn, L.D.; Sundahl, E.; Westropp, J.L. AAFP and ISFM Feline Environmental Needs Guidelines. J. Feline Med. Surg. 2013, 15, 219–230. [Google Scholar] [CrossRef]
- Normando, S.; Gelli, D. Behavioral Complaints and Owners’ Satisfaction in Rabbits, Mustelids, and Rodents Kept as Pets. J. Veter. Behav. 2011, 6, 337–342. [Google Scholar] [CrossRef]
- Howell, T.J.; Mornement, K.; Bennett, P.C. Companion Rabbit and Companion Bird Management Practices Among a Representative Sample of Guardians in Victoria, Australia. J. Appl. Anim. Welf. Sci. 2014, 18, 287–302. [Google Scholar] [CrossRef]
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Langbein, J.; et al. Assessment of Positive Emotions in Animals to Improve Their Welfare. Physiol. Behav. 2007, 92, 375–397. [Google Scholar] [CrossRef]
- Mason, G.J. Stereotypies and Suffering. Behav. Process. 1991, 25, 103–115. [Google Scholar] [CrossRef]
- Ewringmann, A.; Glöckner, B. Leitsymptome bei Meerschweinchen, Chinchilla und Degu, 2nd ed.; Enke in MVS Medizinverlage Stuttgart GmbH & Co. KGw: Stuttgart, Germany, 2012; ISBN 978-3-8304-1091-1. [Google Scholar]
- Baumans, V. Environmental Enrichment for Laboratory Rodents and Rabbits: Requirements of Rodents, Rabbits, and Research. ILAR J. 2005, 46, 162–170. [Google Scholar] [CrossRef]
- Newberry, R.C. Environmental Enrichment: Increasing the Biological Relevance of Captive Environments. Appl. Anim. Behav. Sci. 1995, 44, 229–243. [Google Scholar] [CrossRef]
- Klein, Z.A.; Padow, V.A.; Romeo, R.D. The Effects of Stress on Play and Home Cage Behaviors in Adolescent Male Rats. Dev. Psychobiol. 2009, 52, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Menke, C.; Waiblinger, S.; Fölsch, D.W.; Wiepkema, P.R. Social Behaviour and Injuries of Horned Cows in Loose Housing Systems. Anim. Welf. 1999, 8, 243–258. [Google Scholar] [CrossRef]
- Bekoff, M. The Development of Social Interaction, Play, and Metacommunication in Mammals: An Ethological Perspective. Q. Rev. Biol. 1972, 47, 412–434. [Google Scholar] [CrossRef]
- Brandão, J.; Mayer, J. Behavior of Rodents with an Emphasis on Enrichment. J. Exot. Pet Med. 2011, 20, 256–269. [Google Scholar] [CrossRef]
- Bradley, T.A. Normal Behavior and the Clinical Implications of Abnormal Behavior in Guinea Pigs. Veter. Clin. N. Am. Exot. Anim. Pr. 2001, 4, 681–696. [Google Scholar] [CrossRef]
- Held, S.D.E.; Špinka, M. Animal Play and Animal Welfare. Anim. Behav. 2011, 81, 891–899. [Google Scholar] [CrossRef]
- Waiblinger, S.; Boivin, X.; Pedersen, V.; Tosi, M.-V.; Janczak, A.M.; Visser, E.K.; Jones, R.B. Assessing the Human–Animal Relationship in Farmed Species: A Critical Review. Appl. Anim. Behav. Sci. 2006, 101, 185–242. [Google Scholar] [CrossRef]
- Rault, J.-L.; Waiblinger, S.; Boivin, X.; Hemsworth, P. The Power of a Positive Human–Animal Relationship for Animal Welfare. Front. Vet. Sci. 2020, 7, 590867. [Google Scholar] [CrossRef]
- Veissier, I.; le Neindre, P. Reactivity of Aubrac Heifers Exposed to a Novel Environment Alone or in Groups of Four. Appl. Anim. Behav. Sci. 1992, 33, 11–15. [Google Scholar] [CrossRef]
- Rault, J.-L. Friends with Benefits: Social Support and Its Relevance for Farm Animal Welfare. Appl. Anim. Behav. Sci. 2012, 136, 1–14. [Google Scholar] [CrossRef]
- Salas, M.; Temple, D.; Abáigar, T.; Cuadrado, M.; Delclaux, M.; Enseñat, C.; Almagro, V.; Martínez-Nevado, E.; Quevedo, M.Á.; Carbajal, A.; et al. Aggressive Behavior and Hair Cortisol Levels in Captive Dorcas Gazelles (Gazella dorcas) as Animal-based Welfare Indicators. Zoo Biol. 2016, 35, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Manteca, X.; Amat, M.; Salas, M.; Temple, D. Animal-Based Indicators to Assess Welfare in Zoo Animals. CABI Rev. 2016, 11, 1–10. [Google Scholar] [CrossRef]
- Martins, C.I.M.; Galhardo, L.; Noble, C.; Damsgård, B.; Spedicato, M.T.; Zupa, W.; Beauchaud, M.; Kulczykowska, E.; Massabuau, J.-C.; Carter, T.; et al. Behavioural indicators of welfare in farmed fish. Fish Physiol. Biochem. 2011, 38, 17–41. [Google Scholar] [CrossRef] [PubMed]
- Olsson, I.A.S.; Würbel, H.; Mench, J.A. Behaviour. In Animal Welfare, 2nd ed.; Appleby, M.C., Mench, J.A., Olsson, I.A.S., Hughes, B.O., Eds.; CABI Publishing: Cambridge, UK, 2011; pp. 138–155. ISBN 9781780640808. [Google Scholar]
- Forkman, B.; Keeling, L. Assessment of Animal Welfare Measures for Dairy Cattle, Beef Bulls and Veal Calves; School of City and regional Planning, Cardiff University: Cardiff, UK, 2009; Number 11. [Google Scholar]
- Arhant, C.; Lesch, R.; Heizmann, V.; Schauberger, G.; Windschnurer, I. Risks Associated with Free-Roaming and Collar Use in Cats—An Online Survey. J. Veter. Behav. 2022, 58, 23–36. [Google Scholar] [CrossRef]
- Kormos, C.; Gifford, R. The Validity of Self-Report Measures of Proenvironmental Behavior: A Meta-Analytic Review. J. Environ. Psychol. 2014, 40, 359–371. [Google Scholar] [CrossRef]
- Christiansen, S.B.; Forkman, B. Assessment of Animal Welfare in a Veterinary Context—A Call for Ethologists. Appl. Anim. Behav. Sci. 2007, 106, 203–220. [Google Scholar] [CrossRef]
| Dependent Variables | Included Independent Variables |
|---|---|
| Frequency of affiliative behaviours Occurrence of agonistic behaviours (no/yes) Occurrence of competition for food (no/yes) | Size of the enclosure/accommodation area including elevated areas and exercise areas in case of constant access Roaming frequency outside the enclosure Number of permanently accessible huts/caves/other Food enrichment Cat/dog toys Number of animals in the same enclosure as the focus animal Group size increase within the prior 8 weeks no (0) yes (1) Group size decrease within the prior 8 weeks no (0) yes (1) Daily time spent engaging with focus animal Frequency of lifting up Frequency of carrying and stroking Frequency of talking hand-feeding Female (0) vs. male (1) Age of focus animal |
| Frequency of going back into enclosure and hiding during roaming Frequency of locomotor play and use of enrichment Occurrence of marking behaviour and teeth noises (no/yes) Occurrence of running up and down and bar chewing (no/yes) | Size of the enclosure/accommodation area including elevated areas and exercise areas in case of constant access Roaming frequency outside the enclosure Number of permanently accessible huts/caves/other Food enrichment Cat/dog toys Individual housing yes (0) no (1) Number of animals in the same enclosure as focus animal Group size increase within the prior 8 weeks no (0) yes (1) Group size decrease within the prior 8 weeks no (0) yes (1) Frequency of presence of dogs in the same room Frequency of presence of cats in the same room Daily time spent engaging with focus animal Frequency of lifting up Frequency of carrying and stroking Frequency of talking hand-feeding Frequency of training Frequency of health checks Frequency of cleaning and fur care Duration of residence of the focus animal at the caretaker’s Age of focus animal (only included in the frequency of locomotor play and use of enrichment) |
| Frequency of Roaming Offered | n | % |
|---|---|---|
| Never | 303 | 34.7 |
| 1×/month | 23 | 2.6 |
| Several times per month | 74 | 8.5 |
| 1×/week | 36 | 4.1 |
| 2×/week | 37 | 4.2 |
| 3×/week | 38 | 4.4 |
| 4×/week | 37 | 4.2 |
| 5×/week | 31 | 3.6 |
| 6×/week | 18 | 2.1 |
| 1×/day | 111 | 12.7 |
| 2×/day | 19 | 2.2 |
| 3×/day | 0 | 0 |
| 4×/day | 1 | 0.1 |
| More than 4×/day | 12 | 1.4 |
| Constantly | 132 | 15.1 |
| Enrichment Provided | n | Never | Occasionally | 1×/Week | Several Times/Week | 1×/Day | Several Times/Day | Constantly | |
|---|---|---|---|---|---|---|---|---|---|
| Cardboard box | 923 | 39.3% | 42.1% | 3.6% | 5.3% | 0.7% | 0.8% | 8.2% | |
| Logic toy | 915 | 55.7% | 28.2% | 5.0% | 7.1% | 0.7% | 0.2% | 3.1% | |
| Nibble wood | 898 | 54.1% | 27.2% | 4.2% | 4.8% | 1.0% | 0.3% | 8.4% | |
| Fresh branches | 938 | 5.4% | 35.1% | 15.4% | 25.1% | 4.5% | 1.2% | 13.4% | |
| Tunnel made of fabric, plastic, willow, wood, etc. | 930 | 7.0% | 13.9% | 1.8% | 5.8% | 1.6% | 3.4% | 66.5% | |
| Feeding tree (log with holes to be filled, attached to a wooden plate) | 919 | 66.9% | 19.9% | 2.9% | 3.8% | 0.9% | 0.4% | 5.1% | |
| Dog toys | 918 | 93.7% | 5.0% | 0.3% | 0.2% | 0.1% | 0.0% | 0.7% | |
| Cat toys | 921 | 92.3% | 5.5% | 0.2% | 0.3% | 0.1% | 0.2% | 1.3% | |
| Food ball | 923 | 56.6% | 29.7% | 3.5% | 5.2% | 0.8% | 0.4% | 3.9% | |
| Hay ball | 928 | 52.9% | 33.2% | 2.4% | 2.9% | 1.0% | 0.5% | 7.1% | |
| Human–Animal Interactions | n | Never | Occasionally | 1×/Month | Several Times/Month | 1×/Week | Several Times/Week | 1×/Day | Several Times/Day |
| Stroking | 935 | 22.4% | 31.2% | 0.6% | 2.1% | 2.8% | 8.3% | 9.6% | 22.9% |
| Talking | 942 | 0.5% | 3.4% | 0.0% | 0.1% | 0.1% | 2.3% | 5.3% | 88.2% |
| Clicker training | 924 | 90.3% | 5.8% | 0.2% | 0.8% | 0.4% | 1.1% | 0.9% | 0.5% |
| Target training | 923 | 92.2% | 4.7% | 0.0% | 0.5% | 0.4% | 1.1% | 0.5% | 0.5% |
| Agility | 922 | 90.0% | 6.2% | 0.2% | 0.8% | 0.4% | 0.5% | 1.0% | 0.9% |
| Trick training | 924 | 79.8% | 11.9% | 0.0% | 0.9% | 1.3% | 2.6% | 1.4% | 2.2% |
| Hand-feeding | 940 | 2.0% | 8.2% | 0.1% | 1.2% | 1.3% | 12.4% | 19.9% | 54.9% |
| Carrying around | 932 | 49.8% | 22.7% | 1.4% | 2.0% | 7.2% | 6.5% | 6.2% | 4.1% |
| Food and Supplements | n | Never | Occasionally | 1×/Week | Several Times/ Week | 1×/Day | Several Times/ Day | Constant Access |
|---|---|---|---|---|---|---|---|---|
| Hay | 940 | 0.4% | 1.3% | 0.1% | 1.1% | 2.3% | 4.5% | 90.3% |
| Straw | 925 | 39.5% | 25.6% | 3.4% | 3.2% | 0.4% | 1.1% | 26.8% |
| Dried herbs | 940 | 3.3% | 26.0% | 11.7% | 21.0% | 10.2% | 4.5% | 23.4% |
| Vegetable flakes | 936 | 12.3% | 31.3% | 8.7% | 18.1% | 17.6% | 6.3% | 5.8% |
| Hay pellets | 929 | 56.8% | 25.4% | 3.2% | 4.5% | 3.6% | 1.5% | 5.0% |
| Concentrates with grains | 928 | 84.4% | 8.4% | 0.9% | 1.6% | 1.6% | 0.2% | 2.9% |
| Compound feed without grains | 926 | 39.3% | 28.3% | 5.5% | 7.7% | 8.0% | 1.0% | 10.3% |
| Nuts | 930 | 81.2% | 15.5% | 1.1% | 0.6% | 0.5% | 0.2% | 0.9% |
| Veterinarian food | 920 | 82.0% | 14.5% | 0.7% | 0.9% | 1.1% | 0.4% | 0.5% |
| Green fodder | 937 | 0.9% | 11.5% | 3.4% | 19.3% | 16.5% | 26.6% | 21.8% |
| Vegetables | 941 | 0.0% | 2.1% | 0.5% | 6.0% | 23.6% | 46.1% | 21.7% |
| Culinary herbs | 935 | 2.8% | 22.0% | 8.3% | 34.9% | 12.5% | 11.4% | 8.0% |
| Fruit | 937 | 5.3% | 48.8% | 17.7% | 16.6% | 6.4% | 2.7% | 2.5% |
| Salad | 940 | 1.7% | 11.6% | 2.9% | 21.5% | 18.0% | 28.0% | 16.4% |
| Branches leaves | 938 | 6.3% | 35.9% | 12.8% | 24.0% | 5.7% | 1.8% | 13.5% |
| Bread | 940 | 89.6% | 8.3% | 0.6% | 0.5% | 0.3% | 0.0% | 0.6% |
| Treats | 936 | 59.9% | 27.9% | 2.6% | 2.9% | 4.1% | 2.4% | 0.3% |
| Lime lickstone | 935 | 93.6% | 3.1% | 0.2% | 0.1% | 0.0% | 0.0% | 3.0% |
| Salt lick | 934 | 88.7% | 3.5% | 0.1% | 0.0% | 0.0% | 0.0% | 7.7% |
| Nibble sticks | 934 | 64.7% | 29.0% | 2.8% | 1.2% | 0.3% | 0.0% | 2.0% |
| Nibble woods | 935 | 68.1% | 25.3% | 1.3% | 1.9% | 0.1% | 0.0% | 3.2% |
| Vitamin supplements | 934 | 81.2% | 13.2% | 2.0% | 1.2% | 1.9% | 0.2% | 0.3% |
| (Health) Care Measures | n | Never | Occasionally | 1×/ Month | Several Times/Month | 1×/ Week | Several Times/Week | Daily | |
|---|---|---|---|---|---|---|---|---|---|
| Nail clipping | 928 | 4.6% | 29.5% | 47.2% | 0.0% | 18.3% | 0.2% | 0.1% | |
| Ear check | 929 | 1.8% | 18.1% | 16.9% | 0.0% | 56.9% | 4.0% | 2.3% | |
| Anterior teeth check | 928 | 2.3% | 15.8% | 17.1% | 0.0% | 58.6% | 3.2% | 2.9% | |
| Fur grooming | 926 | 40.4% | 24.6% | 7.6% | 0.0% | 22.6% | 3.6% | 1.3% | |
| Anal region check | 927 | 2.7% | 15.6% | 12.6% | 0.0% | 54.0% | 8.4% | 6.6% | |
| Cleaning eye region | 923 | 37.4% | 32.1% | 4.2% | 0.0% | 18.9% | 3.8% | 3.7% | |
| Cleaning nasal region | 919 | 46.1% | 29.1% | 3.3% | 0.0% | 16.6% | 2.9% | 2.0% | |
| Cleaning of the Following Areas | n | Never | Occasionally | 1×/ Month | Several Times/Month | 1×/ Week | 2×/Week | >2×/Week | Daily |
| Cleaning whole enclosure | 929 | 0.3% | 0.0% | 0.0% | 0.0% | 54.9% | 31.0% | 0.0% | 13.8% |
| Cleaning the whole exercise area | 750 | 11.1% | 0.0% | 0.0% | 0.0% | 46.8% | 23.7% | 0.0% | 18.4% |
| Litter only | 817 | 7.0% | 0.0% | 0.0% | 0.0% | 43.6% | 26.7% | 0.0% | 22.8% |
| Blankets/fleece (incontinence pads, etc.) only | 641 | 27.9% | 0.0% | 0.0% | 0.0% | 23.9% | 17.0% | 0.0% | 31.2% |
| Behaviours Directed Towards Conspecifics or Directed Towards Focus Animal | n | Never | 1×/ Month | Several Times/Month | 1×/ Week | Several Times/Week | 1×/ Day | Several Times/ Day |
|---|---|---|---|---|---|---|---|---|
| Bites conspecifics | 898 | 87.6% | 4.9% | 3.5% | 1.2% | 1.6% | 0.8% | 0.4% |
| Bitten by conspecifics | 896 | 89.7% | 5.4% | 2.5% | 0.6% | 1.2% | 0.2% | 0.4% |
| Chases conspecifics | 897 | 67.0% | 14.0% | 10.0% | 2.9% | 3.5% | 1.2% | 1.3% |
| Chased by conspecifics | 892 | 70.3% | 15.6% | 7.7% | 1.7% | 1.9% | 1.2% | 1.6% |
| Blocks conspecifics from food | 895 | 60.9% | 8.8% | 10.1% | 2.5% | 8.4% | 4.2% | 5.1% |
| Blocked from food by conspecifics | 897 | 67.4% | 8.7% | 9.8% | 2.1% | 6.8% | 2.3% | 2.8% |
| Sprays urine at conspecifics | 894 | 81.7% | 8.6% | 4.9% | 1.1% | 2.6% | 0.7% | 0.4% |
| Sprayed with urine by conspecifics | 893 | 80.6% | 8.5% | 5.9% | 1.2% | 2.0% | 0.8% | 0.9% |
| Plucks out fur from conspecifics | 895 | 96.9% | 1.7% | 0.9% | 0.1% | 0.4% | 0.0% | 0.0% |
| Fur plucked out by conspecifics | 896 | 97.3% | 1.7% | 0.6% | 0.0% | 0.4% | 0.0% | 0.0% |
| Food stolen by conspecifics | 892 | 32.3% | 10.3% | 14.8% | 4.9% | 17.0% | 8.2% | 12.4% |
| Steals food from conspecifics | 894 | 29.0% | 10.3% | 15.9% | 6.3% | 17.0% | 7.8% | 13.8% |
| Mounts on conspecifics | 891 | 63.0% | 14.8% | 12.1% | 2.0% | 5.7% | 0.6% | 1.8% |
| Mounted by conspecifics | 887 | 66.0% | 17.7% | 9.8% | 2.4% | 2.7% | 0.7% | 0.8% |
| Plays with conspecifics | 879 | 24.7% | 4.9% | 13.5% | 2.6% | 21.4% | 5.7% | 27.2% |
| Avoids contact with conspecifics | 889 | 90.7% | 3.6% | 2.5% | 0.3% | 1.7% | 0.3% | 0.9% |
| Avoided by conspecifics | 888 | 92.6% | 2.7% | 2.1% | 0.6% | 1.4% | 0.3% | 0.3% |
| Fighting with conspecifics | 891 | 90.9% | 5.3% | 1.9% | 0.7% | 0.7% | 0.6% | 0.0% |
| Rests together with conspecific(s) (e.g., contact lying, sitting) | 894 | 9.4% | 3.7% | 7.8% | 1.7% | 22.5% | 6.8% | 48.1% |
| Rests alone | 888 | 8.1% | 2.3% | 8.1% | 1.4% | 19.3% | 7.9% | 53.0% |
| Naso-nasal contact | 883 | 13.5% | 4.3% | 12.6% | 3.5% | 23.9% | 8.6% | 33.6% |
| Ano-genital sniffing (control) | 881 | 14.1% | 7.3% | 16.7% | 5.2% | 25.2% | 8.4% | 23.2% |
| Sleeps together with conspecifics in same house/tube | 892 | 18.0% | 5.6% | 11.1% | 2.6% | 19.2% | 7.5% | 36.0% |
| Eats simultaneously and peacefully next to conspecifics | 895 | 0.6% | 0.3% | 1.8% | 0.2% | 6.8% | 2.7% | 87.6% |
| Dependent Variable | Predictor Variables and Model Summary | Estimate a | SE b | Beta c | t | p d |
|---|---|---|---|---|---|---|
| Frequency of affiliative behaviours | Number of permanently accessible huts/caves/other | 0.11 | 0.06 | 0.07 | 1.82 | 0.070 |
| Group size increase within the prior 8 weeks no (0) yes (1) | −0.38 | 0.12 | −0.12 | −3.12 | 0.002 | |
| Food enrichment | 0.22 | 0.05 | 0.18 | 4.55 | <0.001 | |
| Daily time spent engaging with focus animal | 0.10 | 0.05 | 0.07 | 1.89 | 0.059 | |
| Frequency of carrying and stroking | 0.07 | 0.02 | 0.12 | 3.21 | 0.001 | |
| Frequency of talking hand-feeding | 0.07 | 0.04 | 0.07 | 1.84 | 0.067 | |
| Age of focus animal | −0.04 | 0.02 | −0.06 | −1.74 | 0.081 | |
| Female (0) vs. male (1) | 0.23 | 0.09 | 0.09 | 2.52 | 0.012 | |
| Model: adj. R2 = 0.104, F = 10.78, p < 0.001, n = 674 | ||||||
| Dependent Variables | Predictor Variables and Model Summary | B e | SE | OR f | Wald | p d |
| (95% CI) g | ||||||
| Occurrence of agonistic behaviours | Food enrichment | −0.15 | 0.08 | 0.86 (0.73–1.01) | 3.55 | 0.060 |
| Model: R2 = 0.007, Chi2 = 3.61, p = 0.058, n = 676 | ||||||
| Occurrence of competition for food | Frequency of carrying and stroking | −0.17 | 0.47 | 0.84 (0.77–0.93) | 12.92 | <0.001 |
| Frequency of talking hand-feeding | 0.26 | 0.08 | 1.30 (1.11–1.52) | 10.73 | 0.001 | |
| Female (0) vs. male (1) | −0.45 | 0.21 | 0.64 (0.43–0.96) | 4.62 | 0.032 | |
| Model: R2 = 0.056, Chi2 = 23.05, p < 0.001, n = 676 | ||||||
| Behaviour Displayed in the Main Living Area and During Roaming | n | Never | 1×/Month | Several Times/Month | 1×/Week | Several Times/Week | 1×/Day | Several Times/Day |
|---|---|---|---|---|---|---|---|---|
| Fur nibbling | 913 | 67.0% | 5.4% | 7.2% | 3.3% | 7.4% | 3.5% | 6.1% |
| Bar chewing | 913 | 96.7% | 0.9% | 0.5% | 0.4% | 0.3% | 0.4% | 0.7% |
| Running up and down at a certain cage location/between two specific places | 919 | 91.1% | 1.4% | 1.2% | 0.2% | 2.4% | 0.8% | 2.9% |
| Urine spraying | 913 | 80.2% | 6.1% | 6.5% | 1.8% | 3.9% | 0.3% | 1.2% |
| Rubbing anal region over the floor/marking with perianal glands | 913 | 68.7% | 5.8% | 8.3% | 2.4% | 9.1% | 1.8% | 3.9% |
| Teeth grinding | 909 | 78.0% | 6.6% | 6.6% | 1.9% | 4.1% | 1.0% | 1.9% |
| Teeth chattering (threatening behaviour) | 912 | 50.3% | 16.2% | 14.8% | 4.1% | 8.7% | 3.0% | 3.0% |
| Gnawing furniture during free roaming | 838 | 87.5% | 3.3% | 4.3% | 1.1% | 2.1% | 1.0% | 0.7% |
| Hiding during free roaming | 802 | 48.9% | 5.0% | 8.4% | 2.6% | 10.5% | 4.5% | 20.2% |
| Trying to return to cage/enclosure during free roaming | 784 | 73.6% | 2.4% | 4.6% | 1.9% | 3.7% | 1.8% | 12.0% |
| Popcorning (“jumping attacks”) | 895 | 16.6% | 8.4% | 18.0% | 4.9% | 25.3% | 6.9% | 19.9% |
| Using toys/enrichment (e.g., intelligence toys, tunnels) | 883 | 24.6% | 4.1% | 9.4% | 2.7% | 17.8% | 6.0% | 35.4% |
| Housing Types | |||||
|---|---|---|---|---|---|
| Housing Types | Self-Built Enclosure | Larger Fenced Floor Area | Guinea Pig Room | Free Housing | Outdoor Enclosure |
| Frequency of Marking Behaviours and Teeth Noises | |||||
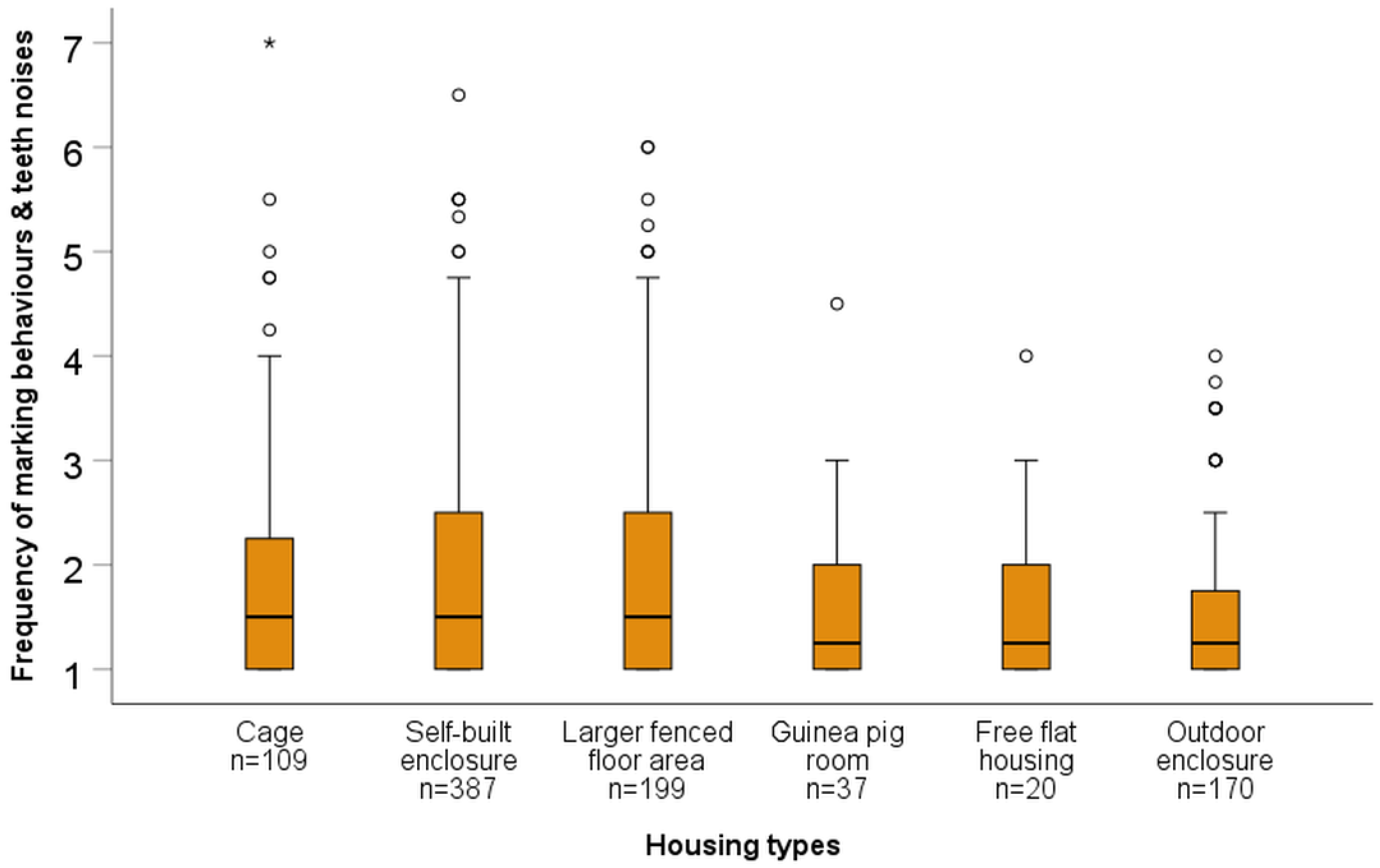
| Cage | Z = −0.27 p = 0.791 | Z = −0.73 p = 0.466 | Z = −1.63 p = 0.103 | Z = −1.10 p = 0.272 | Z = −3.20 p = 0.001 |
| Self-built enclosure | Z = −0.64 p = 0.523 | Z = −1.97 p = 0.048 | Z = −1.31 p = 0.189 | Z = −4.53 p = <0.001 | |
| Larger fenced floor area | Z = −2.16 p = 0.031 | Z = −1.51 p = 0.132 | Z = −4.43 p = <0.001 | ||
| Guinea pig room | Z = −0.11 p = 0.909 | Z = −0.42 p = 0.672 | |||
| Free housing | Z = −0.42 p = 0.674 | ||||
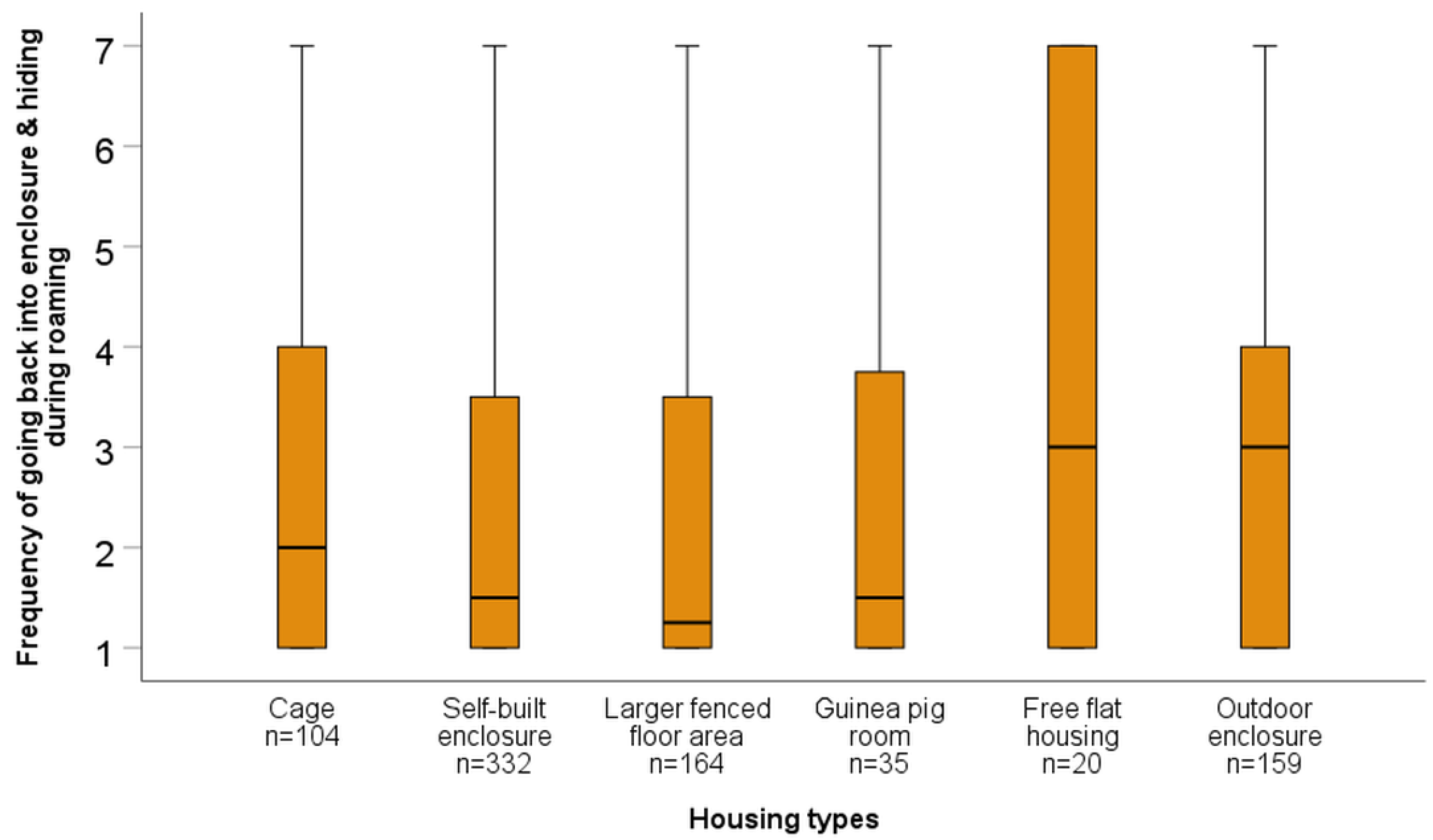
| Frequency of Going Back into the Enclosure and Hiding During Roaming | |||||
| Cage | Z = −2.16 p = 0.031 | Z = −2.06 p = 0.039 | Z = −0.97 p = 0.333 | Z = −1.16 p = 0.248 | Z = −0.85 p = 0.398 |
| Self-built enclosure | Z = −0.22 p = 0.825 | Z = −0.15 p = 0.878 | Z = −2–12 p = 0.034 | Z = −3.36 p = 0.001 | |
| Larger fenced floor area | Z = −0.29 p = 0.775 | Z = −2.15 p = 0.031 | Z = −3.06 p = 0.002 | ||
| Guinea pig room | Z = −1.58 p = 0.114 | Z = −1.48 p = 0.138 | |||
| Free housing | Z = −0.74 p = 0.458 | ||||
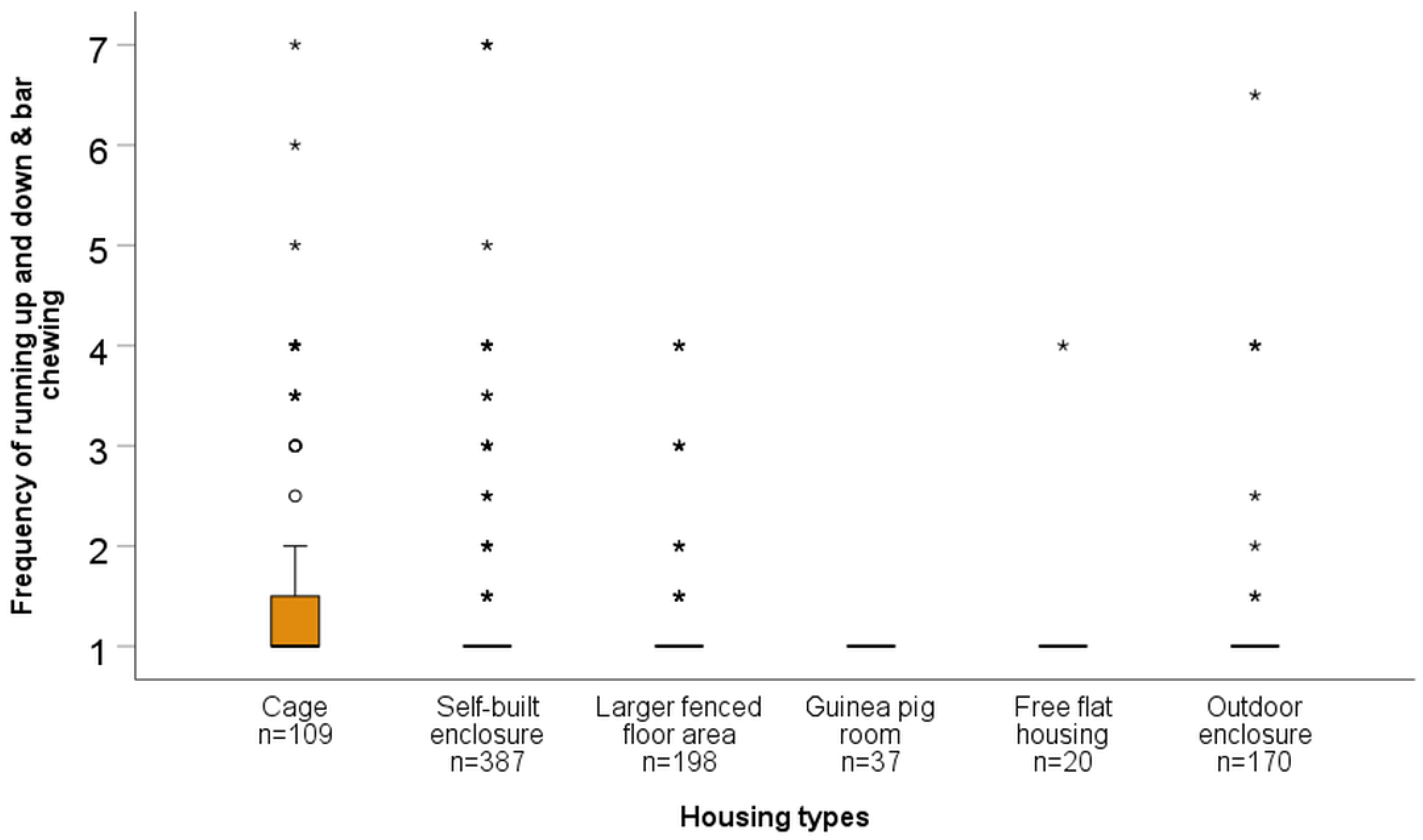
| Frequency of Running Up and Down and Bar Chewing | |||||
| Cage | Z = 4.79 p < 0.001 | Z = −3.71 p < 0.001 | Z = −3.54 p = <0.001 | Z = −2.07 p = 0.038 | Z = −5.42 p = <0.001 |
| Self-built enclosure | Z = −0.64 p = 0.525 | Z = −2.02 p = 0.043 | Z = −0.70 p = 0.486 | Z = −2.09 p = 0.036 | |
| Larger fenced floor area | Z = −2.23 p = 0.026 | Z = −0.88 p = 0.378 | Z = −2.46 p = 0.014 | ||
| Guinea pig room | Z = −1.36 p = 0.174 | Z = −1.34 p = 0.180 | |||
| Free housing | Z = −0.08 p = 0.939 | ||||
| Dependent Variables | Predictor Variables and Model Summary | Estimate a | SE b | Beta c | t | p d |
|---|---|---|---|---|---|---|
| Frequency of going back into enclosure and hiding during roaming | Number of animals in the same enclosure as the focus animal | −0.03 | 0.02 | −0.09 | −2.22 | 0.027 |
| Roaming frequency outside the enclosure | 0.15 | 0.02 | 0.38 | 9.72 | <0.001 | |
| Frequency of health checks | −0.25 | 0.09 | −0.11 | −2.83 | 0.005 | |
| Model: adj. R2 = 0.151, F = 34.27, p < 0.001, n = 560 | ||||||
| Frequency of locomotor play and use of enrichment | Number of animals in the same enclosure as the focus animal | −0.04 | 0.01 | −0.10 | −2.65 | 0.008 |
| Food enrichment | 0.33 | 0.07 | 0.18 | 4.72 | <0.001 | |
| Daily time spent engaging with focus animal | 0.13 | 0.08 | 0.07 | 1.80 | 0.073 | |
| Frequency of talking hand-feeding | 0.21 | 0.06 | 0.14 | 3.60 | <0.001 | |
| Frequency of training | 0.26 | 0.08 | 0.12 | 3.08 | 0.002 | |
| Frequency of health checks | 0.31 | 0.08 | 0.15 | 3.87 | <0.001 | |
| Age of focus animal | −0.21 | 0.03 | −0.22 | −6.02 | <0.001 | |
| Model: adj. R2 = 0.206, F = 23.49, p < 0.001, n = 609 | ||||||
| Dependent Variables | Predictor Variables and Model Summary | B e | SE | OR f | Wald | p d |
| (95% CI) g | ||||||
| Observation of marking behaviour and teeth noises | Daily time spent engaging with focus animal | 0.22 | 0.12 | 1.25 (0.98–1.60) | 3.31 | 0.069 |
| Frequency of training | 0.36 | 0.16 | 1.43 (1.05–1.95) | 5.06 | 0.025 | |
| Frequency of talking hand-feeding | 0.19 | 0.07 | 1.21 (1.05–1.39) | 7.11 | 0.008 | |
| Model: R2 = 0.057, Chi2 = 25.88, p < 0.001, n = 627 | ||||||
| Observation of running up and down and bar chewing | Individual housing yes (0) no (1) | −0.70 | 0.37 | 0.50 (0.24–1.04) | 3.48 | 0.062 |
| Frequency of training | 0.30 | 0.12 | 1.35 (1.07–1.72) | 6.17 | 0.013 | |
| Frequency of carrying and stroking | 0.19 | 0.05 | 1.20 (1.08–1.34) | 11.53 | 0.001 | |
| Model: R2 = 0.069, Chi2 = 22.82, p < 0.001, n = 626 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsbacher, T.; Sommese, A.; Waiblinger, S.; Künzel, F.; Arhant, C.; Windschnurer, I. Guinea Pig (Cavia porcellus) Welfare: Associations Between Husbandry Practices, Human–Animal Interactions, and Animal Behaviour. Animals 2025, 15, 1157. https://doi.org/10.3390/ani15081157
Elsbacher T, Sommese A, Waiblinger S, Künzel F, Arhant C, Windschnurer I. Guinea Pig (Cavia porcellus) Welfare: Associations Between Husbandry Practices, Human–Animal Interactions, and Animal Behaviour. Animals. 2025; 15(8):1157. https://doi.org/10.3390/ani15081157
Chicago/Turabian StyleElsbacher, Tanja, Andrea Sommese, Susanne Waiblinger, Frank Künzel, Christine Arhant, and Ines Windschnurer. 2025. "Guinea Pig (Cavia porcellus) Welfare: Associations Between Husbandry Practices, Human–Animal Interactions, and Animal Behaviour" Animals 15, no. 8: 1157. https://doi.org/10.3390/ani15081157
APA StyleElsbacher, T., Sommese, A., Waiblinger, S., Künzel, F., Arhant, C., & Windschnurer, I. (2025). Guinea Pig (Cavia porcellus) Welfare: Associations Between Husbandry Practices, Human–Animal Interactions, and Animal Behaviour. Animals, 15(8), 1157. https://doi.org/10.3390/ani15081157





