Anti-Inflammatory Effects of Weissella cibaria SDS2.1 Against Klebsiella pneumoniae-Induced Mammary Gland Inflammation
Simple Summary
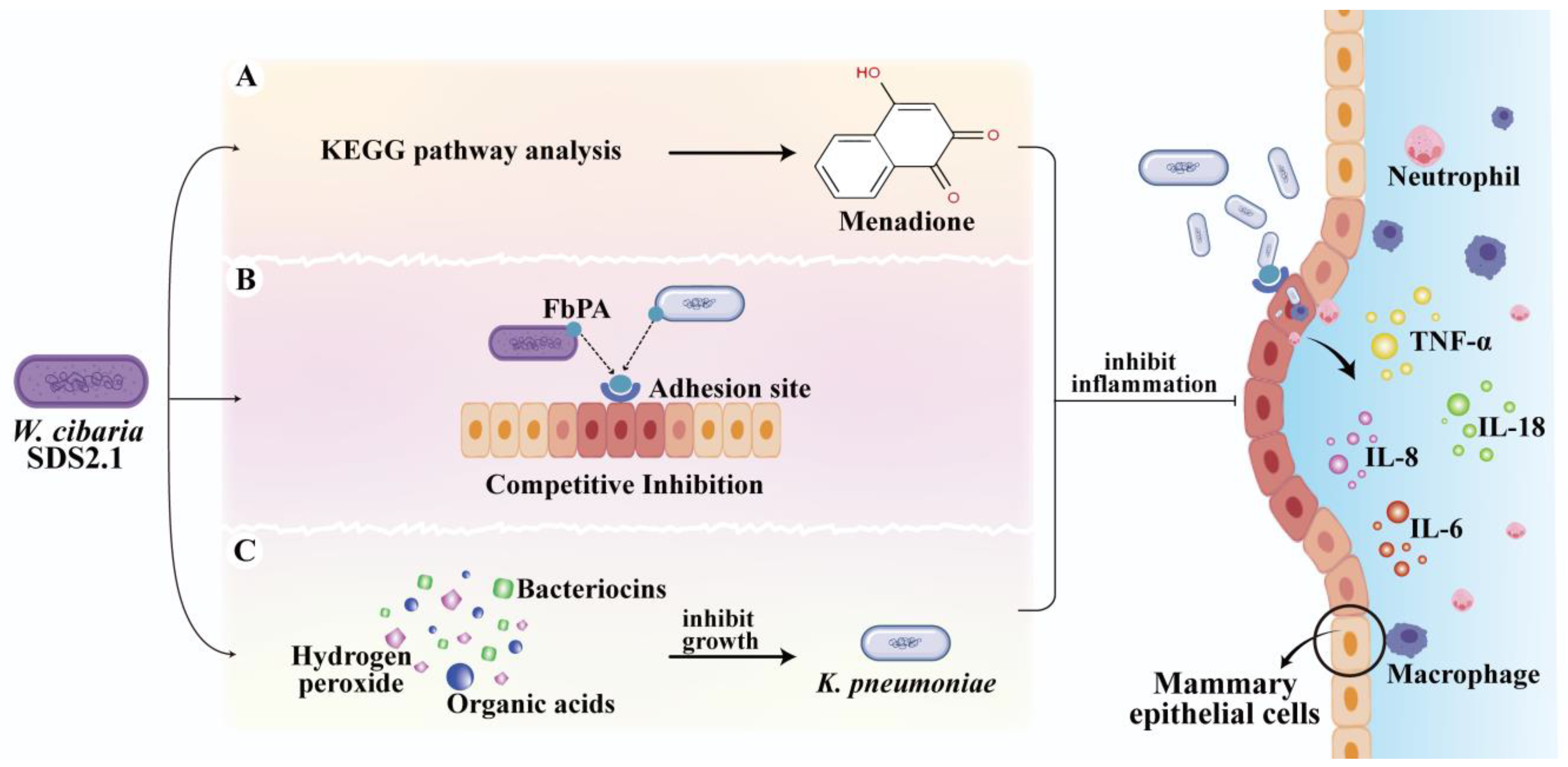
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolation and Source
2.2. Genomic DNA Extraction and Genome Sequencing
2.3. De Novo Genome Assembly and Genome Annotations
2.4. Safety Assessment of W. cibaria SDS2.1
2.4.1. Antibiotic Resistance of W. cibaria SDS2.1
2.4.2. Hemolytic Activity and Cell Viability of W. cibaria SDS2.1
2.4.3. Virulence Genes and Antibiotic Resistance Genes of W. cibaria SDS2.1
2.5. Antibacterial Activity of W. cibaria SDS2.1
2.6. Acid Production Capacity of W. cibaria SDS2.1
2.7. Antibacterial Activity Under Different pH Conditions
2.8. The Impact of pH on the Growth of W. cibaria SDS2.1
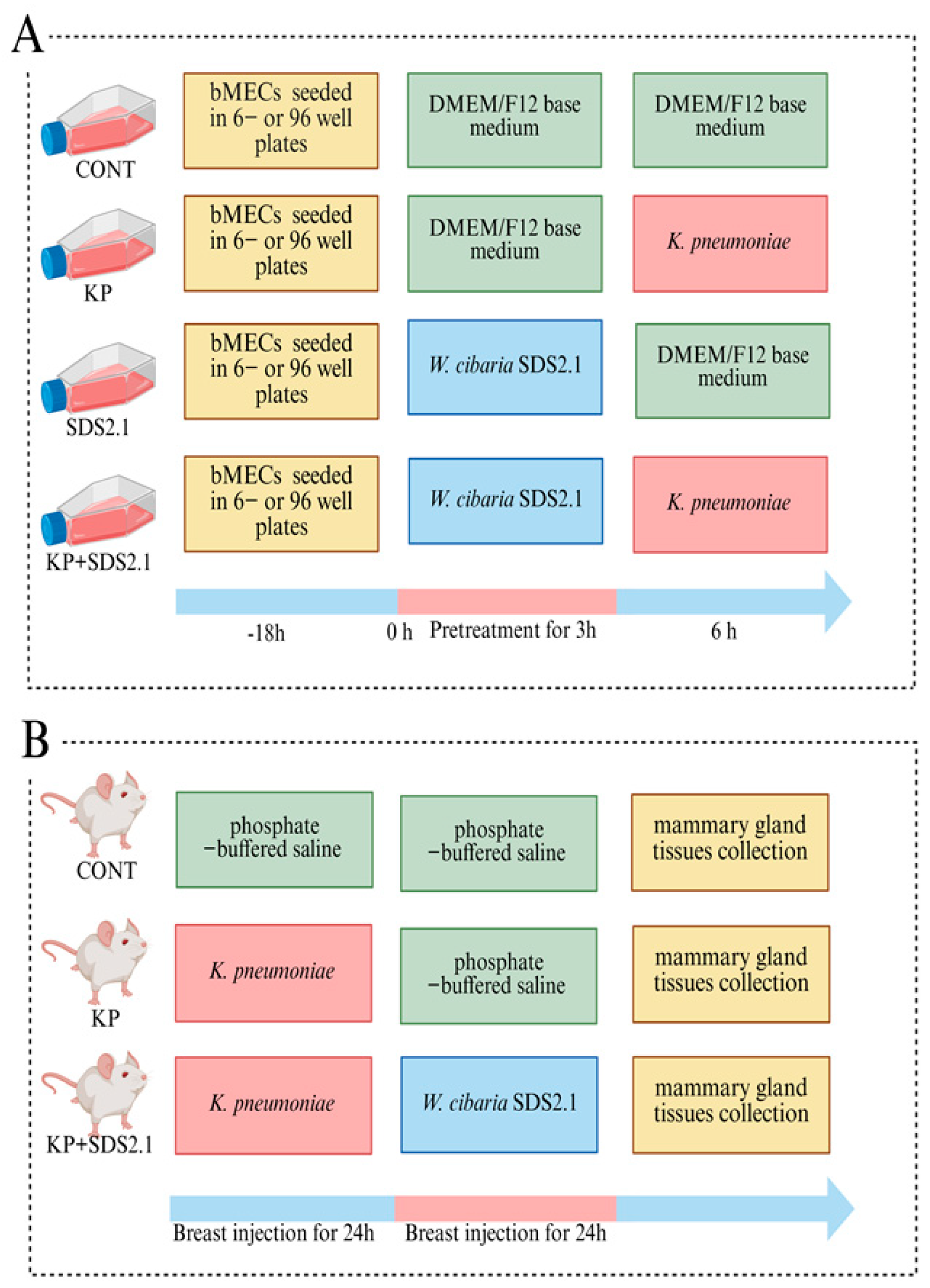
2.9. Bovine Mammary Epithelial Cell (bMEC) Culture and Treatment
2.10. Adhesion and Invasion
2.11. Animals and Experimental Design
2.12. Enumeration of K. pneumoniae in Mammary Gland
2.13. Histopathological Observations
2.14. Myeloperoxidase Evaluation
2.15. Transcriptional Gene Expression of Inflammatory Genes
2.16. Secreted Inflammatory Cytokines Quantification
2.17. Morphology of bMECs
2.18. Lactate Dehydrogenase (LDH) Activity Assay
2.19. Statistical Analyses
3. Results
3.1. Genome Annotation and Gene Ontology-Based Functional Characterization of W. cibaria SDS2.1
3.2. The Safety and Antibacterial Activity of W. cibaria SDS2.1
3.3. W. cibaria SDS2.1 Alleviates K. pneumoniae-Induced Injury to In Vivo Infection Model
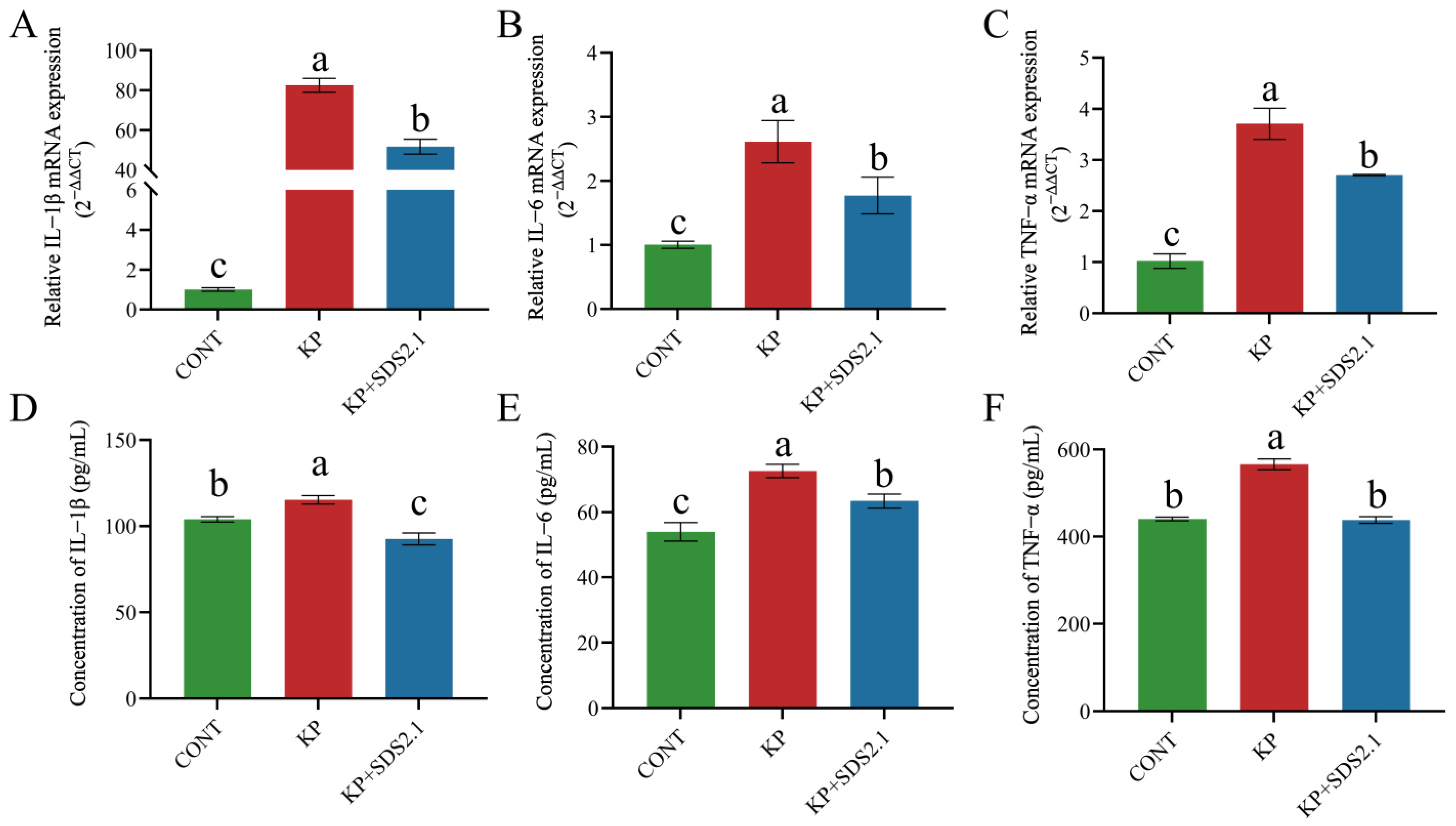
3.4. W. cibaria SDS2.1 Reduced K. pneumoniae-Induced Inflammation in Mice Mastitis
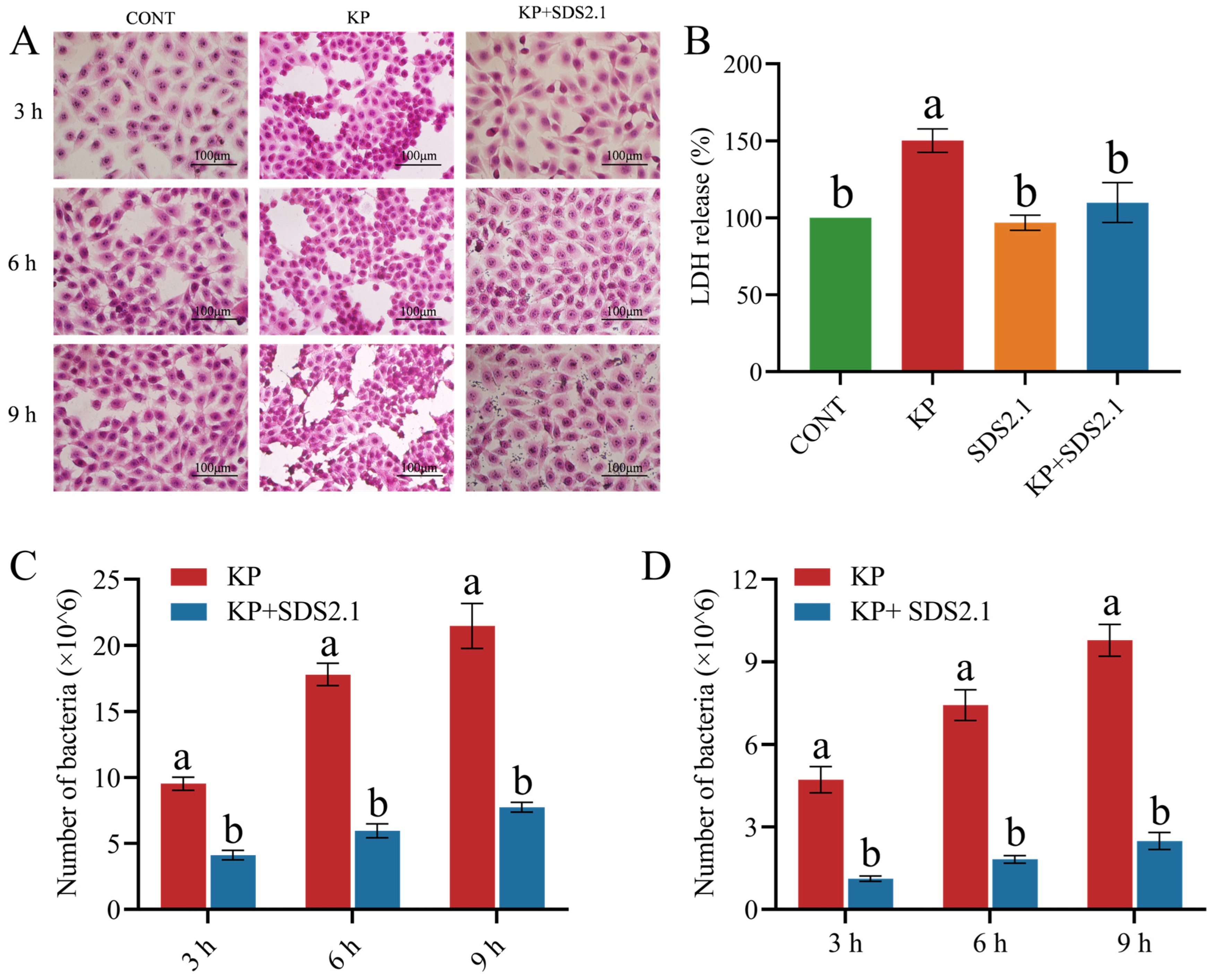
3.5. W. cibaria SDS2.1 Reduced Pathomorphological Changes and LDH Levels in K. pneumoniae-Infected bMECs
3.6. W. cibaria SDS2.1 Reduced Adhesion and Invasion of K. pneumoniae to bMECs
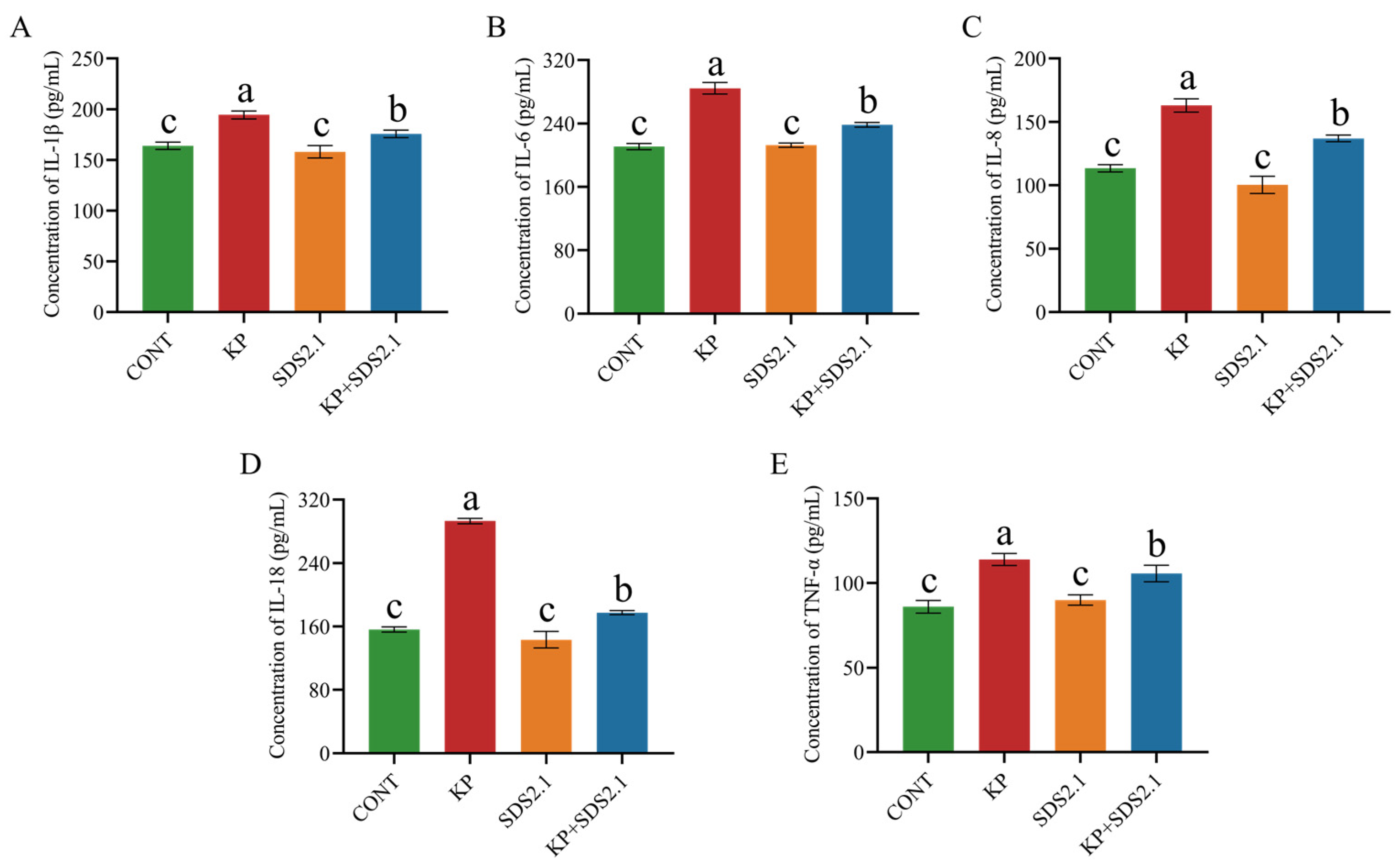
3.7. W. cibaria SDS2.1 Inhibited Inflammatory Responses in Infected bMECs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| K. pneumoniae | Klebsiella pneumoniae |
| bMECs | bovine mammary epithelial cells |
| E. coli | Escherichia coli |
| LAB | lactic acid bacteria |
| W. cibaria | Weissella cibaria |
| S. aureus | Staphylococcus aureus |
| MRS | de Man Rogosa Sharp |
| CFU | colony-forming unit |
| rRNA | ribosomal RNA |
| tRNA | transfer RNA |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| CARD | Comprehensive Antibiotic Resistance Database |
| CAZy | Carbohydrate-Active enZymes Database |
| MICs | minimal inhibitory concentrations |
| MCOFFs | microbiological cut-off values |
| EFSA | European Food Safety Agency |
| TSA | Tryptic Soy Agar |
| MCIC | MacConkey inositol adonitol carbenicillin agar |
| MHA | Mueller–Hinton agar |
| MOI | multiplicity of infection |
| FBS | fetal bovine serum |
| SPF | Specific Pathogen Free |
| PBS | phosphate-buffered saline |
| H&E | hematoxylin and eosin |
| MPO | myeloperoxidase |
| IL | Interleukin |
| TNF | Tumor Necrosis Factor |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| Ct | cycle threshold |
| LDH | lactate dehydrogenase |
| SEMs | standard errors of means |
| AMR | antimicrobial resistance |
| WGS | whole genome sequencing |
References
- Wang, L.; Haq, S.U.; Shoaib, M.; He, J.; Guo, W.; Wei, X.; Zheng, X. Subclinical Mastitis in Small-Holder Dairy Herds of Gansu Province, Northwest China: Prevalence, Bacterial Pathogens, Antimicrobial Susceptibility, and Risk Factor Analysis. Microorganisms 2024, 12, 2643. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Wen, C.; Wang, Z.; Qiu, Y.; Zhang, Y.; Zuo, J.; Xu, Y.; Han, X.; Luo, Z.; Chen, W.; et al. Molecular epidemiology and antimicrobial resistance of outbreaks of Klebsiella pneumoniae clinical mastitis in Chinese dairy farms. Microbiol. Spectr. 2022, 10, e0299722. [Google Scholar] [CrossRef]
- Heng, J.; Zhang, J.; Han, B.; Barkema, H.W.; Cobo, E.R.; Kastelic, J.P.; Zhou, M.; Shi, Y.; Wang, J.; Yang, R.; et al. Klebsiella pneumoniae isolated from bovine mastitis is cytopathogenic for bovine mammary epithelial cels. J. Dairy. Sci. 2020, 103, 3493–3504. [Google Scholar]
- Cheng, J.; Zhang, J.; Yang, J.; Yi, B.; Liu, G.; Zhou, M.; Kastelic, J.P.; Han, B.; Gao, J. Klebsiella pneumoniae infection causes mitochondrial damage and dysfunction in bovine mammary epithelial cells. Vet. Res. 2021, 52, 17. [Google Scholar] [CrossRef]
- Schukken, Y.; Chuff, M.; Moroni, P.; Gurjar, A.; Santisteban, C.; Welcome, F.; Zadoks, R. The “other” gram-negative bacteria in mastitis: Klebsiella, serratia, and more. Vet. Clin. N. Am. Food Anim. Pract. 2012, 28, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Sharun, K.; Dhama, K.; Tiwari, R.; Gugjoo, M.B.; Yatoo, M.I.; Patel, S.K.; Pathak, M.; Karthik, K.; Khurana, S.K.; Singh, R.; et al. Advances in therapeutic and managemental approaches of bovine mastitis: A comprehensive review. Vet. Q. 2021, 41, 107–136. [Google Scholar] [CrossRef]
- Cheng, J.; Qu, W.; Barkema, H.W.; Nobrega, D.B.; Gao, J.; Liu, G.; De Buck, J.; Kastelic, J.P.; Sun, H.; Han, B. Antimicrobial resistance profiles of 5 common bovine mastitis pathogens in large Chinese dairy herds. J. Dairy Sci. 2019, 102, 2416–2426. [Google Scholar] [CrossRef]
- Kober, A.K.M.H.; Saha, S.; Islam, M.A.; Rajoka, M.S.R.; Fukuyama, K.; Aso, H.; Villena, J.; Kitazawa, H. Immunomodulatory Effects of Probiotics: A Novel Preventive Approach for the Control of Bovine Mastitis. Microorganisms 2022, 10, 2255. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, S.; Guo, J.; Xie, Q.; Evivie, S.E.; Song, Y.; Li, B.; Huo, G. The protective effects of Lactobacillus plantarum KLDS 1.0344 on LPS-induced mastitis in vitro and in vivo. Front. Immunol. 2021, 12, 770822. [Google Scholar] [CrossRef]
- Pellegrino, M.; Berardo, N.; Giraudo, J.; Nader-Macías, M.E.F.; Bogni, C. Bovine mastitis prevention: Humoral and cellular response of dairy cows inoculated with lactic acid bacteria at the dry-off period. Benef. Microbes 2017, 8, 589–596. [Google Scholar] [CrossRef]
- Beecher, C.; Daly, M.; Berry, D.P.; Klostermann, K.; Flynn, J.; Meaney, W.; Hill, C.; McCarthy, T.V.; Ross, R.P.; Giblin, L. Administration of a live culture of Lactococcus lactis DPC 3147 into the bovine mammary gland stimulates the local host immune response, particularly IL-1beta and IL-8 gene expression. J. Dairy Res. 2009, 76, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, Y.C.; Wang, Y.; Li, H.; Wang, X.M.; Wu, Y.; Zhang, D.R.; Gao, S.; Qi, Z.L. Impact of yeast and lactic acid bacteria on mastitis and milk microbiota com-position of dairy cows. AMB Express 2020, 10, 22. [Google Scholar] [CrossRef]
- Park, H.E.; Do, K.H.; Lee, W.K. The immune-modulating effects of viable Weissella cibaria JW15 on RAW 264.7 macrophage cells. J. Biomed. Res. 2019, 34, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; You, Y.O.; Kang, J.Y.; Kim, H.J.; Kang, M.S. Weissella cibaria CMU exerts an anti-inflammatory effect by inhibiting Aggregatibacter actinomycetemcomitans-induced NF-κB activation in macrophages. Mol. Med. Rep. 2020, 22, 4143–4150. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, N.; Wang, X.; Yan, H.; Guan, L.; Kong, L.; Chen, J.; Zhang, H.; Ma, H. Weissella cibaria Relieves Gut Inflammation Caused by Escherichia coli through Inflammation Modulation and Gut Microbiota Regulation. Foods 2024, 13, 1133. [Google Scholar] [CrossRef]
- Quintanilla-Pineda, M.; Achou, C.G.; Díaz, J.; Gutiérrez-Falcon, A.; Bravo, M.; Herrera-Muñoz, J.I.; Peña-Navarro, N.; Alvarado, C.; Ibañez, F.C.; Marzo, F. In Vitro Evaluation of Postbiotics Produced from Bacterial Isolates Obtained from Rainbow Trout and Nile Tilapia against the Pathogens Yersinia ruckeri and Aeromonas salmonicida subsp. salmonicida. Foods 2023, 12, 861. [Google Scholar] [CrossRef]
- Wang, L.; Si, W.; Xue, H.; Zhao, X. A fibronectin-binding protein (FbpA) of Weissella cibaria inhibits colonization and infection of Staphylococcus aureus in mammary glands. Cell Microbiol. 2017, 19, e12731. [Google Scholar] [CrossRef]
- Krishnamoorthy, P.; Satyanarayana, M.L.; Shome, B.R.; Rahman, H. Pathological Changes in Experimental Intramammary Infection with Different Staphylococcus Species in Mice. J. Microsc. Ultrastruct. 2018, 6, 93–98. [Google Scholar] [CrossRef]
- Zhong, Y.; Fu, D.; Deng, Z.; Tang, W.; Mao, J.; Zhu, T.; Zhang, Y.; Liu, J.; Wang, H. Lactic acid bacteria mixture isolated from wild pig alleviated the gut inflammation of mice challenged by Escherichia coli. Front. Immunol. 2022, 13, 82275. [Google Scholar] [CrossRef]
- Merenstein, D.; Pot, B.; Leyer, G.; Ouwehand, A.C.; Preidis, G.A.; Elkins, C.A.; Hill, C.; Lewis, Z.T.; Shane, A.L.; Zmora, N.; et al. Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes 2023, l15, 2185034. [Google Scholar] [CrossRef]
- Pradhan, D.; Mallappa, R.H.; Grover, S. Comprehensive approaches for assessing the safety of probiotic bacteria. Food Control. 2020, 108, 106872. [Google Scholar] [CrossRef]
- Shin, E.; Paek, J.J.; Lee, Y. Antimicrobial Resistance of Seventy Lactic Acid Bacteria Isolated from Commercial Probiotics in Korea. J. Microbiol. Biotechnol. 2023, 33, 500–510. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP); Rychen, G.; Aquilina, G. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA J. 2018, 16, e05206. [Google Scholar] [PubMed]
- Zhang, Q.; Wang, M.; Ma, X.; Li, Z.; Jiang, C.; Pan, Y.; Zeng, Q. In vitro investigation on lactic acid bacteria isolated from Yak faeces for potential probiotics. Front. Cell Infect. Microbiol. 2022, 12, 984537. [Google Scholar]
- Kamiloglu, S.; Sari, G.; Ozdal, T.; Capanoglu, E. Guidelines for cell viability assays. Food Front. 2020, 1, 332–349. [Google Scholar] [CrossRef]
- Malberg Tetzschner, A.M.; Johnson, J.R.; Johnston, B.D.; Lund, O.; Scheutz, F. In Silico Genotyping of Escherichia coli Isolates for Extraintestinal Virulence Genes by Use of Whole-Genome Sequencing Data. J. Clin. Microbiol. 2020, 58, e01269-20. [Google Scholar] [CrossRef]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef]
- Tsai, C.C.; Lai, T.M.; Hsieh, Y.M. Evaluation of Lactobacilli for antagonistic activity against the growth, adhesion and invasion of Klebsiella pneumoniae and Gardnerella vaginalis. Indian J. Microbiol. 2019, 59, 81–89. [Google Scholar] [CrossRef]
- Cui, L.B.; Zhou, X.Y.; Zhao, Z.J.; Li, Q.; Huang, X.Y.; Sun, F.Z. The Kunming mouse: As a model for age-related decline in female fertility in human. Zygote 2013, 21, 367–376. [Google Scholar] [CrossRef]
- Mei, X.; Zhang, Y.; Quan, C.; Liang, Y.; Huang, W.; Shi, W. Characterization of the Pathology, Biochemistry, and Immune Response in Kunming (KM) Mice Following Fasciola gigantica Infection. Front. Cell Infect. Microbiol. 2022, 11, 793571. [Google Scholar] [CrossRef]
- Zhao, W.; Shi, Y.; Liu, G.; Yang, J.; Yi, B.; Liu, Y.; Gao, J. Bacteriophage has beneficial effects in a murine model of Klebsiella pneumoniae mastitis. J. Dairy. Sci. 2021, 104, 3474–3484. [Google Scholar] [CrossRef]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Kõressaar, T.; Lepamets, M.; Kaplinski, L.; Raime, K.; Andreson, R.; Remm, M. Primer3_masker: Integrating masking of template sequence with primer design software. Bioinformatics 2018, 34, 1937–1938. [Google Scholar] [CrossRef] [PubMed]
- Zbinden, C.; Pilo, P.; Frey, J.; Bruckmaier, R.M.; Wellnitz, O. The immune response of bovine mammary epithelial cells to live or heat-inactivated Mycoplasma bovis. Vet. Microbiol. 2015, 179, 336–340. [Google Scholar] [CrossRef]
- Lyu, C.C.; Ji, X.Y.; Che, H.Y.; Meng, Y.; Wu, H.Y.; Zhang, J.B.; Zhang, Y.H.; Yuan, B. CGA alleviates LPS-induced inflammation and milk fat reduction in BMECs through the NF-κB signaling pathway. Heliyon 2024, 10, e25004. [Google Scholar] [CrossRef] [PubMed]
- Abdi, R.D.; Gillespie, B.E.; Ivey, S.; Pighetti, G.M.; Almeida, R.A.; Kerro Dego, O. Antimicrobial Resistance of Major Bacterial Pathogens from Dairy Cows with High Somatic Cell Count and Clinical Mastitis. Animals 2021, 11, 131. [Google Scholar] [CrossRef]
- Erskine, R.J.; Bartlett, P.C.; VanLente, J.L.; Phipps, C.R. Efficacy of systemic ceftiofur as a therapy for severe clinical mastitis in dairy cattle. J. Dairy. Sci. 2002, 85, 2571–2575. [Google Scholar] [CrossRef]
- Podder, M.P.; Rogers, L.; Daley, P.K.; Keefe, G.P.; Whitney, H.G.; Tahlan, K. Klebsiella species associated with bovine mastitis in Newfoundland. PLoS ONE 2014, 9, e106518. [Google Scholar] [CrossRef]
- Fukuyama, K.; Islam, M.A.; Takagi, M.; Ikeda-Ohtsubo, W.; Kurata, S.; Aso, H.; Vignolo, G.; Villena, J.; Kitazawa, H. Evaluation of the immunomodulatory ability of lactic acid bacteria isolated from feedlot cattle against mastitis using a bovine mammary epithelial cells in vitro assay. Pathogens 2020, 9, 410. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Z.; Zhao, L.; Zhao, Y.; Yang, G.; Wang, C.; Gao, L.; Niu, C.; Li, S. Lactobacillus plantarum DP189 reduces alpha-SYN aggravation in MPTP-induced parkinson’s disease mice via regulating oxidative damage, inflammation, and gut microbiota disorder. J. Agric. Food Chem. 2022, 70, 1163–1173. [Google Scholar] [CrossRef]
- Teixeira, C.G.; Belguesmia, Y.; da Silva Rodrigues, R.; Lucau-Danila, A.; Nero, L.A.; de Carvalho, A.F.; Drider, D. Assessment of safety and in situ antibacterial activity of Weissella cibaria strains isolated from dairy farms in Minas Gerais State, Brazil, for their food application. Braz. J. Microbiol. 2024, 55, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Marcotte, S.; Zaro, J.L. pH-sensitive antimicrobial peptide with targeted activity in a mildly acidic microenvironment. J. Drug Deliv. Sci. Technol. 2024, 93, 105420. [Google Scholar] [CrossRef]
- Fan, J.; Schiemer, T.; Vaska, A.; Jahed, V.; Klavins, K. Cell via Cell Viability Assay Changes Cellular Metabolic Characteristics by Intervening with Glycolysis and Pentose Phosphate Pathway. Chem. Res. Toxicol. 2024, 37, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Llanco, L.A.; Nakano, V.; Moraes, C.T.P.; Piazza, R.M.F.; Avila-Campos, M.J. Adhesion and invasion of Clostridium perfringens type A into epithelial cells. Braz J Microbiol. 2017, 48, 764–768. [Google Scholar] [CrossRef]
- Cai, L.; Qin, X.; Xu, Z.; Song, Y.; Jiang, H.; Wu, Y.; Ruan, H.; Chen, J. Comparison of Cytotoxicity Evaluation of Anticancer Drugs between Real-Time Cell Analysis and CCK-8 Method. ACS Omega 2019, 4, 12036–12042. [Google Scholar] [CrossRef]
- Patravale, V.; Dandekar, P.; Jain, R. Nanotoxicology: Evaluating toxicity potential of drug-nanoparticles. Nanopart. Drug Deliv. 2012, 4, 123–155. [Google Scholar]
- Kumar, P.; Nagarajan, A.; Uchil, P.D. Analysis of Cell Viability by the Lactate Dehydrogenase Assay. Cold Spring Harb. Protoc. 2018, 6, pdb-prot095497. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, K.; Nie, K.; Deng, M.; Luo, W.; Wu, X.; Wang, X. Assessment of the safety and probiotic properties of Roseburia intestinalis: A potential “Next Generation Probiotic”. Front. Microbiol. 2022, 13, 973046. [Google Scholar] [CrossRef]
- Berardo, N.; Giraudo, J.; Magnano, G.; Nader-Macias, M.E.F.; Bogni, C.; Pellegrino, M. Lactococcus lactis subsp lactis CRL1655 and Schleiferilactobacillus perolens CRL1724 inhibit the adherence of common bovine mastitis pathogens to mammary gland cells, without causing histological changes in the mammary gland. J. Appl. Microbiol. 2022, 133, 733–742. [Google Scholar] [CrossRef]
- Ahn, J.; Ambrosone, C.B.; Kanetsky, P.A.; Tian, C.; Lehman, T.A.; Kropp, S.; Chang-Claude, J. Polymorphisms in genes related to oxidative stress (CAT, MnSOD, MPO, and eNOS) and acute toxicities from radiation therapy following lumpectomy for breast cancer. Clin. Cancer Res. 2006, 12, 7063–7070. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef]
- Islam, A.; Takagi, M.; Fukuyama, K.; Komatsu, R.; Albarracin, L.; Nochi, T.; Suda, Y.; Ikeda-Ohtsubo, W.; Rutten, V.; van Eden, W.; et al. Transcriptome analysis of the inflammatory responses of bovine mammary epithelial cells: Exploring immunomodulatory target genes for bovine mastitis. Pathogens 2020, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Bradford, B.J.; Yuan, K.; Farney, J.K.; Mamedova, L.K.; Carpenter, A.J. Invited review: Inflammation during the transition to lactation: New adventures with an old flame. J. dairy Sci. 2015, 98, 6631–6650. [Google Scholar] [CrossRef]
- Souza, R.F.S.; Rault, L.; Seyffert, N.; Azevedo, V.; Le Loir, Y.; Even, S. Lactobacillus casei BL23 modulates the innate immune response in Staphylococcus aureus-stimulated bovine mammary epithelial cells. Benef. Microbe 2018, 9, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Yang, M.; Tian, M.; Jia, L.; Wu, Y.; Du, J.; Yuan, L.; Li, L.; Ma, Y. The preventive effects of Lactobacillus casei 03 on Escherichia coli-induced mastitis in vitro and in vivo. J. Inflamm. 2024, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- Awaisheh, S.S.; Ibrahim, S.A. Screening of antibacterial activity of lactic acid bacteria against different pathogens found in vacuum-packaged meat products. Foodborne Pathog. Dis. 2009, 6, 1125–1132. [Google Scholar] [CrossRef]
- Xie, Y.; Li, S.; Wu, D.; Wang, Y.; Chen, J.; Duan, L.; Li, S.; Li, Y. Vitamin K: Infection, Inflammation, and Auto-Immunity. J. Inflamm. Res. 2024, 17, 1147–1160. [Google Scholar] [CrossRef]


| Group | Treatment Condition | Times (h) | Experimental Conditions |
|---|---|---|---|
| KP | K. pneumoniae (1 × 109 CFU/mL) | 0 | The solutions were incubated at 37 °C, 180 r/min, and the viable count of K. pneumoniae at each time point was measured using the plate count method. |
| 12 | |||
| 24 | |||
| KP + SDS2.1 | K. pneumoniae + W. cibaria SDS2.1 (1 × 109 CFU/mL) | 0 | |
| 12 | |||
| 24 |
| Clinical Score | Criteria |
|---|---|
| 1 | Active and healthy |
| 2 | Slower reaction, otherwise healthy |
| 3 | Lethargic, but active |
| 4 | Inactive, but responsive |
| 5 | Unresponsive to stimuli |
| Gene | Primer Sequence (5′-3′) | Size | |
|---|---|---|---|
| Bos | |||
| IL-6 | Forward | GCTGAATCTTCCAAAAATGGAGG | 215 |
| Reverse | GCTTCAGGATCTGGATCAGTG | ||
| IL-8 | Forward | ACACATTCCACACCTTTCCAC | 149 |
| Reverse | ACCTTCTGCACCCACTTTTC | ||
| IL-1β | Forward | CCTCGGTTCCATGGGAGATG | 119 |
| Reverse | AGGCACTGTTCCTCAGCTTC | ||
| TNF-α | Forward | TCCAGAAGTTGCTTGTGCCT | 144 |
| Reverse | CAGAGGGCTGTTGATGGAGG | ||
| IL-18 | Forward | TTGCATCAGCTTTGTGGAAA | 78 |
| Reverse | TGGGGTGCATTATCTGAACA | ||
| GAPDH | Forward | GTCTTCACTACCATGGAGAAGG | 201 |
| Reverse | TCATGGATGACCTTGGCCAG | ||
| Mice | |||
| IL-1β | Forward | CCTGGGCTGTCCTGATGAGAG | 188 |
| Reverse | TCCACGGGAAAGACACAGGTA | ||
| IL-6 | Forward | TAGTCCTTCCTACCCCAATTTCC | 142 |
| Reverse | TTGGTCCTTAGCCACTCCTTC | ||
| TNF-α | Forward | CAGGCGGTGCCTATGTCTC | 155 |
| Reverse | CGATCACCCCGAAGTTCAGTAG | ||
| GAPDH | Forward | AGGTCGGTGTGAACGGATTTG | 139 |
| Reverse | TGTAGACCATGTAGTTGAGGTCA | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, M.; Jin, T.; Tong, J.; Song, D.; Xie, Q.; Li, X.; Li, Y.; Liu, K.; Gao, J.; Liu, M.; et al. Anti-Inflammatory Effects of Weissella cibaria SDS2.1 Against Klebsiella pneumoniae-Induced Mammary Gland Inflammation. Animals 2025, 15, 1139. https://doi.org/10.3390/ani15081139
Ren M, Jin T, Tong J, Song D, Xie Q, Li X, Li Y, Liu K, Gao J, Liu M, et al. Anti-Inflammatory Effects of Weissella cibaria SDS2.1 Against Klebsiella pneumoniae-Induced Mammary Gland Inflammation. Animals. 2025; 15(8):1139. https://doi.org/10.3390/ani15081139
Chicago/Turabian StyleRen, Meiyi, Tianxiong Jin, Jingdi Tong, Deyuan Song, Qinna Xie, Xiaohan Li, Yan Li, Kangping Liu, Jian Gao, Mingchao Liu, and et al. 2025. "Anti-Inflammatory Effects of Weissella cibaria SDS2.1 Against Klebsiella pneumoniae-Induced Mammary Gland Inflammation" Animals 15, no. 8: 1139. https://doi.org/10.3390/ani15081139
APA StyleRen, M., Jin, T., Tong, J., Song, D., Xie, Q., Li, X., Li, Y., Liu, K., Gao, J., Liu, M., & Cheng, J. (2025). Anti-Inflammatory Effects of Weissella cibaria SDS2.1 Against Klebsiella pneumoniae-Induced Mammary Gland Inflammation. Animals, 15(8), 1139. https://doi.org/10.3390/ani15081139






