1. Introduction
Insect-derived ingredients are of increasing interest to the pet food industry, providing an opportunity for the bioconversion of feedstocks of low or no value into premium protein and energy ingredients, while having a favorable environmental footprint [
1,
2].
For any ingredient, an evaluation of its safety and efficacy is critical prior to market entry and is required to gain regulatory authorization in different jurisdictions. The safety of insect ingredients for use as nutrient sources in animal feed, including black soldier fly larvae (BSFL)-based ingredients, has been extensively reviewed in the published literature [
3,
4,
5,
6,
7,
8,
9]. The successful demonstration of the safety of larvae (BSFL) ingredients by certain manufacturers in the US has resulted in the Association of American Feed Control Officials (AAFCO) ingredient definitions for BSFL ingredients, in the form of dried whole black soldier fly larvae (DBSFL), partially defatted BSFL meal (DBSFL meal), and BSFL oil. These authorizations are for several species, including finfish, poultry, swine, adult dogs, and adult cats (pending) for DBSFL and DBSFL meal; and finfish, poultry (pending), swine, adult dogs, and adult cats for BSFL oil. The current US authorization [
10] explicitly requires that the BSFL be raised on feed-grade materials, which minimizes the risk of chemical and microbiological hazards accumulating in BSFL ingredients. This authorization does limit the use of some potential feedstocks (e.g., post-consumer food waste) but allows for the use of pre-consumer by-products that may otherwise have low or no value.
It is important to recognize that BSFL-based ingredients can vary in nutrient composition and quality (e.g., digestibility) due to the BSFL diet, life stage at harvest, and processing methods used to create these ingredients. Unlike more traditional ingredients, there is significant potential for variation in quality and composition due to the insect rearing program. The nutrient composition of the BSFL will be significantly influenced by the feedstock [
11,
12]; notably, the fatty acid, mineral, and heavy metal profiles can vary dramatically due to different feeding programs. Similarly, the life stage of the BSFL impacts the nutrient composition and digestibility of those nutrients [
13]. For example, in a previous study, the fat content was low early post-hatch, then increased, then declined again as the BSFL entered the pre-pupal and pupal life stages, although the digestibility of that fat was highest from the pre-pupal and pupal life stages. The crude protein and amino acid contents also varied substantially between life stages, indicating that the age of processing can have a significant impact on the overall nutritional value of BSFL ingredients. The processing methods may also impact the nutritional quality of BSFL, as illustrated by a study that found that DBSFL meal produced from oven-dried BSFL had significantly higher in vitro digestibility compared to meals produced from BSFL that were blanched and then dried or those that were microwaved [
14].
The potential use of BSFL ingredients in pet food is supported by numerous in vitro and in vivo evaluations in relevant animal models. In adult dogs, several trials have demonstrated the safety and efficacy of BSFL ingredients as protein and energy sources for adult dogs at up to 30% inclusion of DBSFL meal and up to 5% inclusion of BSFL oil. In these trials, the dogs maintained their body weight (BW), feed intake, and fecal scores, and the BSFL ingredients generally had similar or slightly lower nutrient digestibility compared to traditional animal protein sources [
15,
16,
17,
18,
19,
20]. Overall, these data support the safety and efficacy of BSFL-derived ingredients as alternatives to traditional protein and energy sources in adult dog diets. Where differences have been observed, such as in digestibility parameters, these may be related to the diet used to rear the BSFL or the processing method used to produce the finished BSFL-derived ingredients.
There are also several published feeding trials in cats. Adult cats fed for 70 days on diets containing either 5% DBSFL partially replacing poultry by-product meal and poultry fat, 10% DBSFL meal replacing poultry by-product meal, or 1.5% BSFL oil replacing poultry fat had similar intake, fecal output, hematology, and blood clinical chemistry results across all treatments, and preferred the BSFL-based diets over the control diet [
21]. As seen in dogs, the protein digestibility was slightly lower than in poultry-based diets. Similar results were seen in cats fed up to 35% DBSFL meal for 6 weeks [
22] and in cats fed 4% DBSFL meal in semi-purified diets. Together, these data support the safety and efficacy of BSFL-derived ingredients in adult cats at maintenance.
While the utility in maintenance diets for dogs and cats is well-documented, the ability of BSFL ingredients to sustain gestation, lactation, and growth has not yet been demonstrated, although numerous trials have demonstrated the acceptability of BSFL ingredients for piglet growth [
23,
24,
25]. The nutritional requirements of these life stages are more demanding than those of adult animals and the diets for these animals require a separate evaluation [
10]. The purpose of this research was to evaluate the safety and efficacy of the protein and lipid components of DBSFL created using a specific feeding program and processing method, to support the gestation and lactation of female cats, as well as the growth of their kittens post-weaning.
2. Materials and Methods
2.1. BSFL Description
The DBSFL used in this trial was derived from 4th–5th instar larvae raised on a proprietary blend of bakery materials and distillery by-products from the bourbon industry, which were also oven-dried (“EnviroBug”, EnviroFlight LLC, Maysville, KY, USA). The nutrient composition of the DBSFL reared on this feedstock and with this method of drying is provided in
Table 1.
2.2. Cat Trials
A gestation, lactation, and growth study was conducted at Ontario Nutri Labs, Inc. (Fergus, ON, Canada), a registered research animal facility. Prior to the start of the study, a palatability test was performed to ensure that all potential queens accepted both the control and the DBSFL diets. A total of 25 queens were fed both control and DBSFL diets for 5 days. All queens consumed both the control and DBSFL diet, and from that cohort 16 queens were selected for the full trial and randomly assigned to the control or DBSFL diet (
n = 8/treatments;
Table 2).
The diets were formulated to be isocaloric and isonitrogenous and to meet or exceed AAFCO and National Research Council (NRC) recommendations for gestation, lactation, and growth [
26,
27]. The DBSFL partially replaced chicken meal and poultry fat, as well as some brewers rice and corn, in the control diet. To minimize bias, the identity of the control and DBSFL diet subjects were blinded to the staff responsible for conducting the study.
All queens were initially fed the control diet for a 7 day acclimatization period and then offered their assigned dietary treatment prior to breeding. The queens (approximately 3.4 kg; at least 1 year old, in at least their 2nd heat) were fed diets containing either 0% (control) or 20% DBSFL during the entire gestation (approximately 10 weeks, weeks 0 to 9) and lactation (approximately 6 weeks, weeks 10 to 15) phases. The cats were fed based on their resting energy requirements based on calculations of their body weight and the energy density of their diet prior to the study starting, whereas after breeding, the queens were fed ad libitum, as energy requirements can vary based on litter size and individual variation [
28]. Fresh water was provided ad libitum. Following parturition, the kittens remained with their dams until weaning (approximately 6 weeks of age).
The cats were group housed for the majority of the study but were housed individually for feeding and study-related purposes where individual data collection, such as urine and stool collection, was required. All rooms were environmentally controlled for lighting, consisting of natural lighting via windows and LED full-spectrum lighting consistent with a natural light cycle, as well as for temperature and ventilation, and were sized in accordance with the Animal Welfare Acts of the US and Canada. A full veterinary physical exam of the queens (including the body condition score (BCS) and haircoat scoring) was undertaken during the acclimation period (baseline), at mid-gestation, within 24 h post-parturition, and at the end of the lactation period. The BWs of the queens were recorded weekly and their food consumption was recorded daily. Blood samples were taken from the queens at baseline (during acclimation period), mid-gestation, post-parturition (5 to 7 days), and at weaning. Prior to blood collection, the queens were fasted for 16 h, which was reduced to 8 h during gestation to limit the time the food was withheld. Their blood samples were analyzed for hematology (red blood cell (RBC), hematocrit, hemoglobin, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), red cell distribution width (RDW), % reticulocyte, reticulocyte, reticulocyte hemoglobin, white blood cell (WBC), % neutrophil, % lymphocyte, % monocyte, % eosinophil, % basophil, neutrophil, lymphocyte, monocyte, eosinophil, basophil, and platelet) and serum biochemistry (albumin, total protein, globulin, albumin/globulin ratio, total bilirubin, conjugated bilirubin, aspartate aminotransferase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), creatine kinase, creatinine, blood urea nitrogen (BUN), glucose, total carbon dioxide (TCO2; bicarbonate), magnesium, sodium, potassium, sodium/potassium [Na/K] ratio, calcium, phosphorus, symmetric dimethylarginine test (SDMA), chloride, cholesterol, triglyceride, amylase, lipase, hemolysis index, icterus index, and lipemia index) data (IDEXX Laboratories Canada, Markham, ON, Canada). Their whole blood and plasma taurine were measured, as well using standard amino acid extraction protocols and a Biochrom 30 amino acid analyzer (UC Davis Amino Acid Lab, Davis, CA, USA).
Stool and urine samples were collected as free catch samples using a 3-tier collection litter box system concurrently with blood samples. The urine was analyzed for its color, protein, glucose, creatinine, ketone, bilirubin, pH, and specific gravity (by refractometer) data, and the urine protein/creatinine ratio (UPCR) was determined, as well as a microscopic sediment examination to identify crystals, casts, red and white blood cells, and epithelial cells (IDEXX Laboratories Canada, Markham, ON, Canada). The stool quality was assessed using the WALTHAM scale, in which each stool was rated on a scale of 1 to 5, with 1 being “bullet-like” and 5 being moist and loose, and with 3 being an optimal score [
29]. Since the urine and stool collection involved being away from group housing for prolonged periods, all cats on collection protocols received supervised enrichment with staff for at least 2 h during these periods.
Upon parturition, the kittens received a full veterinary physical examination (including BCS and haircoat scoring) within 24 h of birth, and again at weaning. Each kitten’s BW was recorded weekly during the lactation phase.
To examine the most rapid period of development and growth of the kittens fed the DBSFL-based diet, a subsample of kittens (n = 8 per dietary treatment, representing at least 3 unique litters and with equal gender distribution) were maintained on the diet assigned to their dam for an additional 10 weeks post-weaning. All kittens were less than 9 weeks old at the time of inclusion in the growth trial and were fed the same diet assigned to their dam between the end of the lactation phase and the start of the growth phase. Their body weights were measured at the start of the growth phase and monitored at seven-day intervals until the end of the 10-week trial. Their blood and urine were collected as described for queens at the start of the growth trial (18–20 days post-weaning), and after 5 and 10 weeks on the growth trial. The blood hematology, clinical chemistry, and urinalysis were assessed as described for the queens. The kittens received a full veterinary physical exam (including BCS and haircoat scoring) at the start of the growth trial (post-weaning), at the mid-point of the growth trial (5 weeks), and at the end of the trial (10 weeks).
2.3. Nutrient Digestibility
Nutrient digestibility was measured via an analysis of diet nutrient concentrations after grinding (dry matter (AOAC 930.15), ash (AOAC: 942.05), crude protein (AOAC: 2001.11), crude fiber (AOCS: Ba6a-05), crude fat (AOAC: 954.02), and calories (Parr Caloric Bomb); UC Davis Amino Acid Lab, Davis, CA, USA). Upon study initiation, a representative sample of each diet was taken, ground, and homogenized independently by the ONL Analytical Lab, and then analyzed for dry matter (AOAC: 930.15), ash (AOAC: 942.05), crude protein (AOAC: 2001.11), crude fiber (AOCS: Ba6a-05), crude fat (AOAC: 954.02), and calorie (Parr Caloric Bomb) data. Samples of both diets were analyzed for amino acids (AOAC 994.12 and AOAC 988.15; UC Davis Amino Acid Lab, Davis, CA, USA). The results from the analyses were used to calculate the digestibility of the diets.
All feces voided throughout days 67 to 71 (120 h) of the growth study were collected. Fecal samples for each cat were weighed and frozen daily for subsequent analyses. The collected feces were dried at 47 °C, weighed, ground, and analyzed for dry matter, ash, crude protein, crude fiber, crude fat, calorie, and amino acid data, as described for the diet analysis. The nitrogen-free extract (NFE%) or carbohydrate data were calculated using the following formula:
and the estimated metabolizable energy (ME) was calculated using the following formula:
For better accuracy, the quantitative collection method without urine collection was used to calculate the metabolizable energy using the fecal and feed analysis data and the following formula:
where a = gross energy of food (kcal/g), b = weight of food consumed (g), c = gross energy of feces in 100% of dry matter (kcal/g), d = weight of feces collected in 100% of dry matter (g), e = percentage of protein consumed as feed (%), f = percentage of protein in the feces in 100% of dry matter (%), and g = correction factor for energy lost in urine (cat) = 0.86 kcal/g.
2.4. Statistical Analysis
The jamovi 2.6.13 statistical software (
www.jamovi.org; accessed on 6 January 2025) with the GALMJ: General Analyses for the Linear Model in jamovi 3.4.2 module was used to analyze all data. The individual queens and kittens were utilized as the experimental units. The litter size and number of still births were analyzed via Kruskal–Wallis tests due to failing the normality assumptions of analysis of variance (ANOVA) tests. The macronutrient and amino acid digestibility were analyzed via an ANOVA, with diet as a factor. All other data were analyzed via linear mixed models with time (timepoint or week) and diet as factors, and the animal ID as a random effect. The residuals from the statistical models were explored using histograms, quantile–quantile plots, and Shapiro–Wilk and Kolmogorov–Smirnov normality tests. Natural log and square root transformations were used for normality violations, although few variables violated normality of residuals assumptions and transformations did not improve the distribution fit of these variables. Therefore, the data were analyzed as is. Planned comparisons were subsequently conducted to compare the treatment diets at every time. Mean, standard error of the mean (SEM), and probability (
p) values were reported for each analysis. Statistical significance was defined as
p ≤ 0.05 and statistical trends were defined as
p > 0.05 and ≤0.10.
3. Results
The veterinary examinations performed during the gestation–lactation phase of the trial showed the queens to be in good physical health. Upon trial commencement, fifteen queens were given the BCS of ideal (5), while one queen, randomly assigned to the DBSFL diet, had a BCS of overweight (6). One queen was removed from the control group as a result of having two kittens that were underdeveloped and had likely died during early pregnancy. Another queen was removed from the DBSFL diet group during the lactation phase of the study as a result of having a large, overdue kitten resulting in a cesarian section and subsequent rejection of the kitten. The kitten was successfully fostered by another queen receiving the 20% DBSFL diet and remained in the trial through the lactation phase but was not included in the growth trial. At the end of the gestation–lactation phase of the study, thirteen queens were given a BCS of ideal (5) and one DBSFL-fed queen had a BCS of underweight (4). All surviving kittens were assessed to be in good physical health within 24 h of birth, and at weaning all kittens were given a BCS of ideal (5). The average litter size from the control queens was 3.63 ± 0.46 and from the DBSFL-fed queens was 2.50 ± 0.46, with no significant difference between treatments (p = 0.11). Similarly, the numbers of stillborn kittens did not differ between treatments (0.38, 0.25 ± 0.257 for control and DBSFL-fed queens, respectively, p = 0.71).
The diet intake, body weight, and fecal quality of the queens during the gestation and lactation phases are presented in
Table 3. The dietary intake of the queens fed the DBSFL diet tended to be lower at weaning on an absolute intake basis (
p = 0.06) but was not significantly different from the control-fed queens at any other timepoint. The energy intake values (on a kcal per day basis) of the queens fed the DBSFL diet were lower than those fed the control diet but only reached statistical significance at weaning (
p = 0.02). The body weights were similar between treatments over time. The fecal quality levels were also consistent between treatments but the queens fed the DBSFL diets tended to have slightly higher and more optimal fecal scores (i.e., closer to a score of “3”) post-partum (
p = 0.07).
The blood hematology parameters at all timepoints were generally within normal ranges (see
Supplementary Materials Table S1), except for the reticulocyte hemoglobin (14.8, 14.9, and 14.4 pg at mid-gestation, post-partum, and weaning, respectively, vs. 15.3–22.9 pg) and WBC (20.9 × 10
9/L at weaning vs. 3.9–19.0 × 10
9/L) counts in the cats receiving the control diet, which varied slightly from the reference ranges (
Table 4). For blood hematology parameters with no significant impact or trend for an impact of diet, data are not presented.
There were a few differences in blood hematology due to dietary treatment. The mean corpuscular volume tended to be higher in the cats fed the DBSFL diets at baseline (p = 0.09) and mid-gestation (p = 0.08) and was significantly higher at weaning (p = 0.05) relative to the controls. The mean corpuscular hemoglobin volume tended to be higher in the queens fed the DBSFL diets at weaning (p = 0.07). Compared to the controls, the absolute number of reticulocytes was higher in the queens fed the DBSFL diet at mid-gestation (p = 0.05), and the reticulocyte hemoglobin volume tended to be higher in the queens fed the DBSFL diet at the post-partum sampling (p = 0.09) and weaning (p = 0.05) points. The white blood cell numbers were higher in the queens fed the control diet at weaning (p < 0.01). The platelet counts tended to be higher in the control-fed queens at mid-gestation (p = 0.07) and were significantly higher at weaning (p = 0.04).
The blood clinical chemistry parameters of the queens were generally within normal ranges (see
Supplementary Materials Table S2) for all parameters throughout the trial, except for glucose, which was lower than the reference range at the post-partum sampling point (3.5 and 3.7 mmol/L vs. 4.0–9.7 mmol/L), and SDMA, which was higher than the reference range at baseline (14.1 and 14.9 μg/dL vs. 0–14 μg/dL) and in the post-partum period (17.0 and 17.8 μg/dL vs. 0–14 μg/dL), irrespective of the treatment (control or DBSFL-containing diets). For blood clinical chemistry parameters with no significant impact or trend for an impact of diet, data are not presented.
The dietary treatment affected the cats’ globulin levels, with the queens fed DBSFL diets tending to have higher globulin levels at mid-gestation (p = 0.09) and significantly higher globulin levels at post-partum sampling (p = 0.03) relative to the controls. The alkaline phosphatase levels also tended to be higher for the DBSFL-fed queens at weaning (p = 0.06).
The whole blood taurine levels in the queens were maintained within the reference ranges [
30] and did not differ significantly between treatments throughout the gestation and lactation periods (mean whole blood taurine 416.89 ± 12.600 nmol/mL; mean plasma taurine 126.26 ± 6.454 nmol/mL). There were no differences in urine pH, urine specific gravity, or urinary creatinine due to dietary treatment (see
Supplementary Materials Table S3). The urinary protein level was significantly (
p = 0.04) higher at post-partum sampling for the queens fed the DBSFL diet compared to those fed the control diet (65.00 and 40.45 mg/dL, respectively) but the results did not differ at any other timepoint.
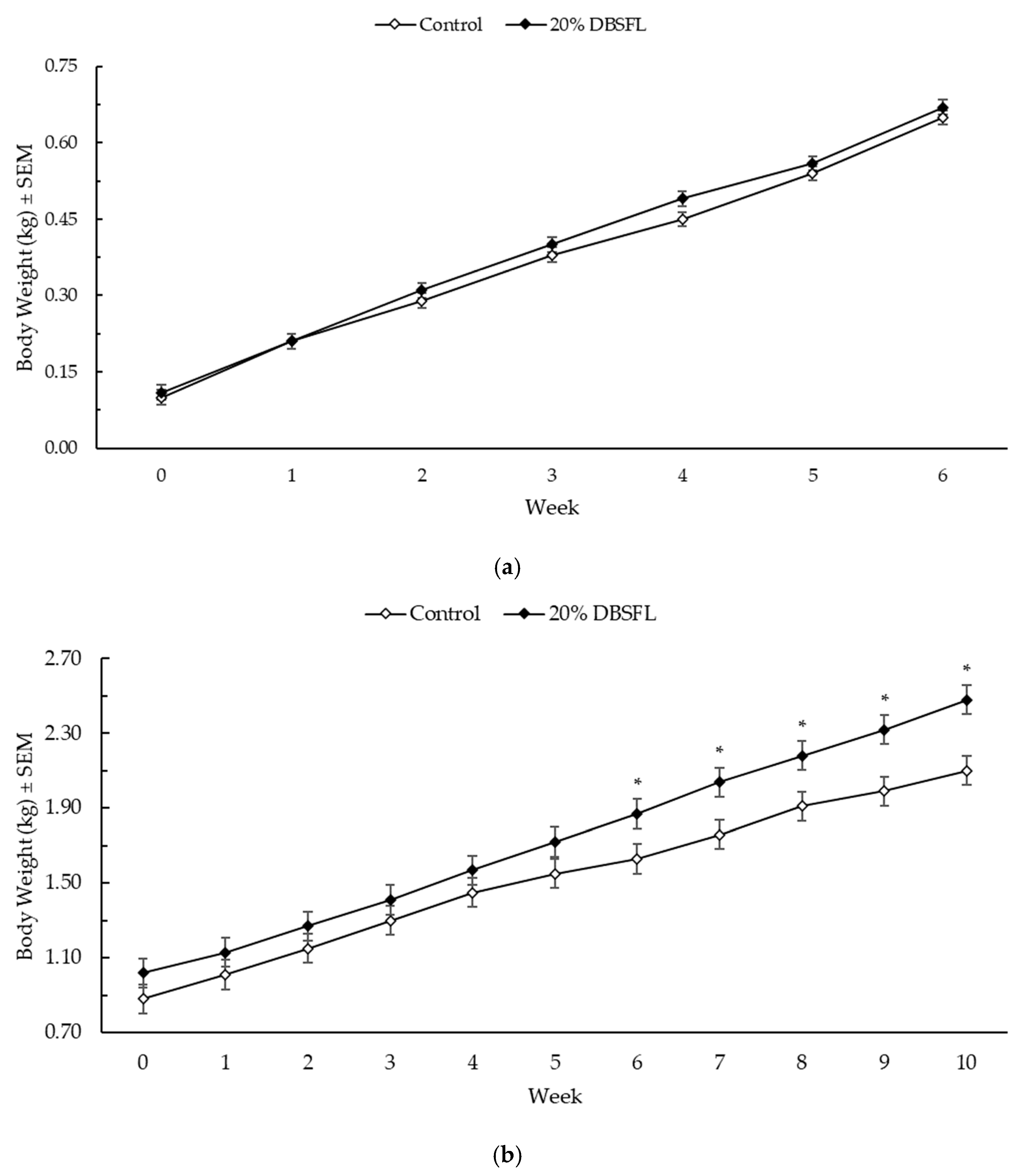
All kittens were given a BCS of 5 (ideal) at the start, mid-point, and at end of the growth phase. The kittens’ body weights during lactation and the post-weaning period are shown in
Figure 1. During the lactation phase, the kittens maintained similar body weights across treatments, with a trend for higher body weight in the kittens from the DBSFL-fed queens at week 4 of lactation (
p = 0.06). During the growth phase, the kittens from the DBSFL-fed queens were significantly heavier at weeks 6 through 10 (
p < 0.05 for each).
The sex of the kittens significantly impacted their body weight, where male kittens were heavier than females through to week 5 (p < 0.01), and kittens from larger litters were heavier than those from smaller litters (p < 0.05).
During the growth period, the kittens fed the DBSFL diet consumed significantly more than those receiving the control diet at week 5 (
p = 0.04) and week 10 (
p = 0.01) (
Table 5). The energy intake, on a kcal per day basis, of the kittens fed the DBSFL diet was numerically greater than for those fed the control diets at week 5 and statistically greater at week 10 (
p = 0.03). The fecal scores did not differ due to dietary treatment (
Table 5).
The dry matter and crude protein digestibility levels were significantly higher in the kittens fed the DBSFL diet (
p = 0.04,
p < 0.0001, respectively,
Table 6) relative to the controls. The ash and energy digestibility tended to be higher for the DBSFL diets than the control diet (
p = 0.10,
p = 0.07, respectively). The taurine digestibility was not affected by dietary treatment (
p = 0.12).
In the absence of historical control data or reference ranges specific to kittens, the values for adult cats were used for comparative purposes, although it is unclear if these values for adult cats are appropriate for the evaluation of kitten hematology or blood chemistry, since differences have been seen due to the age of kittens versus adults [
31].
During the kitten growth period, the blood hematology parameters were generally within the reference ranges described for adult cats, except for reticulocyte hemoglobin, which was lower than the adult reference range in both the control and DBSFL-fed groups (
Table 7).
The dietary treatment was observed to impact several blood hematology parameters. Relative to the controls, several RBC numbers tended to be lower in the DBSFL-fed kittens at the beginning of the growth trial (p = 0.06) but were similar thereafter. Likewise, the RDW was significantly lower in the DBSFL-fed kittens at the beginning of the growth trial (p < 0.01) but was similar thereafter. The absolute number of reticulocytes tended to be higher in the DBSFL-fed kittens at the mid-point of the growth trial (p = 0.06) and was significantly higher at the end of the growth period (p = 0.04). The reticulocyte hemoglobin counts were also higher in the DBSFL-fed kittens at the beginning of the growth trial (p = 0.01) and at the end (p = 0.03). Furthermore, the WBC numbers were higher in the DBSFL-fed kittens at the mid-point of the growth trial compared to the control-fed kittens (p = 0.01). For blood clinical chemistry parameters with no significant impact or trend for an impact of diet, data are not presented.
Several serum chemistry parameters from the kittens on the growth trial were outside the adult cat reference ranges, including the SDMA, globulin, and ALP levels in both the DBSFL-fed kittens and controls (
Table 8). For serum chemistry parameters with no significant impact or trend for an impact of diet, data are not presented. There were several parameters for which the dietary treatment had a significant impact. The blood glucose level was significantly lower in the DBSFL-fed kittens at baseline (
p = 0.03) but was not different thereafter. The blood urea nitrogen level was significantly higher at the start and mid-point of the growth trial for the DBSFL-fed kittens compared to the control-fed kittens (
p = 0.04,
p = 0.01, respectively) and tended to be higher at the end of the growth period for the DBSFL-fed kittens (
p = 0.08). The albumin level tended to be higher at the end of the growth period for the DBSFL-fed kittens (
p = 0.07). The alkaline phosphatase the was significantly higher at baseline in the DBSFL-fed kittens compared to the control-fed kittens (
p = 0.04). Finally, the triglyceride counts were significantly higher at baseline and at the end of the growth period in the DBSFL-fed kittens relative to the controls (
p = 0.04,
p = 0.05, respectively).
The whole blood taurine concentrations were not affected by diet or time (mean = 371.51 ± 13.177 nmol/mL), while the plasma taurine concentration was lower in the DBSFL-fed kittens at baseline (
p = 0.03,
Table 8) but was similar thereafter.
With respect to the urinalysis parameters in the kittens, there was no impact of dietary treatment on the urine pH or specific gravity (
Table 9). The urinary creatinine level was significantly lower in the DBSFL-fed kittens compared to the control-fed kittens at the mid-point of the growth trial (
p = 0.04) and tended to be lower at the end of the growth trial (
p = 0.06). The urinary protein the tended to be lower in the kittens fed the DBSFL diet compared to those fed the control diet at the mid-point of the growth trial (
p = 0.07).
4. Discussion
Together, the findings of the study demonstrate that DBSFL has the ability to sustain gestation, lactation, and growth; therefore, it is suitable for inclusion in the food of cats of all life stages. The design of the study followed the general principles laid down in the minimum feeding protocol for proving a gestation–lactation claim for a cat food (AAFCO, 2024 [
27]). With this design, the queens must be in at least their second heat period and at least one year or age and the kittens are to be followed for at least the first 6 weeks of age, independent of when they are weaned. The AAFCO protocol was designed for complete food for cats but was considered relevant to this study on the basis that DBSFL and its components, defatted BSFL and BSFL oil, have the potential to be primary nutrient sources in cat food. Key differences were introduced to expand the value of the study, particularly the use of a control group rather than use of historical control colony data only, to allow a direct comparison of DBSFL with a diet containing conventional protein and fat sources, i.e., chicken meal and poultry fat. Additionally, the duration of the study of growth in kittens was extended from 6 to 10 weeks to include the most sensitive period of life and also that of the highest growth. These data further allow the bridging of maintenance data in adult dogs and cats (typically greater than 1 year old) in the published literature to these more demanding life stages.
Previous evaluations using the precision-fed cecectomized rooster assay suggested that DBSFL is a high-quality protein and amino acid source [
13]. Similarly, in studies in adult cats and dogs, the digestibility of DBSFL meal has generally been similar to, or slightly below, poultry-based control diets [
15,
17,
21]. This study provided an opportunity to evaluate the quality of the protein source in vivo under periods of high nutrient demand. The queens included in the trial had similar food intake, BW, fecal quality, and litter size levels when fed a DBSFL-based diet as compared to a chicken meal and poultry fat-based (control) diet, indicative of DBSFL being of sufficient quality to maintain health during gestation and lactation. The kittens nursing from the queens fed the DBSFL diet or directly consuming that diet post-weaning had higher dietary intake and BW levels and also had higher protein digestibility levels. Together, the improved growth performance and protein digestibility of the kittens fed DBSFL are indicative of the better palatability and nutrient availability of DBSFL when contributing significantly to the protein and energy levels of the diet compared to chicken meal and poultry fat. The basis for the improved nutrient digestibility seen in this trial may be related to the exposure to this ingredient at an early life stage, allowing for better adaptation of the developing gut microflora to BSFL-based nutrients. There is evidence of modulation of gut microflora with BSFL-based diets. For example, when 20% DBSFL meal was introduced into the diet of adult dogs, after one month there were significant changes observed in short-chain fatty acid concentration, microbial populations, and the interruption of a number of metabolic pathways relative to the controls fed a chicken-meal-based diet [
17]. Additionally, on an as-fed basis, the control and DBSFL diets contained 0.01% and 2.08%, respectively, of lauric acid. The presence of lauric acid in this DBSFL ingredient may provide a readily available energy source in young animals for which gut development is not complete, and which has been demonstrated in other species [
32]. Lauric acid has also been implicated in mammary gland development and milk fat production in ruminants [
33], as well as improved gut morphology and epithelial cell turnover in weaned piglets [
34,
35]. Thus, there are several potential mechanisms by which DBSFL-based diets may contribute to improved nutrient availability.
There were a few differences in blood parameters between the DBSFL-fed and control groups that were most likely due to normal biological variation and the physiological adaptations to pregnancy, lactation, and rapid growth and not related to diet. In the queens, all values remained within the reference ranges, although the reticulocyte concentrations were variable during the trial in both dietary groups, with a significant difference between the controls and DBSFL-fed queens observed at mid-gestation. In cats, mature red blood cells serve as a primary source of iron for the developing fetus [
36]. Thus, it is likely these changes are a result of the need to support the developing fetus and regenerate mature erythrocytes for the queen rather than any impact of dietary treatment. Additionally, the SDMA values were slightly above the reference ranges during the baseline and post-partum assessments in both dietary groups. Symmetric dimethylarginine is used as a marker of renal function, and elevated SDMA levels can be of concern [
37]. However, in this trial, no other markers of renal function were elevated (e.g., creatinine, BUN, urinalysis), indicating normal physiological variation or a response to pregnancy rather than pathology. Moreover, the urinary protein levels were higher post-partum in the DBSFL-fed queens but the protein/creatinine ratios were also well within the normal ranges, consistent with normal renal function [
38]. The additional variation in blood values among the groups of queens was also likely a consequence of pregnancy rather than an effect of diet.
In the kittens on the trial, several hematology and blood clinical chemistry values deviated from the standard reference ranges, although as these ranges were derived from adult cats and all kittens presented as healthy at the veterinary inspections, these differences are unlikely to be a cause for concern. For example, the ALP concentration was dramatically higher than the reference range but has been shown to be substantially higher in kittens and to decline as they reach adult body size [
31]. Similarly, the creatinine concentration was lower than the reference range but this also is typical of growing kittens [
31]. Higher numbers of reticulocytes and reticulocyte hemoglobin counts, as well as higher BUN concentrations, were generally observed throughout the growth period in kittens consuming the DBSFL diet relative to those fed the control diet. These findings are likely to reflect the nutritional differences of the two diets, although the changes were not considered to be biologically significant, falling well within the reference ranges. The data from this trial provide valuable additional information on the standard blood chemistry and hematology profiles of both kittens and queens during gestation and lactation.
The urinary creatinine and protein levels were lower in the kittens fed the DBSFL diet, although the kittens from both treatments had urinary protein/creatinine ratios in the normal range [
38]. Protein sources can vary significantly in their creatinine content [
39], and the availability to the animal is influenced by the protein digestibility and other factors [
39,
40,
41]. Thus, these effects are attributed to a nutritional effect, with no biological relevance.
Finally, it is important to reiterate that the life stage, feedstock, and processing methods used to create BSFL-based ingredients can impact the nutrient profile and availability of those ingredients [
12]. Amino acid profiles are largely taxon-based, and although the diet can influence the fatty acid profile, typically lauric acid is always present. Therefore, these nutrients from DBSFL are likely to be similar between sources provided the larvae are reared under controlled conditions using nutritionally balanced, feed-grade materials. However, other factors will contribute to the overall quality of the DBSFL, including the relative amounts of protein and fat, which are driven to some extent by the feedstock, the mineral profile, the presence of heavy metals, and other contaminants from the feedstock. Careful control of the production process is an established critical factor in the production of feed-grade DBSFL, and the source and rearing methods used to generate BSFL ingredients should be considered when extrapolating these results to other BSFL ingredients sources [
12,
42,
43].
Considering the importance of alternative protein sources in the diet of cats, these data contribute not only to the knowledge of DBSFL as an ingredient but also provide valuable insight into the overall health of cats during gestation and lactation and of kittens pre- and post-weaning under controlled conditions in a study designed to meet AAFCO guidelines for proving the adequacy of complete food for these life stages. As mentioned above, the comparative data using the AAFCO feeding protocol are based on historical controls, and the results of the study above, particularly with regards to blood parameters, contribute to the body of knowledge under current feeding practices using conventional and novel protein sources.