A New Species of Sinospelaeobdella from China: Sinospelaeobdella jiangxiensis sp. n. (Hirudinda, Arhynchobdellida, Haemadipsidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
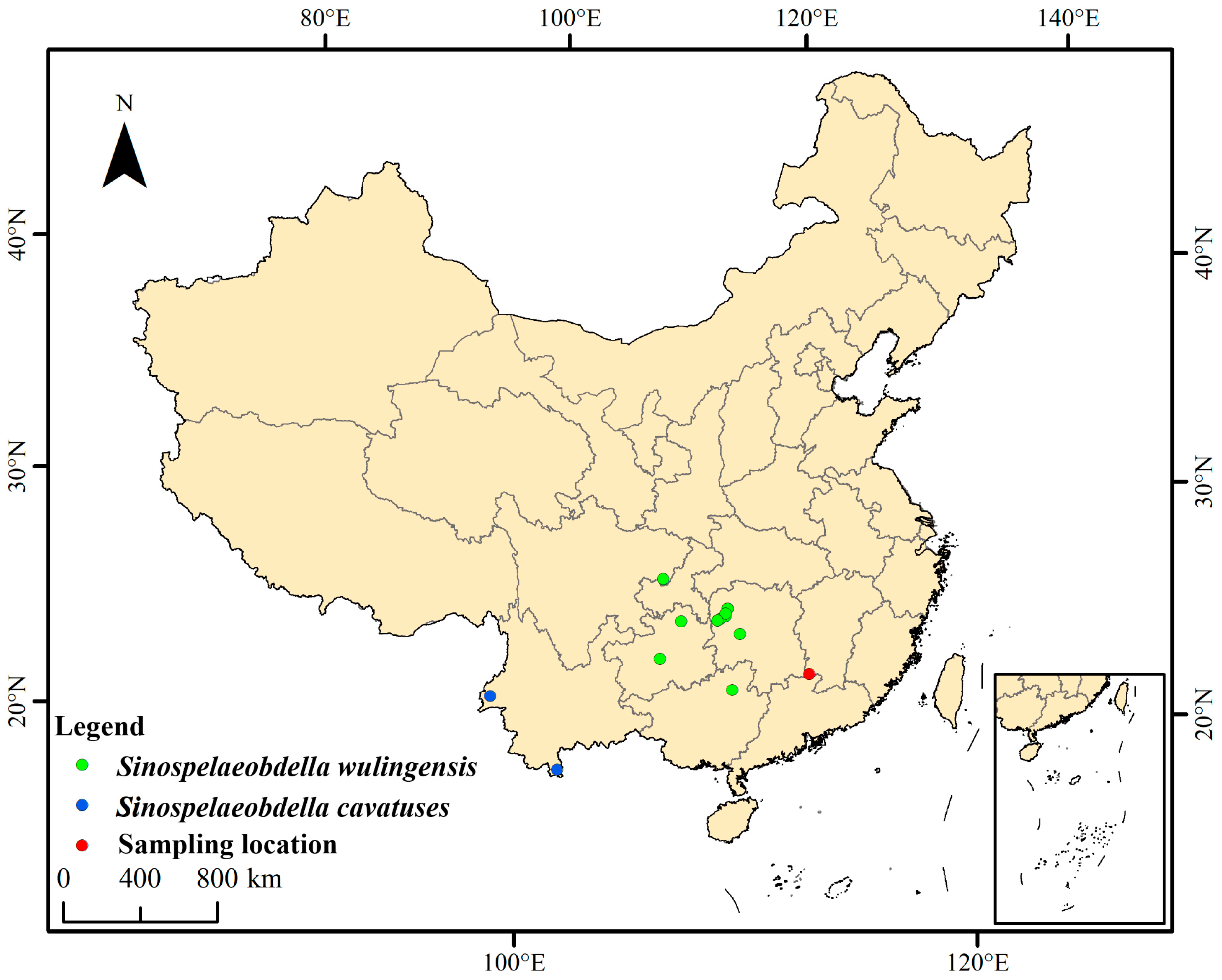
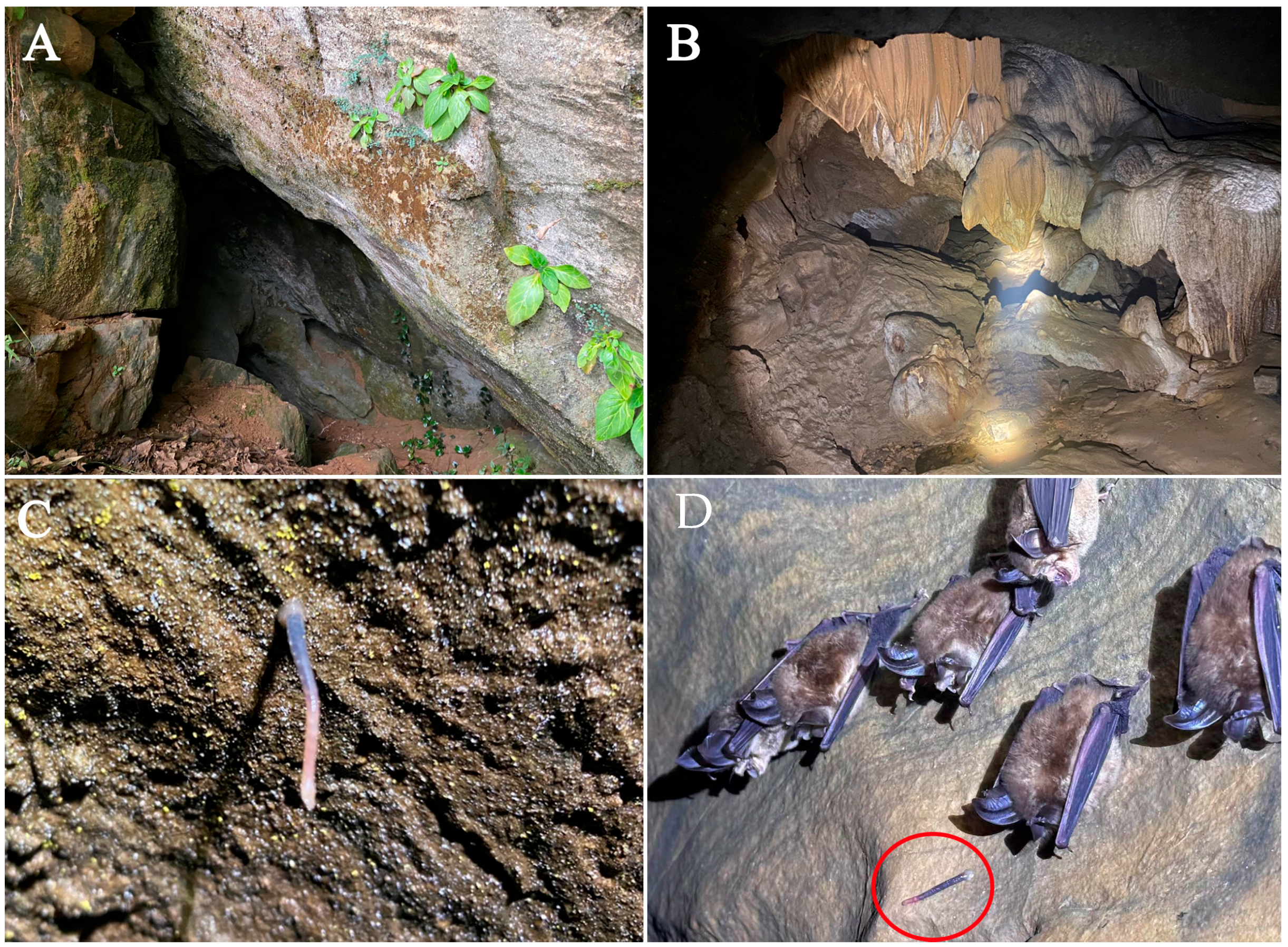
2.1. Sampling
2.2. Morphological Analysis
2.3. Phylogenetic Analysis
3. Results
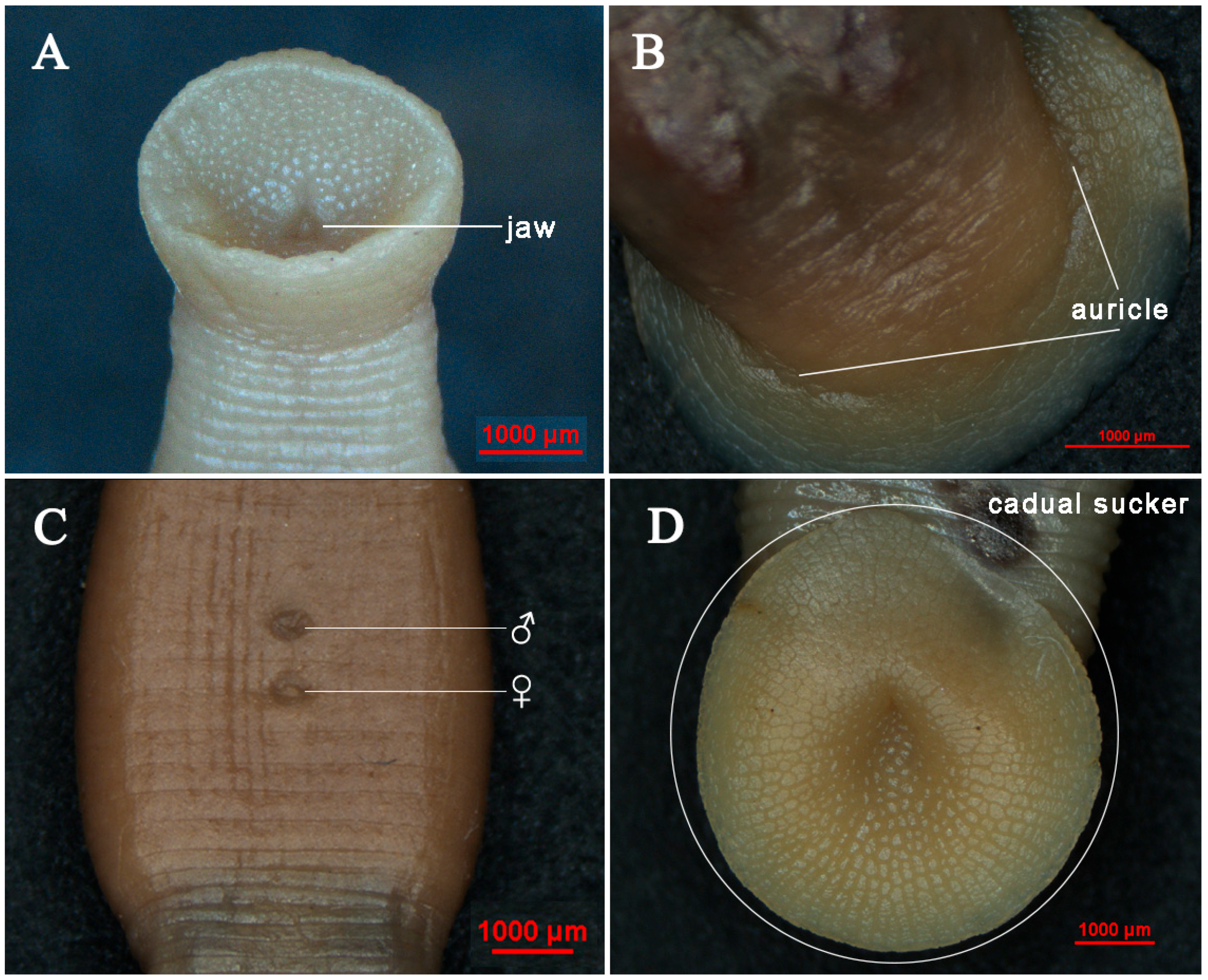
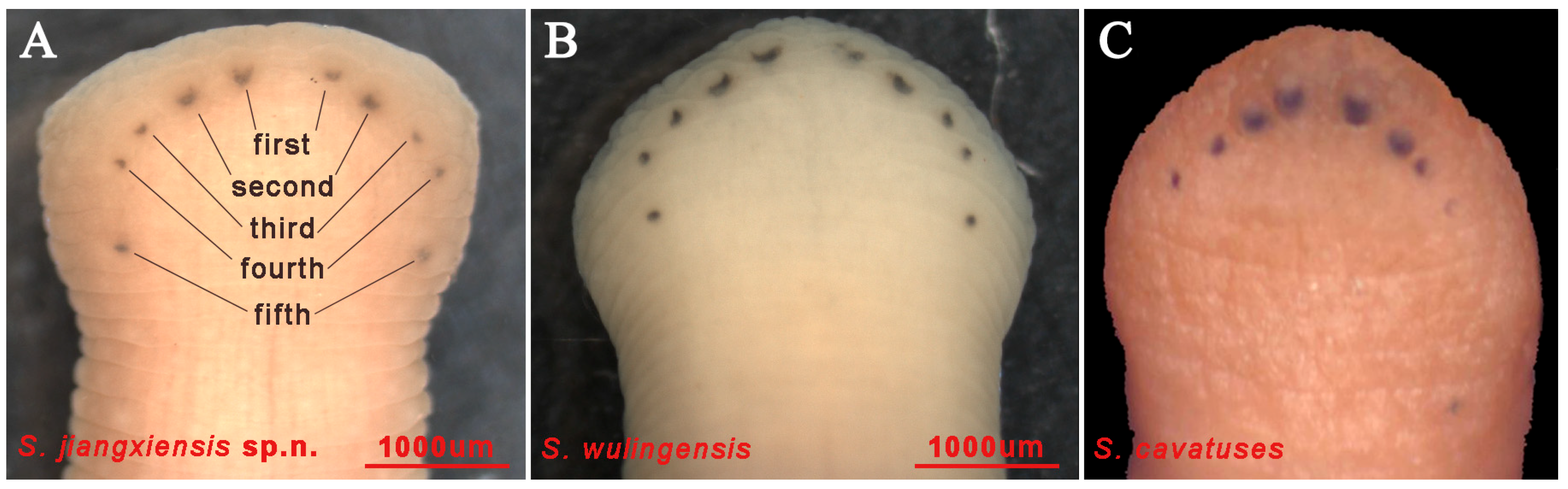
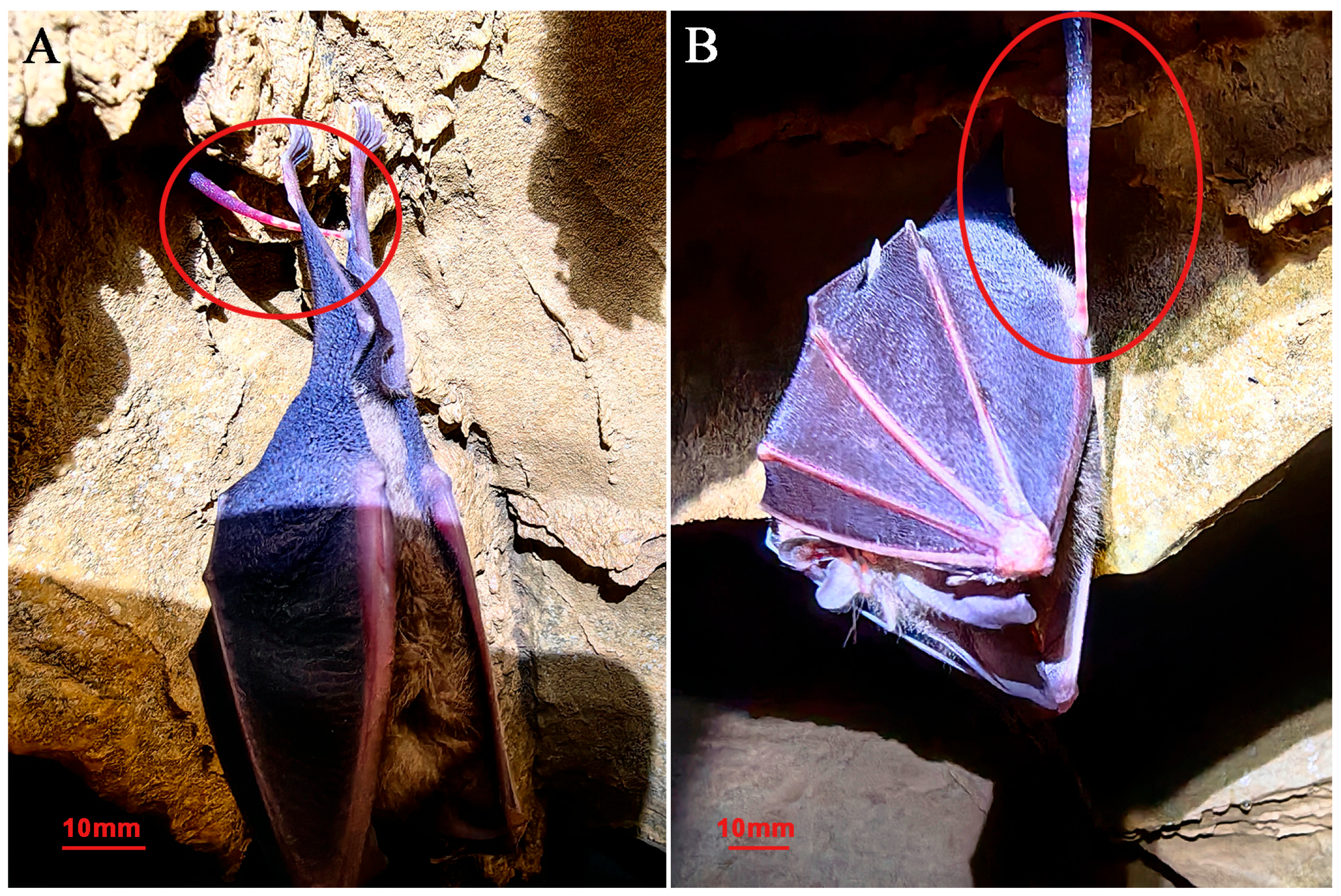
3.1. Taxonomy
3.2. Phylogenetic Analysis and Genetic Distance Comparison
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borda, E.; Oceguera-figueroa, A.; Siddall, M.E. On the classification, evolution and biogeography of terrestrial haemadipsoid leeches (Hirudinida: Arhynchobdellida: Hirudiniformes). Mol. Phylogenetics Evol. 2008, 46, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Borda, E.; Siddall, M.E. Insights into the evolutionary history of Indo-Pacific bloodfeeding terrestrial leeches (Hirudinida: Arhynchobdellida: Haemadipisdae). Invertebr. Syst. 2010, 24, 456–472. [Google Scholar] [CrossRef]
- Tessler, M.; Barrio, A.; Borda, E. Description of a Soft-Bodied Invertebrate with Microcomputed Tomography and Revision of the Genus Chtonobdella (Hirudinea: Haemadipsidae). Zool. Scr. 2016, 45, 552–565. [Google Scholar] [CrossRef]
- Wu, Q.J.; Lan, X.Y.; Wu, T.; Wu, L.Y.; Liu, Z.X. Wuling Cave Leeches (Sinospelaeobdella wulingensis) Were First Discovered in Guizhou Province, China. Int. J. Ecol. 2023, 12, 94–100. (In Chinese) [Google Scholar] [CrossRef]
- Wu, Q.J.; Xiang, D.Q.; Xiang, H.Y.; Liao, J.P.; Li, J.M.; Liu, Z.X. On the Batblood-sucking Exclusiveness of Sinospelaeodella wulingensis based on Bloodmeal iDNA Technology. Life Sci. Res. 2024, 28, 1–13. (In Chinese) [Google Scholar] [CrossRef]
- Huang, T.F.; Liu, Z.W.; Gong, X.Y.; Wu, T.; Liu, H.; Deng, J.X.; Zhang, Y.X.; Peng, Q.Z.; Zhang, L.B.; Liu, Z.X. Vampire in the darkness: A new genus and species of land leech exclusively bloodsucking cave-dwelling bats from China (Hirudinda: Arhynchobdellida: Haemadipsidae). Zootaxa 2019, 4560, zootaxa.4560.2.2. [Google Scholar] [CrossRef]
- Ren, B.S.; Huang, T.F.; Wu, L.Y.; Yuan, X.Y.; Liu, Z.X. Sinospelaeobdella wulingensis (Hirudinea, Haemadipsidae) Found in Linshui County, Sichuan Province. Sichuan J. Zool. 2020, 39, 679–689. (In Chinese) [Google Scholar] [CrossRef]
- Yang, T.; Mo, X.; Wang, D.B. A new species of cvernous blood-sucking land leech (Hirudinea, Haemadipsidae) in the west of Yunnan Province, China. Zool. Syst. 2009, 34, 125–129. (In Chinese) [Google Scholar]
- Huang, T.F. Census of Cave-Dwelling Bats and a New Genus & Species of Batblood-Sucking Landleech. Master’s Thesis, Jishou University, Jishou, China, 2019. (In Chinese). [Google Scholar] [CrossRef]
- Wu, L.Y. Preliminary Study on the Population Dynamics and Behavioral Ecology of Sinospelaeobdella wulingensis. Master’s Thesis, Jishou University, Jishou, China, 2022. (In Chinese). [Google Scholar] [CrossRef]
- Wu, Q.J. Genetic Diversity of Sinospelaeobdella wulingensis and Its Host Species Identification of Blood Meal-iDNA. Master’s Thesis, Jishou University, Jishou, China, 2024. (In Chinese). [Google Scholar] [CrossRef]
- Folmer, O.; Back, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [PubMed]
- Nakano, T.; Lai, Y.T. First record of Poecilobdella nanjingensis (Hirudinida: Arhynchobdellida: Hirudinidae) from Taiwan and its molecular phylogenetic position within the family. Species Divers. 2016, 21, 127–134. [Google Scholar] [CrossRef]
- Kappes, H. Genetics and morphology of the genus Tritetrabdella (Hirudinea, Haemadipsidae) from the mountainous rain forests of Sabah, Borneo, reveal a new species with two new subspecies. Contrib. Zool. Bijdr. Tot De Dierkd. 2013, 82, 185–197. [Google Scholar] [CrossRef]
- Chatterjee, N.; Dhar, B.; Bhattarcharya, D.; Deori, S.; Doley, J.; Bam, J.; Das, J.; Bera, K.; Deb, M.; Devi, N. Genetic assessment of leech species from yak (Bos grunniens) in the tract of Northeast India. Mitochondrial Dna Part A Dna Mapp. Seq. Anal. 2018, 29, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Hu, Y.; Xia, J.; Zhang, K.; Ma, L.; Xu, Z.; Ma, J. Morphological and Phylogenetic Analyses Reveal Three New Species of Didymella (Didymellaceae, Pleosporales) from Jiangxi, China. J. Fungi 2024, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Ewers, W.H. Trypanosoma aunawa sp. n. from an insectivorous bat, Miniopterus tristris, in New Guinea, which may be transmitted by a leech. J. Parasitol. 1974, 60, 172–178. [Google Scholar] [PubMed]
- Calisher, C.H.; Childs, J.E.; Holmes, K.V.; Schountz, T. Bats: Important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006, 19, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Feng, Y.; Chen, X.; Shi, M.; Fu, S.H.; Yang, W.H.; Liu, W.J.; Gao, G.F.; Liang, G.D. Virome of Bat-Infesting Arthropods: Highly Divergent Viruses in Different Vectors. J. Virol. 2022, 96, e0146421. [Google Scholar] [CrossRef]
- Szubert-Kruszynska, A.; Stanczak, J.; Cieniuch, S.; Podsiadly, E.; Postawa, T.; Michalik, J. Bartonella and Rickettsia infections in haematophagous Spinturnix myoti mites (Acari: Mesostigmata) and their bat host, Myotis myotis (Yangochiroptera: Vespertilionidae), from Poland. Microbialecology 2019, 77, 759–768. [Google Scholar] [CrossRef]
- Tendu, A.; Hughes, A.C.; Berthet, N.; Wong, G. Viral Hyperparasitism in Bat Ectoparasites: Implications for Pathogen Maintenance and Transmission. Microorganisms 2022, 10, 1230. [Google Scholar] [CrossRef] [PubMed]


| Species | Sample No. | Location | GenBank Acc | Reference |
|---|---|---|---|---|
| Sinospelaeobdella | ||||
| S. jiangxiensis sp. n. | JXCS6 | Jiangxi, China | PV383319 | This study |
| S. jiangxiensis sp. n. | JXCS7 | Jiangxi, China | PV383320 | This study |
| S. wulingensis | HNSND | Hunan, China | MG195986 | Huang et al. 2019 [6] |
| S. wulingensis | HNTLD1 | Hunan, China | MG195990 | Huang et al. 2019 [6] |
| S. wulingensis | HNJZPD | Hunan, China | MG195991 | Huang et al. 2019 [6] |
| S. wulingensis | HNTLD2 | Hunan, China | MG195992 | Huang et al. 2019 [6] |
| S. cavatuses | HABL | Luang Namtha, Laos | HQ203168 | Borda et al. 2010 [2] |
| S. cavatuses | YNLD1 | Yunnan, China | MG195987 | Huang et al. 2019 [6] |
| S. cavatuses | YNSD | Yunnan, China | MG195993 | Huang et al. 2019 [6] |
| Chtonobdella | ||||
| C. tanae | AU79 | Australia | HQ203164 | Borda et al. 2010 [2] |
| C. cf. grandis | EB-2010 | Australia (Tasmania) | HQ203190 | Borda et al. 2010 [2] |
| C. bilineata | Australia (New South Wales) | AF003267 | Siddall 1998 | |
| Tritetrabdella | ||||
| T. taiwana | TICH | Guanxi, China | HQ203195 | Borda et al. 2010 [2] |
| T. scandens | TI49 | Thailand | HQ203194 | Borda et al. 2010 [2] |
| T. longiducta | VNMN04733 | Vietnam | LC099537 | Nakano et al. 2016 [13] |
| T. kinabaluensi inobongensis | SP13380 | Malaysia (Sabah) | KF839944 | Kappes 2013 [14] |
| Haemadipsa | ||||
| H. japonica | HAJA | Japan | HQ203171 | Borda et al. 2010 [2] |
| H. zeylanica subagilis | HAN04 | Thailand | HQ203173 | Borda et al. 2010 [2] |
| H. montana | HZKI | Nepal | HQ203182 | Borda et al. 2010 [2] |
| Outgroup | ||||
| Poecilobdella nanjingensis | Taiwan, China | LC145741 | Nakano et al. 2016 [13] |
| Items | S. cavatuses [8] n = 13 | S. wulingensis [4,6,7] n = 25 | S. jiangxiensis sp. n. n = 5 |
|---|---|---|---|
| Body length (mm) | 31~44 | 15~28 | 22.66~28.89 |
| Maximum body breadth (mm) | 6.0~8.0 | 3.5~5.4 | 3.75~5.67 |
| Anterior sucker breadth (mm) | 2.6~3.0 | 1.8~2.8 | 2.25~3.42 |
| Posterior sucker diameter (mm) | 6.0~7.5 | 4.1~6.7 | 3.91~5.95 |
| Jaws | 3 | 3 | 3 |
| Complete somites | 5 annuli | 5 annuli | 5 annuli |
| Largest eyes | first pair | second pair | second pair |
| Fifth pair eyes | small or absent | present | present |
| The dorsum papillae | large and obvious | small | large and obvious |
| Caeca pairs | 10 | 14 | 16 |
| Testisac pairs | 10 | 10 | 10 |
| Friction rays | 78 | 78 | 79~90 |
| Genbank no. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| S. jiangxiensis | PV383319 | |||||||||
| PV383320 | 0.0030 | |||||||||
| S. wulingensis | MG195991 | 0.0893 | 0.0892 | |||||||
| MG195990 | 0.0943 | 0.0943 | 0.0162 | |||||||
| MG195992 | 0.0893 | 0.0892 | 0.0000 | 0.0162 | ||||||
| MG195986 | 0.0943 | 0.0943 | 0.0177 | 0.0015 | 0.0177 | |||||
| MG195994 | 0.0943 | 0.0943 | 0.0177 | 0.0015 | 0.0177 | 0.0000 | ||||
| S. cavatuses | MG195987 | 0.1094 | 0.1094 | 0.1080 | 0.1063 | 0.1080 | 0.1046 | 0.1046 | ||
| HQ203168 | 0.1094 | 0.1094 | 0.1097 | 0.1080 | 0.1097 | 0.1097 | 0.1097 | 0.0404 | ||
| MG195993 | 0.1111 | 0.1111 | 0.1063 | 0.1046 | 0.1063 | 0.1063 | 0.1063 | 0.0162 | 0.0389 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Liu, Y.; Zhang, C.; Gu, H.; Cheng, Z.; Peng, J.; Feng, J.; Liu, Y. A New Species of Sinospelaeobdella from China: Sinospelaeobdella jiangxiensis sp. n. (Hirudinda, Arhynchobdellida, Haemadipsidae). Animals 2025, 15, 1079. https://doi.org/10.3390/ani15081079
Li T, Liu Y, Zhang C, Gu H, Cheng Z, Peng J, Feng J, Liu Y. A New Species of Sinospelaeobdella from China: Sinospelaeobdella jiangxiensis sp. n. (Hirudinda, Arhynchobdellida, Haemadipsidae). Animals. 2025; 15(8):1079. https://doi.org/10.3390/ani15081079
Chicago/Turabian StyleLi, Tianyi, Yuhang Liu, Chen Zhang, Hao Gu, Zheng Cheng, Jie Peng, Jiang Feng, and Ying Liu. 2025. "A New Species of Sinospelaeobdella from China: Sinospelaeobdella jiangxiensis sp. n. (Hirudinda, Arhynchobdellida, Haemadipsidae)" Animals 15, no. 8: 1079. https://doi.org/10.3390/ani15081079
APA StyleLi, T., Liu, Y., Zhang, C., Gu, H., Cheng, Z., Peng, J., Feng, J., & Liu, Y. (2025). A New Species of Sinospelaeobdella from China: Sinospelaeobdella jiangxiensis sp. n. (Hirudinda, Arhynchobdellida, Haemadipsidae). Animals, 15(8), 1079. https://doi.org/10.3390/ani15081079






