Use of Caffeine and Inducing Parturition with Dual Administration of Prostaglandin F2α in Gilts and Its Effect on Neonatal Vitality and Performance at Birth
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Installations and Environment
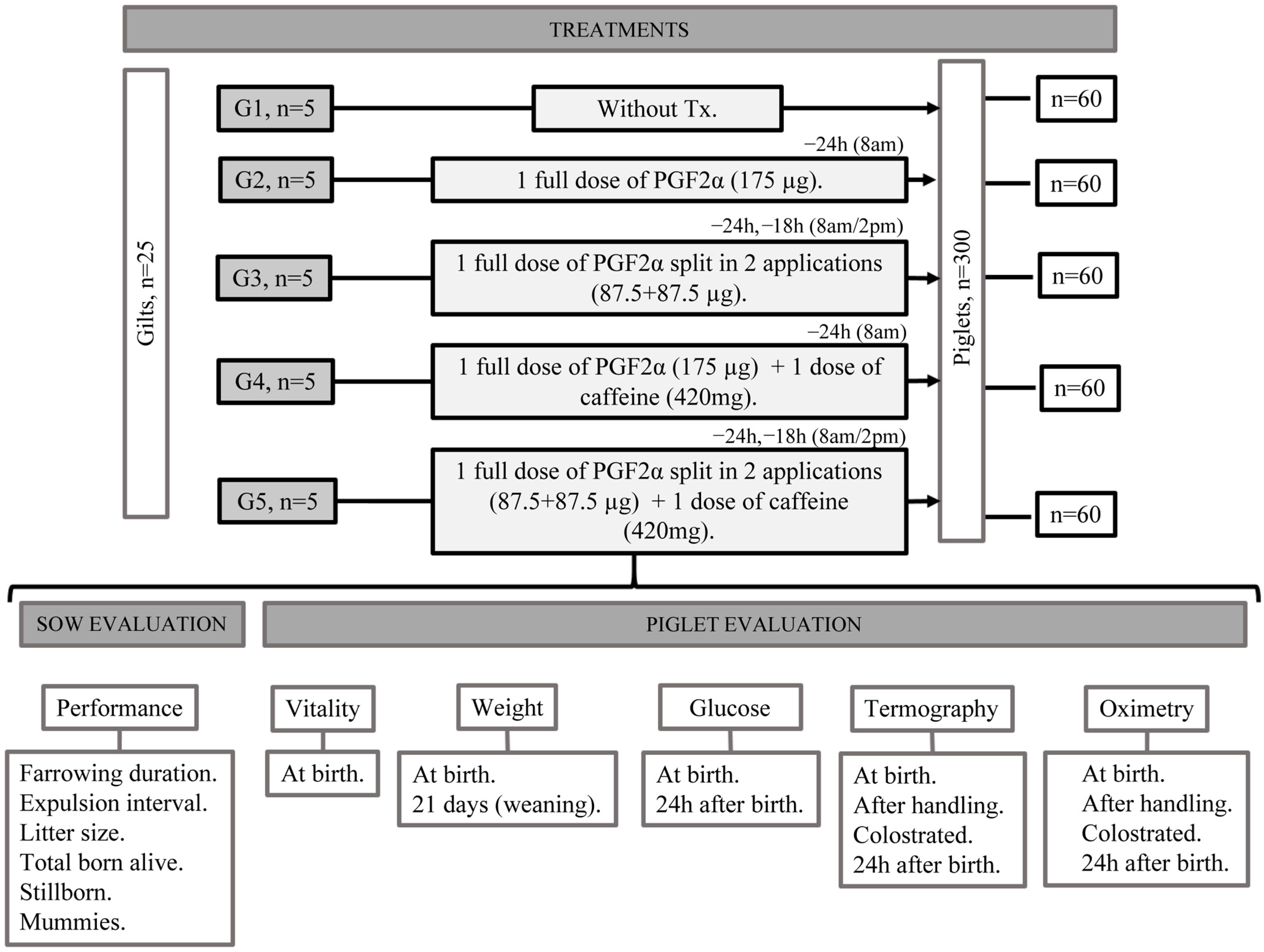
2.2. Animals and Treatments
2.3. Sow Performance
2.4. Neonate Vitality
2.5. Blood Sampling
2.6. Thermography
2.7. Oximetry
2.8. Weight
2.9. Analysis Statistical
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PGF2α | Prostaglandin F2alpha |
| FD | Full dose |
| SD | Split dose |
References
- Edwards, S.A.; Baxter, E.M.; Farmer, C. The gestating and lactating sow. In Piglet Mortality: Causes and Prevention; Wageningen Academic: Wageningen, The Netherlands, 2015; pp. 253–278. [Google Scholar]
- Menegat, M.; Tokach, M.D. 263 Review of Current Nutrition Knowledge and Practices for Gilt Development. J. Anim. Sci. 2021, 99, 105–106. [Google Scholar] [CrossRef]
- Redmer, D.; Wallace, J.; Reynolds, L. Effect of nutrient intake during pregnancy on fetal and placental growth and vascular development. Domest. Anim. Endocrinol. 2004, 27, 199–217. [Google Scholar] [CrossRef]
- Whittemore, C. Nutrition reproduction interactions in primiparous sows. Livest. Prod. Sci. 1996, 46, 65–83. [Google Scholar] [CrossRef]
- Malavé, T.; Alfaro, M.; Hurtado, E. Efecto del número de partos, tamaño y peso de la camada al destete sobre el intervalo destete-estro en cerdas. Rev. Unellez De Cienc. Y Tecnol. 2007, 25, 10–15. [Google Scholar]
- Leite, C.; Lui, J.; Albuquerque, L.G.d.; Alves, D. Environmental and genetic factors affecting the weaning-estrus interval in sows. Genet. Mol. Res. 2011, 10, 2692–2701. [Google Scholar] [CrossRef] [PubMed]
- Ruediger, K.; Schulze, M. Post-farrowing stress management in sows by administration of azaperone: Effects on piglets performance. J. Anim. Sci. 2012, 90, 2331–2336. [Google Scholar] [CrossRef]
- Roldán-Santiago, P.; Mota-Rojas, D.; Martínez-Burnes, J.; Velarde, A.; Muns, R.; López-Mayagoitia, A. Neurophysiological development of newborn pigs: Effect of the sow’s parity number in eutocic farrowings. Anim. Prod. Sci. 2019, 59, 216–224. [Google Scholar] [CrossRef]
- Schild, S.-L.A.; Foldager, L.; Rangstrup-Christensen, L.; Pedersen, L.J. Characteristics of Piglets Born by Two Highly Prolific Sow Hybrids. Front. Vet. Sci. 2020, 7, 355. [Google Scholar] [CrossRef]
- Roldán-Santiago, P.; Martínez-Burnes, J.; López-Mayagoitia, A.; Ramírez-Necoechea, R.; Mota-Rojas, D. Relationship of vitality and weight with the temperature of newborn piglets born to sows of different parity. Livest. Sci. 2019, 220, 26–31. [Google Scholar] [CrossRef]
- Tospitakkul, P.; Kraomkaew, K.; Thammasin, K.; Uttarak, P.; Nuntapaitoon, M.; De Rensis, F.; Tummaruk, P. Induction of parturition by double administration of prostaglandin F2alpha in sows reduces the variation of gestation length without affecting the colostrum yield and piglet performance. J. Vet. Med. Sci. 2019, 81, 1334–1340. [Google Scholar] [CrossRef]
- Zotti, E.; Resmini, F.A.; Schutz, L.G.; Volz, N.; Milani, R.P.; Bridi, A.M.; Alfieri, A.A.; Silva, C.A.d. Impact of piglet birthweight and sow parity on mortality rates, growth performance, and carcass traits in pigs. Rev. Bras. Zootec. 2017, 46, 856–862. [Google Scholar]
- Will, K.J.; Magoga, J.; De Conti, E.R.; da Rosa Ulguim, R.; Mellagi, A.P.G.; Bortolozzo, F.P. Relationship between dexamethasone treatment around parturition of primiparous sows and farrowing performance and newborn piglet traits. Theriogenology 2023, 198, 256–263. [Google Scholar] [CrossRef]
- Decaluwe, R.; Janssens, G.; Declerck, I.; de Kruif, A.; Maes, D. Induction of parturition in the sow. Vlaams Diergeneeskd. Tijdschr. 2012, 81, 158–165. [Google Scholar] [CrossRef]
- Nowland, T.L.; Kind, K.; Hebart, M.L.; van Wettere, W.H.E.J. Caffeine supplementation at birth, but not 8 to 12 h post-birth, increased 24 h pre-weaning mortality in piglets. Animal 2019, 14, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Gregorio, H.; Bonilla-Jaime, H.; Mota-Rojas, D.; Trujillo-Ortega, M.E.; Roldan-Santiago, P.; Martínez-Rodríguez, R.; Borderas-Tordesillas, F.; Flores-Peinado, S.; Mora-Medina, P.; Ramírez-Necoechea, R. Effects of subcutaneous administration of caffeine on the physiometabolic profile of low-birthweight neonate piglets. Anim. Prod. Sci. 2012, 52, 981–990. [Google Scholar] [CrossRef]
- Superchi, P.; Mazzoni, C.; Zanardelli, P.; Piancastelli, C.; Zambini, E.M.; Beretti, V.; Sabbioni, A. Effects of oral caffeine administration to sows with induced parturition on hypoxia in piglets. Livest. Sci. 2013, 157, 372–377. [Google Scholar] [CrossRef]
- Dearlove, B.A.; Kind, K.L.; Gatford, K.L.; van Wettere, W.H.E.J. Oral caffeine administered during late gestation increases gestation length and piglet temperature in naturally farrowing sows. Anim. Reprod. Sci. 2018, 198, 160–166. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, J.A.; Orozco-Gregorio, H.; González-Lozano, M.; Roldán-Santiago, P.; González-Hernández, M.; Ballesteros-Rodea, G.; Bonilla-Jaime, H. Caffeine administered to pregnant sows improves piglet vitality, gas exchange and body weight gain. Anim. Reprod. Sci. 2019, 208, 106120. [Google Scholar] [CrossRef]
- Superchi, P.; Saleri, R.; Farina, E.; Cavalli, V.; Riccardi, E.; Sabbioni, A. Effects of oral administration of caffeine on some physiological parameters and maternal behaviour of sows at farrowing. Res. Vet. Sci. 2016, 105, 121–123. [Google Scholar] [CrossRef]
- Aranda, J.V.; Beharry, K.D. Pharmacokinetics, pharmacodynamics and metabolism of caffeine in newborns. Semin. Fetal Neonatal Med. 2020, 25, 101183. [Google Scholar] [CrossRef]
- Council, N.R. Nutrient Requirements of Swine: 10th Revised Edition; The National Academies Press: Washington, DC, USA, 1998; p. 212. [Google Scholar]
- Orozco-Gregorio, H.; Mota-Rojas, D.; Bonilla-Jaime, H.; Trujillo-Ortega, M.E.; Becerril-Herrera, M.; Hernández-González, R.; Villanueva-García, D. Effects of administration of caffeine on metabolic variables in neonatal pigs with peripartum asphyxia. Am. J. Vet. Res. 2010, 71, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Westgate, J.; Garibaldi, J.M.; Greene, K.R. Umbilical cord blood gas analysis at delivery: A time for quality data. BJOG Int. J. Obstet. Gynaecol. 1994, 101, 1054–1063. [Google Scholar] [CrossRef]
- Nuntapaitoon, M.; Tummaruk, P. Neonatal piglet survival associated with blood glucose concentration. Thai J. Vet. Med. 2014, 44, 159–160. [Google Scholar]
- Martínez-Miró, S.; Tecles, F.; Ramón, M.; Escribano, D.; Hernández, F.; Madrid, J.; Orengo, J.; Martínez-Subiela, S.; Manteca, X.; Cerón, J.J. Causes, consequences and biomarkers of stress in swine: An update. BMC Vet. Res. 2016, 12, 171. [Google Scholar] [CrossRef]
- Pulido-Rodriguez, L.F.; Titto, E.; Henrique, F.L.; Longo, A.; Hooper, H.B.; Pereira, T.L.; Pereira, A.; Titto, C.G. Infrared thermography of the ocular surface as stress indicator for piglets postweaning. Pesqui. Veterinária Bras. 2017, 37, 453–458. [Google Scholar] [CrossRef][Green Version]
- Schmitt, O.; O’Driscoll, K. Use of infrared thermography to noninvasively assess neonatal piglet temperature. Transl. Anim. Sci. 2020, 5, txaa208. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.A.; Mendler, M.; Maurer, M.; Waitz, M.; Huang, L.; Hummler, H.D. Reliability of Pulse Oximetry during Cardiopulmonary Resuscitation in a Piglet Model of Neonatal Cardiac Arrest. Neonatology 2014, 107, 113–119. [Google Scholar] [CrossRef]
- Vongsariyavanich, S.; Sundaraketu, P.; Sakulsirajit, R.; Suriyapornchaikul, C.; Therarachatamongkol, S.; Boonraungrod, N.; Pearodwong, P.; Tummaruk, P. Effect of carbetocin administration during the mid-period of parturition on farrowing duration, newborn piglet characteristics, colostrum yield and milk yield in hyperprolific sows. Theriogenology 2021, 172, 150–159. [Google Scholar] [CrossRef]
- Ward, S.A.; Kirkwood, R.N.; Plush, K.J. Are Larger Litters a Concern for Piglet Survival or An Effectively Manageable Trait? Animals 2020, 10, 309. [Google Scholar] [CrossRef]
- Kobek-Kjeldager, C.; Larsen, M.L.V.; Pedersen, L.J. Changes in piglet and litter characteristics across parities in two highly prolific sow hybrids in an outdoor organic herd. Anim. Sci. J. 2023, 94, e13840. [Google Scholar] [CrossRef]
- Bortolozzo, F.P.; Zanin, G.P.; Ulguim, R.d.R.; Mellagi, A.P.G. Managing Reproduction in Hyperprolific Sow Herds. Animals 2023, 13, 1842. [Google Scholar] [CrossRef]
- Oliviero, C.; Heinonen, M.; Valros, A.; Peltoniemi, O. Environmental and sow-related factors affecting the duration of farrowing. Anim. Reprod. Sci. 2010, 119, 85–91. [Google Scholar] [CrossRef]
- Jarratt, L.; James, S.E.; Kirkwood, R.N.; Nowland, T.L. Effects of Caffeine and Glucose Supplementation at Birth on Piglet Pre-Weaning Growth, Thermoregulation, and Survival. Animals 2023, 13, 435. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Spilsbury, M.a.; Mota-Rojas, D.; Martínez-Burnes, J.; Arch, E.; Mayagoitia, A.L.; Ramírez-Necoechea, R.; Olmos, A.; Trujillo, M.a.E. Use of oxytocin in penned sows and its effect on fetal intra-partum asphyxia. Anim. Reprod. Sci. 2004, 84, 157–167. [Google Scholar]
- Ngo, C.; Boonprakob, R.; Tummaruk, P. Factors contributing to stillbirth in young hyper-prolific sows in a tropical free-farrowing system. Reprod. Domest. Anim. 2024, 59, e14693. [Google Scholar] [CrossRef] [PubMed]
- Tilley, S.L. Methylxanthines in asthma. In Methylxanthines; Fredholm, B.B., Ed.; Springer: Heidelberg, Germany, 2011; pp. 439–456. [Google Scholar]
- Mathew, O.P. Apnea of prematurity: Pathogenesis and management strategies. J. Perinatol. 2011, 31, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Mannan, M.; Afroze, S. Caffeine Administration in Preterm Neonates: A Review. JMRKSH 2022, 3, 64–67. [Google Scholar]
- Walther, F.J.; Erickson, R.; Sims, M.E. Cardiovascular Effects of Caffeine Therapy in Preterm Infants. Am. J. Dis. Child. 1990, 144, 1164–1166. [Google Scholar] [CrossRef]
- Panzardi, A.; Bernardi, M.L.; Mellagi, A.P.; Bierhals, T.; Bortolozzo, F.P.; Wentz, I. Newborn piglet traits associated with survival and growth performance until weaning. Prev. Vet. Med. 2013, 110, 206–213. [Google Scholar] [CrossRef]
- Herpin, P.; Le Dividich, J.; Hulin, J.C.; Fillaut, M.; De Marco, F.; Bertin, R. Effects of the level of asphyxia during delivery on viability at birth and early postnatal vitality of newborn pigs. J. Anim. Sci. 1996, 74, 2067–2075. [Google Scholar] [CrossRef]
- Sapolsky, R.M. Endocrinology of the stress-response. In Behavioral Endocrinology, 2nd ed.; MIT Press: Cambridge, MA, USA, 2002; pp. 409–450. [Google Scholar]
- Natarajan, G.; Lulic-Botica, M.; Aranda, J.V. Pharmacology Review: Clinical Pharmacology of Caffeine in the Newborn. NeoReviews 2007, 8, e214–e221. [Google Scholar] [CrossRef]
- Paredes-Flores, M.A.; Rahimi, N.; Mohiuddin, S.S. Biochemistry, glycogenolysis. In StatPearls [Internet]; StatPearls Publishing: Petersburg, FL, USA, 2024. [Google Scholar]
- Keijzers, G.B.; De Galan, B.E.; Tack, C.J.; Smits, P. Caffeine Can Decrease Insulin Sensitivity in Humans. Diabetes Care 2002, 25, 364–369. [Google Scholar] [CrossRef]
- Reddy, V.S.; Shiva, S.; Manikantan, S.; Ramakrishna, S. Pharmacology of caffeine and its effects on the human body. Eur. J. Med. Chem. Rep. 2024, 10, 100138. [Google Scholar] [CrossRef]
- Tuchscherer, M.; Puppe, B.; Tuchscherer, A.; Tiemann, U. Early identification of neonates at risk: Traits of newborn piglets with respect to survival. Theriogenology 2000, 54, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Schoos, A.; Muro, B.B.D.; Carnevale, R.F.; Chantziaras, I.; Biebaut, E.; Janssens, G.P.J.; Maes, D. Relationship between piglets’ survivability and farrowing kinetics in hyper-prolific sows. Porc. Health Manag. 2023, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Halldner, L.; Ådén, U.; Dahlberg, V.; Johansson, B.; Ledent, C.; Fredholm, B.B. The adenosine A1 receptor contributes to the stimulatory, but not the inhibitory effect of caffeine on locomotion: A study in mice lacking adenosine A1 and/or A2A receptors. Neuropharmacology 2004, 46, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Nuntapaitoon, M.; Muns, R.; Theil, P.K.; Tummaruk, P. Factors influencing colostrum consumption by piglets and their relationship with survival and growth in tropical climates. Livest. Sci. 2019, 224, 31–39. [Google Scholar] [CrossRef]
- Theil, P.K.; Lauridsen, C.; Quesnel, H. Neonatal piglet survival: Impact of sow nutrition around parturition on fetal glycogen deposition and production and composition of colostrum and transient milk. Animal 2014, 8, 1021–1030. [Google Scholar] [CrossRef]
- Mazzoni, C.; Sabbioni, A.; Bertini, S.; Menozzi, A.; Piancastelli, C.; Catelli, E.; Zanardelli, P.; Zambini, E.M.; Saleri, R.; Superchi, P. Estudio preliminar de la utilizaciòn de la cafeina en las cerdas en el momento del parto. Suis 2012, 90, 32–35. [Google Scholar]
- Thorens, B. Neuronal regulation of glucagon secretion and gluconeogenesis. J. Diabetes Investig. 2022, 13, 599–607. [Google Scholar] [CrossRef]
- Menozzi, A.; Mazzoni, C.; Serventi, P.; Zanardelli, P.; Bertini, S. Pharmacokinetics of oral caffeine in sows: A pilot study. Large Anim. Rev 2015, 21, 207–210. [Google Scholar]
| Control (n = 5 Gilts) | PGF2α FD (n = 5 Gilts) | PGF2α SD (n = 5 Gilts) | PGF2α FD+ Caffeine (n = 5 Gilts) | PGF2α SD + Caffeine (n = 5 Gilts) | p-Value | |
|---|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | ||
| Farrowing duration (min) | 200.40 ± 30.72 AB | 239.40 ± 30.72 A | 122.40 ± 30.72 B | 237.80 ± 30.72 A | 136.00 ± 30.72 B | 0.0329 * |
| Expulsion interval (min) | 14.02 ± 2.13 B | 19.68 ± 2.89 A | 12.89 ± 1.97 B | 15.57 ± 2.26 AB | 9.65 ± 2.13 B | 0.0204 * |
| Total number of piglets born per litter TB (n) | 12.8 ± 0.7 BC | 14.0 ± 0.5 A | 13.6 ± 0.4 B | 13.4 ± 0.3 B | 13.6 ± 0.2 BC | 0.7470 |
| Number of piglets born alive per litter (n) | 11.8 ± 0.5 A | 12.8 ± 0.6 A | 13.0 ± 0.4 A | 13.0 ± 0.71 A | 13.00 ± 0.9 A | 0.7470 |
| Number of stillborn (n) | 0.89 ± 0.1 A | 0.99 ± 0.19 A | 0.69 ± 0.15 AB | 0.42 ± 0.15 B | 0.49 ± 0.1 B | <0.0001 * |
| Number of mummies per litter (n) | 0.21 ± 0.01 B | 0.25 ± 0.1 A | 0.12 ± 0.4 C | 0 ± 0.0 D | 0.24 ± 0.1 A | <0.0001 * |
| Control | PGF2α FD | PGF2α SD | PGF2α FD + Caffeine | PGF2α SD + Caffeine | p-Value | |
|---|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | ||
| Heart rate (bpm) | 135.10 ± 3.03 B | 142.20 ± 3.06 B | 155.27 ± 2.20 A | 158.24 ± 3.52 A | 164.37 ± 3.39 A | <0.0001 * |
| Latency to first respiration (s) | 5.52 ± 0.33 B | 5.59 ± 0.34 B | 4.51 ± 0.24 B | 5.34 ± 0.38 B | 7.14 ± 0.37 A | <0.0001 * |
| Latency to stand up (s) | 116.78 ± 6.62 AB | 133.42 ± 6.43 A | 124.64 ± 7.41 AB | 98.03 ± 6.47 B | 102.08 ± 7.92 B | 0.0014 * |
| Latency to connecting to the dam’s teat (min) | 23.64 ± 1.30 A | 18.97 ± 1.32 A | 18.76 ± 0.95 A | 15.05 ± 1.48 B | 14.90 ± 1.44 B | <0.0001 * |
| Control | PGF2α FD | PGF2α SD | PGF2α FD + Caffeine | PGF2α SD + Caffeine | p-Value | ||
|---|---|---|---|---|---|---|---|
| Presentation (%) | |||||||
| Cranial | 78.44 * | 77.55 | 81.05 * | 72.98 | 71.42 | <0.0001 * | |
| Caudal | 21.56 | 22.45 * | 18.95 | 27.02 | 28.58 * | <0.0001 * | |
| Meconium staining (%) | |||||||
| Absent | 78 * | 51.86 | 60.64 * | 36.12 | 61.76 | <0.0001 * | |
| Moderate | 20 | 22.22 | 22.34 | 61.11 * | 26.2 | <0.0001 * | |
| Severe | 2 | 25.92 * | 17.02 | 2.77 | 12.04 * | <0.0001 * | |
| Skin color (%) | |||||||
| Pink | 100 | 100 | 100 | 91.66 * | 100 | <0.0001 * | |
| Pale | 0 | 0 | 0 | 8.34 | 0 | NA | |
| Cyanotic | 0 | 0 | 0 | 0 | 0 | NA | |
| Umbilical cord condition (%) | |||||||
| Adhered | 90 * | 75.51 | 88.03 | 87.12 * | 87.34 | <0.0001 * | |
| Rupture | 10 | 24.49 * | 11.97 | 12.88 | 12.66 | <0.0001 * | |
| Variable | Time | Control | PGF2α FD | PGF2α SD | PGF2α FD + Caffeine | PGF2α SD + Caffeine | p-Value |
|---|---|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | |||
| SpO2 (%) | At birth | 84.74 ± 1.32 A | 80.85 ± 1.37 A | 82.31 ± 0.97 A | 74.72 ± 1.54 B | 85.02 ± 1.50 A | <0.0001 * |
| After handling | 82.28 ± 1.54 A | 79.95 ± 1.64 A | 79.36 ± 1.14 A | 83.00 ± 1.81 A | 84.66 ± 1.74 A | 0.077 | |
| Colostrated | 89.36 ± 0.94 A | 85.93 ± 0.97 AB | 85.45 ± 0.71 B | 87.54 ± 1.12 AB | 89.87 ± 1.06 A | 0.0009 * | |
| 24 h after birth | 84.76 ± 0.89 C | 88.34 ± 0.92 AB | 86.58 ± 0.66 BC | 91.10 ± 1.04 A | 91.87 ± 1.01 A | <0.0001 * | |
| Glucose (mg/dL) | At birth | 52.51 ± 1.66 A | 58.85 ± 1.79 A | 42.98 ± 1.29 B | 53.07 ± 1.86 A | 42.55 ± 1.94 B | <0.0001 * |
| 24 h after birth | 99.24 ± 2.43 B | 113.62 ± 2.71 A | 107.76 ± 1.91 A | 111.56 ± 2.75 A | 110.84 ± 2.78 A | 0.0007 * |
| Control | PGF2α FD | PGF2α SD | PGF2α FD + Caffeine | PGF2α SD + Caffeine | p-Value | |
|---|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | ||
| At birth | 34.84 ± 0.24 B | 36.18 ± 0.22 A | 34.44 ± 0.20 B | 34.21 ± 0.32 B | 34.40 ± 0.31 B | <0.0001 * |
| After handling | 31.61 ± 0.25 B | 34.03 ± 0.28 A | 29.37 ± 0.21 D | 30.74 ± 0.32 BC | 30.21 ± 0.31 CD | <0.0001 * |
| Colostrated | 32.95 ± 0.35 AB | 34.27 ± 0.42 A | 30.58 ± 0.29 C | 31.55 ± 0.51 C | 30.97 ± 0.42 C | <0.0001 * |
| 24 h after birth | 34.69 ± 0.18 CD | 36.27 ± 0.21 A | 34.19 ± 0.16 D | 35.15 ± 0.28 BC | 35.60 ± 0.23 B | <0.0001 * |
| Control | PGF2α FD | PGF2α SD | PGF2α FD + Caffeine | PGF2α SD + Caffeine | p-Value | |
|---|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | ||
| At birth | 34.73 ± 0.29 B | 36.39 ± 0.32 A | 34.44 ± 0.25 B | 34.46 ± 0.40 B | 34.11 ± 0.37 B | <0.0001 * |
| After handling | 31.23 ± 0.29 B | 33.40 ± 0.33 A | 29.16 ± 0.25 C | 29.26 ± 0.39 C | 28.55 ± 0.38 C | <0.0001 * |
| Colostrated | 33.47 ± 0.37 A | 34.56 ± 0.44 A | 30.85 ± 0.30 B | 31.51 ± 0.54 B | 30.87 ± 0.45 B | <0.0001 * |
| 24 h after birth | 36.38 ± 0.17 B | 36.80 ± 0.20 AB | 36.28 ± 0.15 B | 36.61 ± 0.27 AB | 37.20 ± 0.22 A | 0.0115 * |
| Control | PGF2α FD | PGF2α SD | PGF2α FD + Caffeine | PGF2α SD + Caffeine | p-Value | |
|---|---|---|---|---|---|---|
| Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | Mean ± SE | ||
| Weight 0 d | 1318.00 ± 36.41 B | 1381.63 ± 36.78 AB | 1315.46 ± 26.14 B | 1491.03 ± 41.23 A | 1406.10 ± 40.21 AB | 0.0038 * |
| Weight 21 d | 6133.67 ± 87.81 BC | 5929.38 ± 88.72 C | 6120.37 ± 68.30 BC | 6357.89 ± 99.71 AB | 6591.07 ± 116.16 A | <0.0001 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrales-Hernández, A.A.; Bonilla-Jaime, H.; Orozco-Gregorio, H.O.; Limón-Morales, O.; de la Cruz-Cruz, L.A.; Chávez-Delgadillo, E.; Roldán-Santiago, P. Use of Caffeine and Inducing Parturition with Dual Administration of Prostaglandin F2α in Gilts and Its Effect on Neonatal Vitality and Performance at Birth. Animals 2025, 15, 984. https://doi.org/10.3390/ani15070984
Corrales-Hernández AA, Bonilla-Jaime H, Orozco-Gregorio HO, Limón-Morales O, de la Cruz-Cruz LA, Chávez-Delgadillo E, Roldán-Santiago P. Use of Caffeine and Inducing Parturition with Dual Administration of Prostaglandin F2α in Gilts and Its Effect on Neonatal Vitality and Performance at Birth. Animals. 2025; 15(7):984. https://doi.org/10.3390/ani15070984
Chicago/Turabian StyleCorrales-Hernández, Adrián Alejandro, Herlinda Bonilla-Jaime, Héctor O. Orozco-Gregorio, Ofelia Limón-Morales, Luis Alberto de la Cruz-Cruz, Elías Chávez-Delgadillo, and Patricia Roldán-Santiago. 2025. "Use of Caffeine and Inducing Parturition with Dual Administration of Prostaglandin F2α in Gilts and Its Effect on Neonatal Vitality and Performance at Birth" Animals 15, no. 7: 984. https://doi.org/10.3390/ani15070984
APA StyleCorrales-Hernández, A. A., Bonilla-Jaime, H., Orozco-Gregorio, H. O., Limón-Morales, O., de la Cruz-Cruz, L. A., Chávez-Delgadillo, E., & Roldán-Santiago, P. (2025). Use of Caffeine and Inducing Parturition with Dual Administration of Prostaglandin F2α in Gilts and Its Effect on Neonatal Vitality and Performance at Birth. Animals, 15(7), 984. https://doi.org/10.3390/ani15070984





