Dietary Glyceryl Polyethylene Glycol Ricinoleate as an Additive to Improve Intestinal Health in Post-Weaning Piglets
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Sample Collection and Processing
2.2.1. Plasma
2.2.2. Gastrointestinal Tract
2.3. Statistical Analysis
3. Results
3.1. Plasma
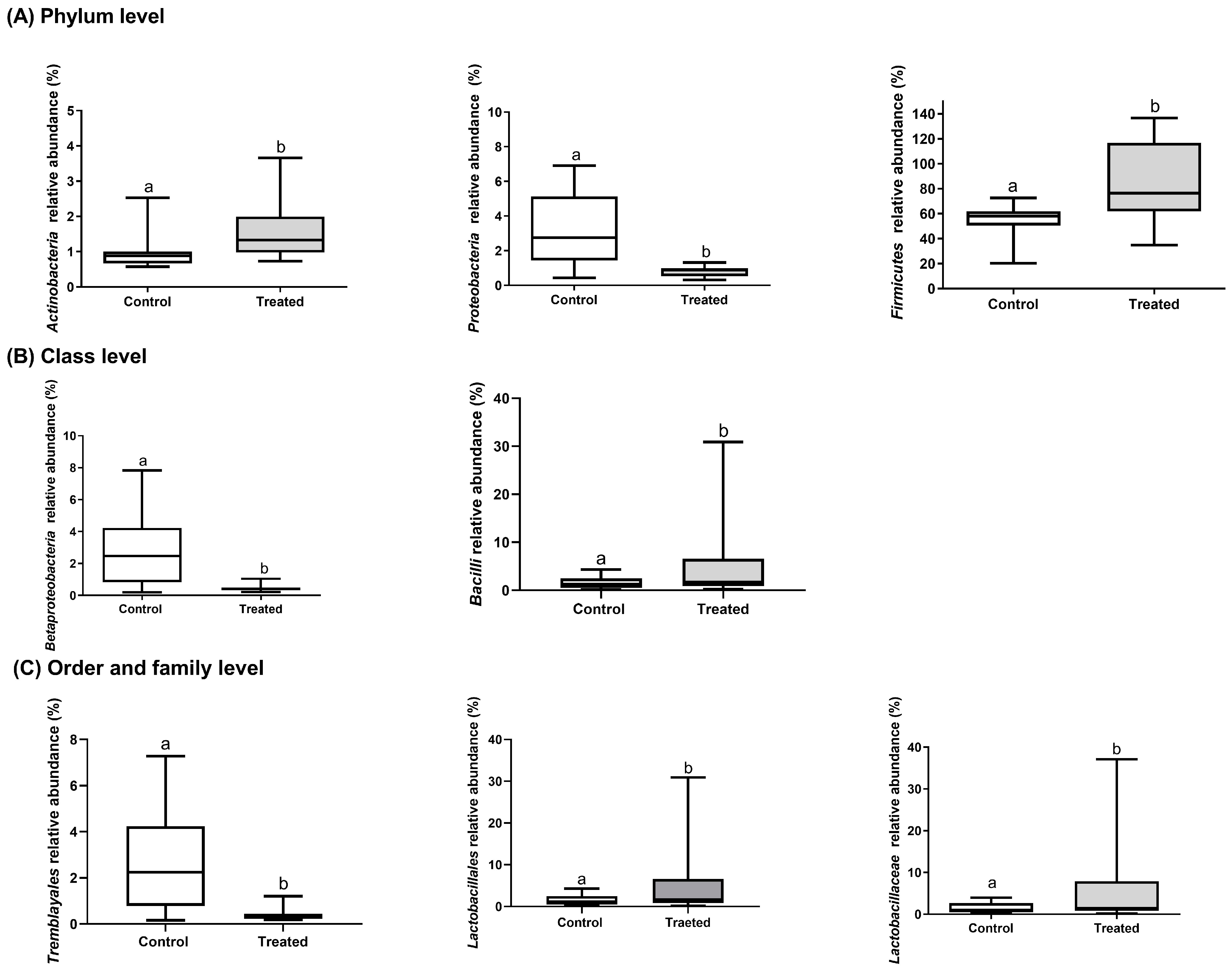
3.2. Gastrointestinal Tract
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar]
- Heo, J.M.; Opapeju, F.O.; Pluske, J.R.; Kim, J.C.; Hampson, D.J.; Nyachoti, C.M. Gastrointestinal health and function in weaned pigs, a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. 2013, 97, 207–237. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Xiong, K.; Fang, R.; Li, M. Weaning stress and intestinal health of piglets: A review. Front. Immunol. 2022, 13, 1042778. [Google Scholar] [CrossRef]
- Hedemann, M.S.; Jensen, B.B. Variations in enzyme activity in stomach and pancreatic tissue and digesta in piglets around weaning. Arch. Anim. Nutr. 2004, 58, 47–59. [Google Scholar] [PubMed]
- Jones, D.B.; Hancock, J.D.; Harmon, D.L.; Walker, C.E. Effects of exogenous emulsifiers and fat sources on nutrient digestibility, serum lipids, and growth performance in weanling pigs. J. Anim. Sci. 1992, 70, 3473–3482. [Google Scholar] [PubMed]
- Lewis, D.S.; Oren, S.; Wang, X.; Moyer, M.L.; Beitz, D.C.; Knight, T.J.; Mott, G.E. Developmental changes in cholesterol 7α-and 27-hydroxylases in the piglet. J. Anim. Sci. 2000, 78, 943–951. [Google Scholar]
- Price, K.L.; Lin, X.; Van Heugten, E.; Odle, R.; Willis, G.; Odle, J. Diet physical form, fatty acid chain length, and emulsification alter fat utilization and growth of newly weaned pigs. J. Anim. Sci. 2013, 91, 783–792. [Google Scholar] [CrossRef]
- He, Y.; Liu, N.; Ji, Y.; Tso, P.; Wu, Z. Weaning stress in piglets alters the expression of intestinal proteins involved in fat absorption. J. Nutr. 2022, 152, 2387–2395. [Google Scholar]
- Bai, G.; He, W.; Yang, Z.; Fu, H.; Qiu, S.; Gao, F.; Shi, B. Effects of different emulsifiers on growth performance, nutrient digestibility, and digestive enzyme activity in weanling pigs. J. Anim. Sci. 2019, 97, 4235–4241. [Google Scholar] [CrossRef]
- Bontempo, V.; Comi, M.; Jiang, X.R. The effects of a novel synthetic emulsifier product on growth performance of chickens for fattening and weaned piglets. Animal 2016, 10, 592–597. [Google Scholar]
- Van Kinh, L.; Vasanthakumari, B.L.; Sugumar, C.; Thanh, H.L.T.; Van Thanh, N.; Wealleans, A.L.; Ngoan, L.D.; Loan, N.V.T.H. Effect of a Combination of Lysolecithin, Synthetic Emulsifier and Monoglycerides on the Apparent Ileal Digestibility, Metabolizable Energy and Growth Performance of Growing Pigs. Animals 2022, 13, 88. [Google Scholar] [CrossRef]
- Song, M.; Zhang, F.; Chen, L.; Yang, Q.; Su, H.; Yang, X.; He, H. Dietary chenodeoxycholic acid improves growth performance and intestinal health by altering serum metabolic profiles and gut bacteria in weaned piglets. Anim. Nutr. 2021, 7, 365–375. [Google Scholar]
- Sun, H.Y.; Kim, I.H. Evaluation of an emulsifier blend on growth performance, nutrient digestibility, blood lipid profiles, and fecal microbial in growing pigs fed low energy density diet. Livest. Sci. 2019, 227, 55–59. [Google Scholar] [CrossRef]
- Udomprasert, P.; Rukkwamsuk, T. Effect of an exogenous emulsifier on growth performance in weanling pigs. Agric. Nat. Resour. 2006, 40, 652–656. [Google Scholar]
- Zhao, P.Y.; Li, H.L.; Hossain, M.M.; Kim, I.H. Effect of emulsifier (lysophospholipids) on growth performance, nutrient digestibility and blood profile in weanling pigs. Anim. Feed. Sci. Technol. 2015, 207, 190–195. [Google Scholar] [CrossRef]
- Ganna, S.; Abdel-Latif, M.; Ahmed, H. Effect of Dietary Supplementation of Some Emulsifiers on Growth Performance, Carcass Traits, Lipid Peroxidation and Some Nutrients Digestibility in Broiler Chickens. Damanhour J. Vet. Sci. 2022, 7, 16–23. [Google Scholar] [CrossRef]
- Michels, D.; Verkempinck, S.H.; Staes, E.; Spaepen, R.; Vermeulen, K.; Wealleans, A.; Grauwet, T. Unravelling the impact of emulsifier blends on interfacial properties and in vitro small intestinal lipolysis of oil-in-water emulsions. Food Hydrocoll. 2023, 141, 108735. [Google Scholar] [CrossRef]
- National Research Council; Division on Earth and Life Studies; Board on Agriculture and Natural Resources; Committee on Nutrient Requirements of Swine. Nutrient Requirements of Swine, 11th ed.; The National Academies Press: Washington, DC, USA, 2012. [Google Scholar]
- Li, L.A.; Yang, J.J.; Li, Y.; Lv, L.; Xie, J.J.; Du, G.M.; Jiao, X.L. Effect of weaning age on cortisol release in piglets. Genet. Mol. Res. 2016, 15, gmr7693. [Google Scholar]
- Martínez-Miró, S.; Tecles, F.; Ramón, M.; Escriban, D.; Hernánde, F.; Madrid, J.; Cerón, J.J. Causes, consequences and biomarkers of stress in swine, an update. BMC Vet. Res. 2016, 12, 171. [Google Scholar]
- Dieguez, S.N.; Decundo, J.M.; Martínez, G.; Amanto, F.A.; Bianchi, C.P.; Pérez Gaudio, D.S.; Soraci, A.L. Effect of dietary oregano (Lippia origanoides) and clover (Eugenia caryophillata) essential oils’ formulations on intestinal health and performance of pigs. Planta Medica 2022, 88, 324–335. [Google Scholar]
- Pluschke, A.M.; Williams, B.A.; Zhang, D.; Anderson, S.T.; Roura, E.; Gidley, M.J. Male grower pigs fed cereal soluble dietary fibers display biphasic glucose response and delayed glycaemic response after an oral glucose tolerance test. PLoS ONE 2018, 13, e0193137. [Google Scholar] [CrossRef] [PubMed]
- Berkeveld, M.; Langendijk, P.; Verheijden, J.H.M.; Taverne, M.A.M.; Van Nes, A.; Van Haard, P.; Koets, A.P. Citrulline and intestinal fatty acid-binding protein, Longitudinal markers of postweaning small intestinal function in pigs? J. Anim. Sci. 2008, 86, 3440–3449. [Google Scholar]
- Crenn, P.; Messing, B.; Cynober, L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin. Nutr. 2008, 27, 328–339. [Google Scholar] [PubMed]
- Soraci, A.L.; Decundo, J.M.; Dieguez, S.N.; Martínez, G.; Pérez Gaudio, D.S.; Amanto, F.A. Citrullinemia is a suitable biomarker for post weaning performance in piglets under intensive farming. J. Am. Vet. Med. Assoc. 2023, 261, 858–864. [Google Scholar]
- Wu, G.; Meininger, C.J. Analysis of citrulline, arginine, and methylarginines using high-performance liquid chromatography. Methods Enzymol. 2008, 440, 177–189. [Google Scholar]
- Ohara, T.E.; Colonna, M.; Stappenbeck, T.S. Adaptive differentiation promotes intestinal villus recovery. Dev. Cell 2022, 57, 166–179. [Google Scholar] [PubMed]
- Kisielinski, K.; Willis, S.; Prescher, A.; Klosterhalfen, B.; Schumpelick, V. A simple new method to calculate small intestine absorptive surface in the rat. Clin. Exp. Med. 2002, 2, 131–135. [Google Scholar]
- Canal, A.M.; Cubillos, V.; Zamora, J.; Reinhardt, G.; Paredes, E.; Ildefonso, R.; Alberdi, A. Lesiones macro y microscópicas de intestino delgado de cerdos neonatos sin calostrar inoculados experimentalmente con cepas de E. coli fimbriadas. Arch. Med. Vet. 1999, 31, 69–79. [Google Scholar]
- Bai, X.; Liu, X.; Su, Y. Inhibitory effects of intestinal mucus on bacterial adherence to cultured intestinal epithelial cells after surface burns. Chin. Med. J. 2000, 113, 449–450. [Google Scholar]
- Pluske, J.R.; Williams, I.H.; Aherne, F.X. Maintenance of villous height and crypt depth in piglets by providing continuous nutrition after weaning. Anim. Sci. 1996, 62, 131–144. [Google Scholar]
- Solaymani-Mohammadi, S.; Singer, S.M. Host immunity and pathogen strain contribute to intestinal disaccharidase impairment following gut infection. J. Immunol. 2011, 187, 3769–3775. [Google Scholar] [CrossRef] [PubMed]
- Dahlqvist, A. Method for Assay of Intestinal Disaccharidases. Anal. Biochem. 1964, 7, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Jouany, J.P. Volatlile fatty acid and alcohol determination in digestive contents, silage juices, bacterial cultures and anaerobic fermentor contents. Sci. Aliment. 1982, 2, 131–144. [Google Scholar]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project, improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, 590–596. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. phyloseq, an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Farrier, D. PK Solutions. Non Compartimental Pharmacokinetics Data Analysis; User Guide; Summit Research Services: Ashland, OH, USA, 1997. [Google Scholar]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Lallès, J.P.; Bosi, P.; Smidt, H.; Stokes, C.R. Weaning—A challenge to gut physiologists. Livest. Sci. 2007, 108, 82–93. [Google Scholar] [CrossRef]
- Saleh, A.A.; Amber, K.A.; Mousa, M.M.; Nada, A.L.; Awad, W.; Dawood, M.A.; Abd El-Moneim, A.E.M.E. A mixture of exogenous emulsifiers increased the acceptance of broilers to low energy diets, Growth performance, blood chemistry, and fatty acids traits. Animals 2020, 10, 437. [Google Scholar] [CrossRef]
- Siyal, F.A.; El-Hack, M.E.; Alagawany, M.; Wang, C.; Wan, X.; He, J.; Wang, M.; Zhang, L.; Zhong, X.; Wang, T.; et al. Effect of soy lecithin on growth performance, nutrient digestibility and hepatic antioxidant parameters of broiler chickens. In CABI Compendium; CAB International: Wallingford, UK, 2017. [Google Scholar]
- Franklin, M.A.; Mathew, A.G.; Vickers, J.R.; Clift, R.A. Characterization of microbial populations and volatile fatty acid concentrations in the jejunum, ileum, and cecum of pigs weaned at 17 vs. 24 days of age. J. Anim. Sci. 2002, 80, 2904–2910. [Google Scholar]
- Li, L.; Wang, H.; Zhang, N.; Zhang, T.; Ma, Y. Effects of α-glycerol monolaurate on intestinal morphology, nutrient digestibility, serum profiles, and gut microbiota in weaned piglets. J. Anim. Sci. 2022, 100, skac046. [Google Scholar]
- Mitchaothai, J.; Yuangklang, C.; Vasupen, K.; Wongsuthavas, S.; Beynen, A.C. Effect of dietary calcium and lecithin on growth performance and small intestinal morphology of young wild pigs. Livest. Sci. 2010, 134, 106–108. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Zhang, Q.; Zheng, T.; Li, Q.; Yang, S.; Shao, J.; Guan, W.; Zhang, S. Nutritional strategies to reduce intestinal cell apoptosis by alleviating oxidative stress. Nutr. Rev. 2024, 83, nuae023. [Google Scholar]
- Rosero, D.S.; Odle, J.; Moeser, A.J.; Boyd, R.D.; van Heugten, E. Peroxidised dietary lipids impair intestinal function and morphology of the small intestine villi of nursery pigs in a dose-dependent manner. Br. J. Nutr. 2015, 114, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Cai, X.; Guo, Q.; Chen, X.; Zhu, S.; Xu, J. Effect of N-acetyl cysteine on enterocyte apoptosis and intracellular signalling pathways’ response to oxidative stress in weaned piglets. Br. J. Nutr. 2013, 110, 1938–1947. [Google Scholar]
- Lock, J.Y.; Carlson, T.L.; Wang, C.M.; Chen, A.; Carrier, R.L. Acute exposure to commonly ingested emulsifiers alters intestinal mucus structure and transport properties. Sci. Rep. 2018, 8, 10008. [Google Scholar]
- Kubiś, M.; Kołodziejski, P.; Pruszyńska-Oszmałek, E.; Sassek, M.; Konieczka, P.; Górka, P.; Flaga, J.; Katarzyńska-Banasik, D.; Hejdysz, M.; Wiśniewska, Z.; et al. Emulsifier and xylanase can modulate the gut microbiota activity of broiler chickens. Animals 2020, 10, 2197. [Google Scholar] [CrossRef]
- Kelly, D.; Smyth, J.A.; McCracken, K.J. Digestive development of the early-weaned pig, 1. Effect of continuous nutrient supply on the development of the digestive tract and on changes in digestive enzyme activity during the first week post-weaning. Br. J. Nutr. 1991, 65, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Blachier, F. Metabolism of dietary substrates by intestinal bacteria and consequences for the host intestine. In Metabolism of Alimentary Compounds by the Intestinal Microbiota and Health; Springer International Publishing: Cham, Switzerland, 2023; pp. 45–144. [Google Scholar]
- Camp Montoro, J.; Solà-Oriol, D.; Muns, R.; Gasa, J.; Llanes, N.; Manzanilla, E.G. Blood and faecal biomarkers to assess dietary energy, protein and amino acid efficiency of utilization by growing and finishing pigs. Porc. Health Manag. 2022, 8, 32. [Google Scholar]
- Ángel-Isaza, J.A.; Herrera Franco, V.; López-Herrera, A.; Parra-Suescun, J.E. Nutraceutical Additives Modulate Microbiota and Gut Health in Post-Weaned Piglets. Vet. Sci. 2024, 11, 332. [Google Scholar] [CrossRef]
- Guevarra, R.B.; Hong, S.H.; Cho, J.H.; Kim, B.R.; Shin, J.; Lee, J.H.; Kang, B.N.; Kim, Y.H.; Wattanaphansak, S.; Isaacson, R.E.; et al. The dynamics of the piglet gut microbiome during the weaning transition in association with health and nutrition. J. Anim. Sci. Biotechnol. 2018, 9, 54. [Google Scholar]
- Wang, L.; Zou, L.; Li, J.; Yang, H.; Yin, Y. Effect of dietary folate level on organ weight, digesta pH, short-chain fatty acid concentration, and intestinal microbiota of weaned piglets. J. Anim. Sci. 2021, 99, skab015. [Google Scholar] [CrossRef] [PubMed]
- Gresse, R.; Chaucheyras Durand, F.; Dunière, L.; Blanquet-Diot, S.; Forano, E. Microbiota composition and functional profiling throughout the gastrointestinal tract of commercial weaning piglets. Microorganisms 2019, 7, 343. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef]
- McCormack, U.M.; Curião, T.; Buzoianu, S.G.; Prieto, M.L.; Ryan, T.; Varley, P.; Crispie, F. Exploring a possible link between the intestinal microbiota and feed efficiency in pigs. Appl. Environ. Microbiol. 2017, 83, e00380-17. [Google Scholar] [CrossRef]
- Mulder, I.E.; Schmidt, B.; Stokes, C.R.; Lewis, M.; Bailey, M.; Aminov, R.I.; Prosser, J.I. Environmentally-acquired bacteria influence microbial diversity and natural innate immune responses at gut surfaces. BMC Biol. 2009, 7, 79. [Google Scholar] [CrossRef]
- Qin, Y.; Roberts, J.D.; Grimm, S.A.; Lih, F.B.; Deterding, L.J.; Li, R.; Chrysovergis, K. An obesity-associated gut microbiome reprograms the intestinal epigenome and leads to altered colonic gene expression. Genome Biol. 2018, 19, 7. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria, microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, A.; Lennon, G.; O’Sullivan, O.; Docherty, N.; Balfe, A.; Maguire, A.; Mulcahy, H.E.; Doherty, G.; O’Donoghue, D.; Hyland, J.; et al. Spatial variation of the colonic microbiota in patients with ulcerative colitis and control volunteers. Gut 2015, 64, 1553–1561. [Google Scholar] [CrossRef]
- Morgan, X.C.; Tickle, T.L.; Sokol, H.; Gevers, D.; Devaney, K.L.; Ward, D.V.; Reyes, J.A.; Shah, S.A.; LeLeiko, N.; Snapper, S.B.; et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012, 13, R79. [Google Scholar] [CrossRef]
| Nutritional Specifications | Feed First Week | Feed Second Week |
|---|---|---|
| Dry Matter (%) | 92.50 | 92.50 |
| Crude protein (%) | 21.85 | 20.56 |
| Fat (%) | 5.80 | 6.53 |
| Starch (%) | 25.80 | 31.29 |
| Crude Fiber (%) | 1.50 | 1.65 |
| Ash (%) | 5.49 | 5.35 |
| Calcium | 0.74 | 0.90 |
| Available phosphorus (%) | 0.57 | 0.48 |
| Metabolizable Energy (kcal) | 3394.41 Kcal | 3410.40 Kcal |
| Net Energy (kcal) | 2436.35 Kcal | 2489.17 Kcal |
| Lactose (%) | 12.60 | 10.54 |
| Digestible lysine (%) | 1.52 | 1.39 |
| Digestible methionine (%) | 0.61 | 0.46 |
| Methionine + cystine (%) | 0.94 | 0.59 |
| Digestible threonine (%) | 0.98 | 0.84 |
| Digestible tryptophan (%) | 0.29 | 0.24 |
| Digestible arginine (%) | 1.28 | 1.27 |
| Digestible valine (%) | 0.98 | 0.92 |
| Digestible isoleucin (%) | 0.82 | - |
| Digestible leucine (%) | 1.61 | - |
| Plasma | Piglets | Day Mean | ||
|---|---|---|---|---|
| Concentrations | Days | Control | GPGR | |
| Cortisol (nmol/L) | ||||
| 0 | 184.30 ± 92.38 | 205.08 ± 132.13 | 201.90 ± 112.63 a | |
| 4 | 208.46 ± 68.04 | 225.64 ± 70.85 | 217.05 ± 69.62 b | |
| 8 | 194.59 ± 70.31 | 179.66 ± 62.74 | 187.13 ± 67.92 ab | |
| 12 | 174.88 ± 51.83 | 168.96 ± 54.92 | 171.91 ± 53.14 a | |
| Treatment mean | 192.26 ± 72.09 | 194.93 ± 89.52 | ||
| AUCcor | 2344.26 ± 526.86 | 2384.59 ± 601.88 | ||
| Citrulline (µmol/L) | ||||
| 0 | 57.69 ± 23.56 | 69.30 ± 28.75 | 63.22 ± 26.65 a | |
| 4 | 33.75 ± 11.44 | 35.56 ± 12.00 | 34.62 ± 11.67 b | |
| 8 | 30.34 ± 10.98 | 33.54 ± 13.88 | 31.88 ± 12.49 b | |
| 12 | 40.31 ± 19.53 | 50.85 ± 20.22 | 45.40 ± 20.44 c | |
| 15 | 37.15 ± 17.15 | 46.57 ± 17.81 | 41.39 ± 17.93 c | |
| Treatment mean | 39.85 ± 19.55 a | 47.23 ± 23.44 b | ||
| AUCcit | 563.82 ± 205.97 a | 661.85 ± 209.97 b | ||
| Piglets | ||||
|---|---|---|---|---|
| Zone | Variables | Control | GPGR | p-Value |
| Jejunum | ||||
| Vh (μm) | 299.54 ± 34.62 a | 379.02 ± 57.95 b | <0.001 | |
| Cd (μm) | 99.52 ± 8.64 | 105.21 ± 15.73 | 0.241 | |
| Vh:Cd | 3.02 ± 0.37 a | 3.64 ± 0.62 b | 0.007 | |
| IAA | 5.83 ± 0.66 a | 6.71 ± 0.62 b | 0.006 | |
| vGC | 834.13 ± 144.59 | 1002.46 ± 343.84 | 0.219 | |
| cGC | 1156.58 ± 373.66 | 968.94 ± 154.09 | 0.122 | |
| Ileum | ||||
| Vh (μm) | 251.72 ± 42.32 a | 300.39 ± 33.21 b | 0.005 | |
| Cd (μm) | 99.10 ± 9.02 | 96.54 ± 6.65 | 0.437 | |
| Vh:Cd | 2.55 ± 0.40 a | 3.12 ± 0.41 b | <0.001 | |
| IAA | 4.86 ± 0.66 a | 5.47 ± 0.74 b | 0.044 | |
| vGC | 1600.83 ± 220.18 | 1482.67 ± 344.49 | 0.088 | |
| cGC | 1022.08 ± 298.00 | 1354.96 ± 509.14 | 0.328 | |
| Adherence (%) | 90.08 ± 6.20 | 93.45 ± 3.70 | 0.325 | |
| Piglets | ||||
|---|---|---|---|---|
| Disaccharidases | Zone | Control | GPGR | p-Value |
| Sucrase (U/mg) | ||||
| Duodenum | 80.01 ± 44.97 a | 158.24 ± 109.82 b | 0.013 | |
| Proximal jejunum | 203.01 ± 137.27 | 248.09 ± 146.75 | 0.478 | |
| Mid jejunum | 1378.66 ± 498.76 | 1220.89 ± 425.25 | 0.413 | |
| Ileum | 485.37 ± 339.47 | 565.00 ± 417.56 | 0.671 | |
| Lactase (U/mg) | ||||
| Duodenum | 489.72 ± 203.06 | 419.46 ± 314.40 | 0.478 | |
| Proximal jejunum | 782.97 ± 283.25 | 667.66 ± 345.99 | 0.178 | |
| Mid jejunum | 2238.15 ± 819.07 | 1825.63 ± 870.00 | 0.244 | |
| Ileum | 119.21 ± 63.65 | 145.46 ± 119.90 | 0.551 | |
| Maltase (U/mg) | ||||
| Duodenum | 572.27 ± 393.39 a | 1351.15 ± 708.17 b | <0.001 | |
| Proximal jejunum | 648,01 ± 283.62 a | 1860.62 ± 958.63 b | <0.001 | |
| Mid jejunum | 1579.36 ± 631.36 a | 3817.85 ± 1328.14 b | <0.001 | |
| Ileum | 1370.43 ± 886.72 a | 2405.74 ± 1007.43 b | 0.014 | |
| Piglets | |||
|---|---|---|---|
| VFA (mmol/L) | Control | GPGR | p-Value |
| Acetic | 60.60 ± 19.38 | 64.18 ± 15.45 | 0.625 |
| Propionic | 21.29 ± 6.32 | 26.08 ± 6.72 | 0.086 |
| Butyric | 7.63 ± 3.55 | 7.50 ± 2.83 | 0.921 |
| Valeric | 1.09 ± 0.59 | 1.26 ± 1.32 | 0.792 |
| Total | 90.62 ± 27.87 | 99.02 ± 22.02 | 0.421 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Decundo, J.M.; Dieguez, S.N.; Martínez, G.; Amanto, F.A.; Maté, M.L.; Lirón, J.P.; Pérez Gaudio, D.S.; Bianchi, C.P.; Montagnon, A.; Soraci, A.L. Dietary Glyceryl Polyethylene Glycol Ricinoleate as an Additive to Improve Intestinal Health in Post-Weaning Piglets. Animals 2025, 15, 983. https://doi.org/10.3390/ani15070983
Decundo JM, Dieguez SN, Martínez G, Amanto FA, Maté ML, Lirón JP, Pérez Gaudio DS, Bianchi CP, Montagnon A, Soraci AL. Dietary Glyceryl Polyethylene Glycol Ricinoleate as an Additive to Improve Intestinal Health in Post-Weaning Piglets. Animals. 2025; 15(7):983. https://doi.org/10.3390/ani15070983
Chicago/Turabian StyleDecundo, Julieta M., Susana N. Dieguez, Guadalupe Martínez, Fabián A. Amanto, María L. Maté, Juan P. Lirón, Denisa S. Pérez Gaudio, Carolina P. Bianchi, Aurélie Montagnon, and Alejandro L. Soraci. 2025. "Dietary Glyceryl Polyethylene Glycol Ricinoleate as an Additive to Improve Intestinal Health in Post-Weaning Piglets" Animals 15, no. 7: 983. https://doi.org/10.3390/ani15070983
APA StyleDecundo, J. M., Dieguez, S. N., Martínez, G., Amanto, F. A., Maté, M. L., Lirón, J. P., Pérez Gaudio, D. S., Bianchi, C. P., Montagnon, A., & Soraci, A. L. (2025). Dietary Glyceryl Polyethylene Glycol Ricinoleate as an Additive to Improve Intestinal Health in Post-Weaning Piglets. Animals, 15(7), 983. https://doi.org/10.3390/ani15070983








