1. Introduction
The global demand for inexpensive and highly palatable protein has driven the tremendous growth of the poultry sector [
1]. For decades, antibiotics were administered to promote growth and to protect chickens against pathogenic microbes [
2]. Antibiotics are banned in the European Union and China because of their unwanted effects, including antibiotic-resistant bacteria, residue contamination of animal products, and ecosystem contamination [
3]. The replacement of synthetic growth enhancers and antibiotics with organic nutritional supplements was reported to improve the birds’ immunity, growth, and carcass quality [
4,
5]. EOs have emerged as a potential substitute for antibiotics in broiler production because of their natural antibacterial and anti-inflammatory characteristics. Thus, utilizing these plant-extracted compounds can enhance performance and gut health, as well as meat quality and oxidative stability, without the risks associated with antibiotic residue and antibiotic-resistant bacteria proliferation [
6].
One of the natural strategies that has been widely used in broiler production is using oregano essential oil (OEO), which has contributed to positive results in several studies [
7]. These studies indicate that OEO acts as a growth promoter, a natural antibiotic, and acts to elevate beneficial bacteria in the digestive tract [
8,
9]. OEO has also been suggested as an efficient mechanism for enhancing broiler meat quality [
10]. Carvacrol and thymol are principal volatile compounds present in OEO, which contribute to the biological activity of the oil. Orange EO contains a plethora of compounds and is composed of 85–99% volatile constituents, which are extracted from the peel of the fruit [
11]. Most of these volatile compounds are terpenoids and their oxygenated derivatives. EOs of orange and thyme were reported to also inhibit lipid oxidation in meat without compromising quality [
12]. Cinnamon spice is achieved from the inner surface of
Cinnamomum verum, a hardy, evergreen plant that is part of the Lauraceae family and has natural aromatic properties. Cinnamon and its EOs and bioactive compounds, including cinnamaldehyde and eugenol, are commonly used in poultry production as dietary additives. Cinnamon supplementation has been found to enhance meat quality and improve its shelf life [
13].
Many researchers have observed the influence of EOs by adding them to broiler feeds and studying their impact on performance and meat quality data [
7,
14]. Another approach is to include EOs in DW to observe how EOs affect broiler performance. The EOB supplementation has been shown to increase the carcass yield, tenderness, and WHC in chickens [
15]. EOs are reported to have anti-oxidative properties on lipids in tissues and serum, meanwhile enhancing meat quality and extending shelf life [
16]. Based on previous studies, EOs in DW have the potential to enhance the growth, health, and meat quality of birds. EO supplementation has demonstrated improvements in productivity and antioxidant capacity in chickens [
17]. Lipid peroxidation (MDA) is a common indication of oxidative stress in poultry [
18], suggesting that EOs may reduce MDA levels, improve health tissues, and thus improve meat quality. Chickens that were administered EOs have also reported decreased TC, TG, and LDL-C [
19], indicating that EOs may contribute to lipid metabolism regulation. Lipid content is closely related to metabolic disorders, like atherosclerosis, coronary heart disease, and fatty liver. Both total lipid intake and the ratio of FAs in the daily diet, when properly managed, can reduce the incidence of cardiovascular disease [
20]. Moreover, lipid oxidation could also influence the quality and flavor of meat during FA production [
21]. As an example, chicken breast meat, which has high levels of unsaturated fatty acids (UFAs), can decrease both the thrombogenicity and atherogenicity indices [
22]. Oregano oil and cinnamon oil have been shown to have antibacterial and growth-promoting effects, and are widely used in poultry production. In addition, limonene has also been shown to improve antioxidant properties and gut health in broilers [
23].
Few studies have been conducted to investigate whether the EOB extract supplementation from oregano leaves, cinnamon, and orange peels in broiler DW affects their meat quality and lipid metabolism. Thus, the purpose of this experiment is to thoroughly assess the influence of the EOB supplements in the DW on growth performance, breast meat quality, oxidative functions, FA profiles, and lipid quality indexes. This study applies valuable insight into broiler production.
4. Discussion
The present study describes the impacts of varying levels of EOB supplementation on growth attributes, carcass traits, meat FA composition, and oxidative stability. The results of this study showed that there were no significant differences in growth performance between the treatments throughout the experimental period. Conversely, along with our findings, ref. [
37] observed higher chicken profitability and similar growth efficiency measures across all dietary regimens when essential oil blends were included as feed additives. The antioxidant properties of EO supplements, along with the regulation of digestive enzyme activity, may support intestinal function and consequently higher nutrient utilization, leading to superior growth rates [
38]. Earlier research has discussed similar results with no negative effects on ADFI or BW that were observed for Ross broilers that received DW supplemented with a total of two types of Mexican OEO at 400 mg/L [
27]. Variability in EO dosage, plant source, chemical composition, oil type, and environment could account for these differences in experimental findings.
Slaughter characteristics are an important performance indicator of meat production for both poultry and livestock, and are directly related to the profit of the chicken industry. The breast muscle index in the EOB
250 group showed an increase compared to the CON group. This result is consistent with {Formatting Citation}, who found that a mixture of EO supplementation increased the carcass yield of birds, while other slaughter characteristics were not affected among treatments. EOs include carvacrol, which promotes pancreatic secretions, increasing digestive efficiency and the assimilation of nutrients. This process ultimately leads to higher carcass production [
39]. The researchers believed an increase in valuable muscle mass was caused by EOs’ support of muscle development. However, other research has shown that EOs and organic acid supplementation did not affect the carcass characteristics in chickens [
40]. The differences in EO composition and dosage levels could lead to variability in carcass traits observed in chickens fed with EOs.
Excessive amounts of lipids lead to a chronic disease known as hyperlipidemia, which increases the risk of developing heart disease and stroke [
41]. Our experiment indicated that the EOB supplementation increased HDL content and decreased TG, TC, and LDL-C concentrations in both the liver and serum of the birds. This decrease helps to avoid metabolic diseases. The supplementation of EOs in diet was reported to decrease plasma TG levels and enhance HDL concentrations, adjust the FA composition profile of the breast muscle, decrease drip loss, and improve the meat quality of the chickens [
42]. Therefore, we speculated that the potential function of the EOB in improving meat quality might relate to its regulation of the lipid metabolism. Studies show that the administration of an EOB to birds reduces serum TC concentrations, which may be associated with the inhibitory effects of the EO compounds on the activity of 3-hydroxy-3-methylglutaryl coenzyme A reductase, a key enzyme in the regulatory pathway of TC synthesis [
43].
The sensory and physical characteristics of meat, including color, texture, and flavor, are critical factors influencing consumer consumption choices [
44]. We studied how the EOB supplementation influenced the breast meat quality, and found that the EOB
250 treatment significantly elevated the yellow intensity of the breast fillets. Various factors regulate the color of broiler meat, including myoglobin and other sarcoplasmic proteins (including hemoglobin, cytochromes, and catalases), diet, age, pH, breed, sex, and management practices [
45]. The yellowness of the breast fillets was found to be higher when the chickens were given an EOB of cinnamaldehyde, thymol, and carvacrol [
46]. In another study, it was reported that the inclusion of thymol, cinnamaldehyde, and carvacrol showed an increase in yellowness, with or without curcumin added [
47]. Besides the enhancement of color, the WHC was improved by the EOB supplementation, with decreased cooking and drip loss. In agreement with our findings, ref. [
48] also reported that thyme EO in a broiler diet improved the WHC and minimized drip loss, thereby increasing tenderness. This improvement seems to be directly related to an increased oxidative defense system in the muscle [
49]. Therefore, oxidation is a natural process that will affect meat quality by changing its pigments, fats, and proteins, resulting in a reduction in its shelf-life reduction [
50]. Free radicals generated during lipid oxidation disrupt the membrane structure by targeting unsaturated fatty acids, lipoproteins, and other components within the phospholipid bilayer of cell membranes. This damage increases membrane permeability, compromising cellular integrity. Maintaining the structural integrity of cell membranes can help restrict the production of free radicals, reduce oxidative reactions, decrease the leakage of sarcoplasmic fluid, and enhance the WHC [
51,
52]. EOs contain bioactive compounds, including phenolics and terpenoids, which exhibit medicinal properties by reducing oxidative stress in broilers [
52]. The increased levels of T-AOC, CAT, and GSH-Px suggested that improvement of the WHC, a factor contributing to lower cooking loss of the chicken meat for the EOB-supplemented group, also came from improvement of the muscle antioxidant capacity. Furthermore, higher muscle redox status and muscle water retention ability may be considered additional factors that could help to achieve improved meat quality results [
53].
Texture profile analysis, a widely used method, uses a double compression cycle to mimic chewing, enabling the analysis of textural properties in the chicken meat [
54]. This study also contributes to the understanding of the texture profile of the meat, specifically examining the effects of the EOB addition on cohesiveness and resilience. The EOB has the potential to improve meat tenderness, according to the findings. Similar findings [
55] showed that the meat of chickens raised on diets with oregano or anise EOs had improved tenderness and juiciness. Two types of Mexican OEO in DW were tested in broiler chickens, and they improved meat quality by reducing cooking loss and increasing cohesiveness and resilience [
27]. Physicochemical characteristics of meat, in general, can enhance the quality of chicken meat making it more palatable to consumers.
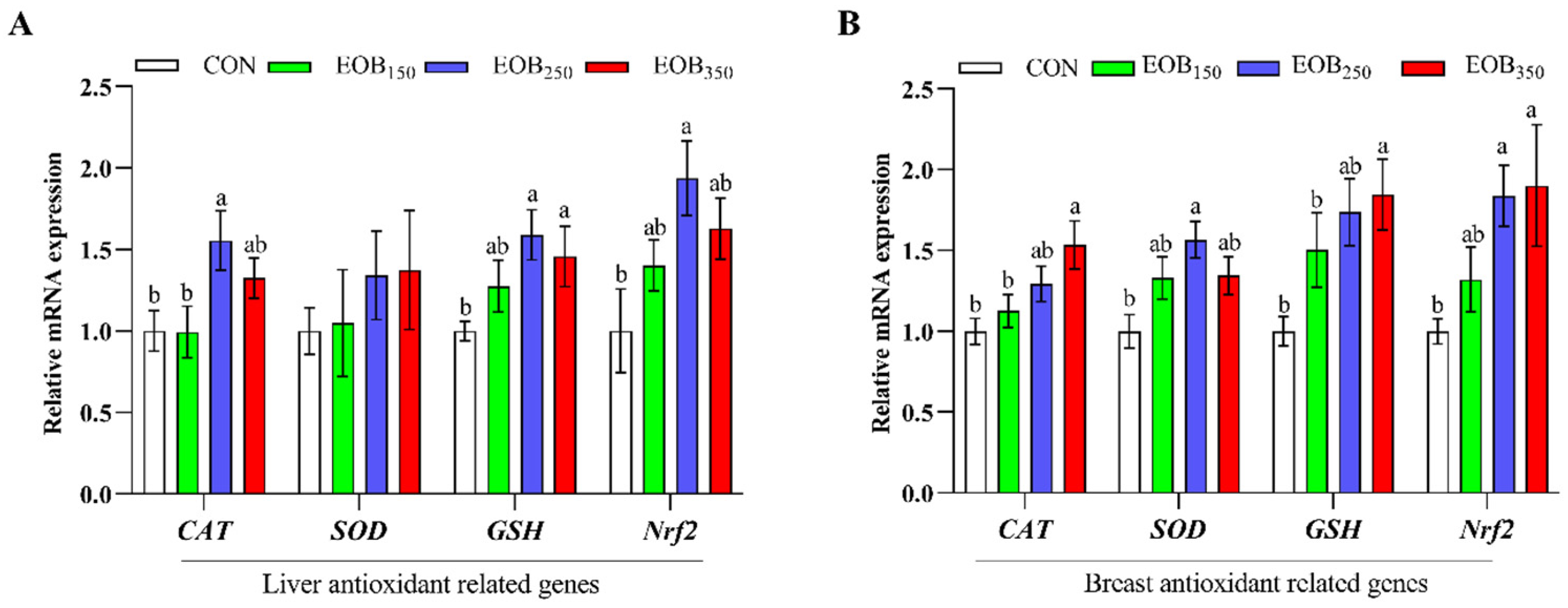
The activity of antioxidant defense enzymes in the body that reflects the physiological response of an organism to oxidative stress is tightly related to the general state of health [
56]. Our results suggested that the EOB supplementation can improve antioxidant activity by increasing the T-SOD, CAT, GSH-Px, and T-AOC of the serum, liver, and breast muscle in broiler chickens. Several studies have reported that EOs can improve the oxidative stability of chicken tissues [
57]. This study also found that the EOB supplementation lowered MDA levels, a marker of oxidative stress, in the liver and breast muscle tissues. MDA is a byproduct of the peroxidation of PUFAs in cells, and its excessive formation is driven by an increase in free radicals [
58]. Consistent with our findings, similar studies have reported that the inclusion of aromatic plant extracts enhances GPx activity and reduces MDA levels [
59,
60]. Additionally, a balance was observed between the levels of reactive oxygen species and antioxidants in the chickens. The absence of secondary lipid peroxidation (MDA) and protein oxidation suggests that the broilers exhibited a robust antioxidant defense capacity, which effectively interrupted oxidative reactions and contained ROS. This indicates a potential protective effect of the EOB against oxidative damage. Nrf2 is a transcription factor that is a master regulator of cell antioxidant defense pathways. It induces cells to activate the expression of antioxidant response elements that regulate the expression of genes that control oxidative stress [
61]. Broilers fed with EOB
250 had a significantly higher mRNA level of
Nrf2, demonstrating the stimulation of their intrinsic antioxidant system. Nrf2 upregulation suggests that the EOB supplementation may trigger a defensive mechanism against oxidative damage, thereby enhancing meat quality and stability. This antioxidant effect is combined by synergistic actions of T-SOD and CAT, in addition to GSH-Px, which work together to prevent reactive oxygen species (ROS) from inducing oxidative stress to muscle tissues. This study revealed that some antioxidants in the EOB entered the blood, remained in tissues, and protected the body from oxidative stress by building antioxidant enzymes.
Meat from PUFA-rich diets, especially n-3 PUFAs, provides health-promoting benefits. However, Our findings demonstrated that the PUFA content of muscle increased when the EOB was administered. The observed changes in fatty acid composition may be associated with the antioxidant properties of plant-derived compounds, such as flavonoids and terpenoids, and/or their ability to modify the gut microbial community [
62,
63], as well as the reduction of biohydrogenation of unsaturated fatty acids, which led to an increase in the PUFA/SFA rate [
64]. Moreover, an upregulation of the expression of
FADS2 mRNA in birds supplied with the EOB was also detected, indicating that in birds receiving the EOB, the rise in PUFA levels was associated with upregulation of the
FADS2 pathway for PUFA synthesis. In the lipid metabolic pathway,
FADS2 is responsible for desaturating the FAs linoleate and alpha-linolenate into PUFAs [
65]. Moreover, the birds that were supplemented with EOB
250 and EOB
350 had less SFA fractions, as shown by decreased C14:0, C16:0, and C18:0 contents. SFA is suggested to promote high levels of LDL-C and TC [
66]. Consuming more SFAs in diets might lead to the hypercholesterolemic effects associated with coronary artery disease [
67].
Meat health indices are valuable for analyzing the influence of a dietary FA profile on susceptibility to common chronic diseases. These indices are more cost-effective than lengthy laboratory procedures [
68]. Whereas SFAs have been shown to have pro-inflammatory effects, EPA and DHA are considered anti-inflammatory [
69]. The UI indicator for the level of unsaturation of FAs causing its structure, which is useful for assessing oxidative stability and suggesting possible oxidative protection approaches for livestock feed [
70]. Also, the PI evaluates the stability of PUFAs in food, contributing to the prevention of oxidation processes [
71]. In the relationship to health, NVI gives information about possible health consequences of the lipid profiles that positively correlate with the quality of the FAs [
72]. More consistent with our findings, the elevated amounts of EPA + DHA, UI, and PI in the supplemented groups indicated a positive impact of the EOB supplementation on the health-promoting properties of chicken meat. IA and TI indices and h/H are good indicators of lipid health and nutritional quality. A lower IA and TI indicate a healthier fatty acid profile, which decreases the risk of platelet clumping and coronary diseases, while a high h/H ratio denotes a better quality of nutrition [
73]. The EOB supplementation reduced TI and IA while elevating h/H in meat, indicating improved meat quality; it was demonstrated herein that the EOB
350 and EOB
250 levels of supplementation were the most effective treatment among the supported levels. Additionally, DFA, FLQ, and NVI serve as key markers of the overall health-promoting properties of the meat [
36]. These results indicate that the EOB supplementation can improve meat quality, usually by modifying the FA profile in the breast meat, which is beneficial for consumer health.
To analyze the molecular mechanisms that led to alterations in the FA profile, we measured the expression of key genes associated with the liver lipid metabolism. A transcription factor,
SREBP-1c, regulates genes associated with FA and TG synthesis, like
SCD, FAS, and
ACC [
74]. In our study, the mRNA abundance level of
SREBP-1c was reduced in the EOB-treated chickens compared to the CON, which was supported with lower levels of blood TG and TC. The activity of the EOB reduced blood TC and TG levels in experimental groups, which was related to decreased expression of
FAS and
ACC, especially in the EOB-treated groups. Another study reported similar findings, which revealed that carvacrol inhibits fat accumulation by downregulating
SREBP-1c and
FAS, thereby inhibiting lipogenesis and increasing
CPT1 expression to promote FA oxidation in high-fat diet-fed mice [
75]. PPARγ is a nuclear receptor required for adipogenesis and acts by recruiting
C/EBPα to generate a transcriptional complex regulating adipocyte differentiation and lipid storage [
76]. In the present study,
PPARγ mRNA expression level was higher in the CON group than the EOB
250. This finding follows other studies [
77] that cinnamaldehyde inhibits the development of adipocytes and modulates adipose tissue metabolism by downregulating
PPARγ and
C/EBPα. This regulation leads to the prevention of adipocyte differentiation and fat accumulation. Cinnamaldehyde also restricted the expression of
SREBP-1c and
FAS, which can also inhibit FA synthesis and lipid storage.
To further understand the impact of the EOB on lipid metabolism, we assessed the expression level of enzymes involved in FA catabolism in liver tissue.
PPARα regulates numerous metabolic processes that facilitate cellular uptake of FA and metabolism via FA
β-oxidation, ultimately promoting cellular respiration [
78], including expression of
CPT1, which is responsible for FA transport into mitochondria for
β-oxidation. ACOX1 is another important enzyme for FA catabolism in the peroxisome, which is considered a rate-limiting step during peroxisomal β-oxidation [
78]. In this study, supplementation of the EOB increased the mRNA expression of
PPARα,
ACOX1, and
CPT1 in liver tissue compared to the CON. These results are in agreement with previous findings, which showed that cinnamon can upregulate PPARα gene expression in liver tissue while decreasing serum and liver TC levels as well as serum TG. Generally, these findings suggested that the EOB modulates FA β-oxidation, at least in part by activating the
PPARα pathway, leading to enhanced FA β-oxidation [
79]. To summarize, the EOB supplementation increased ABW, FCR, and breast weight compared to the CON, which may have a major economic impact on the industry.