First Case Report of Successful Treatment of Mycobacterium abscessus Infection in a Cat in Thailand
Simple Summary
Abstract
1. Introduction
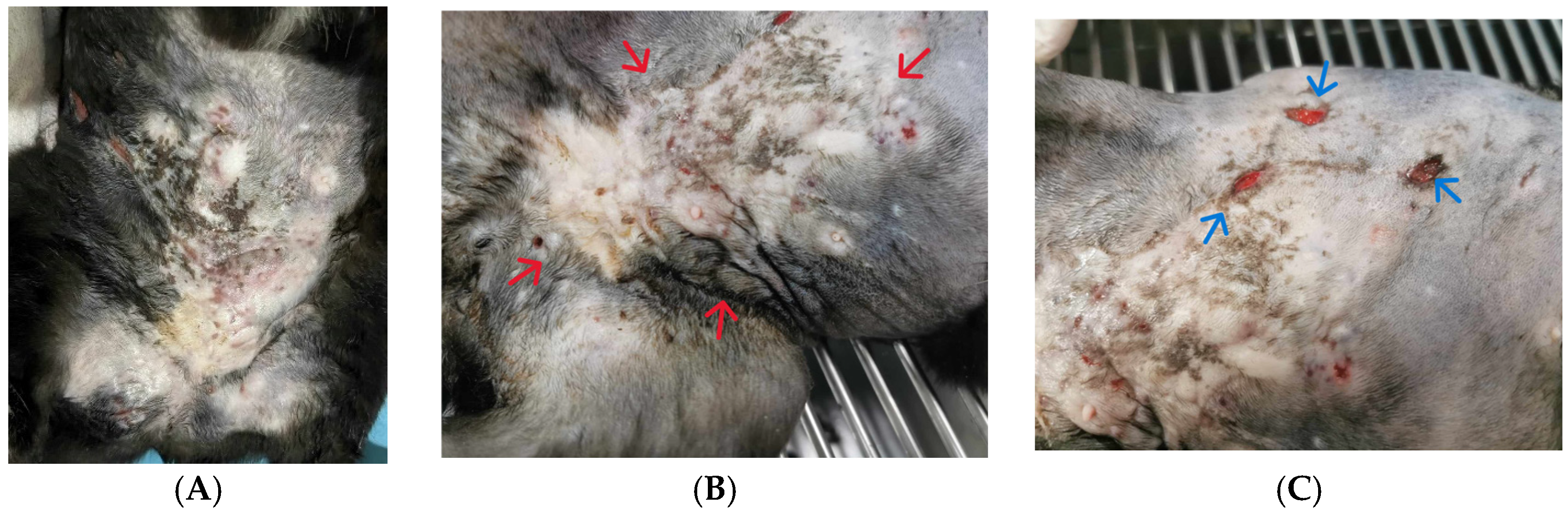
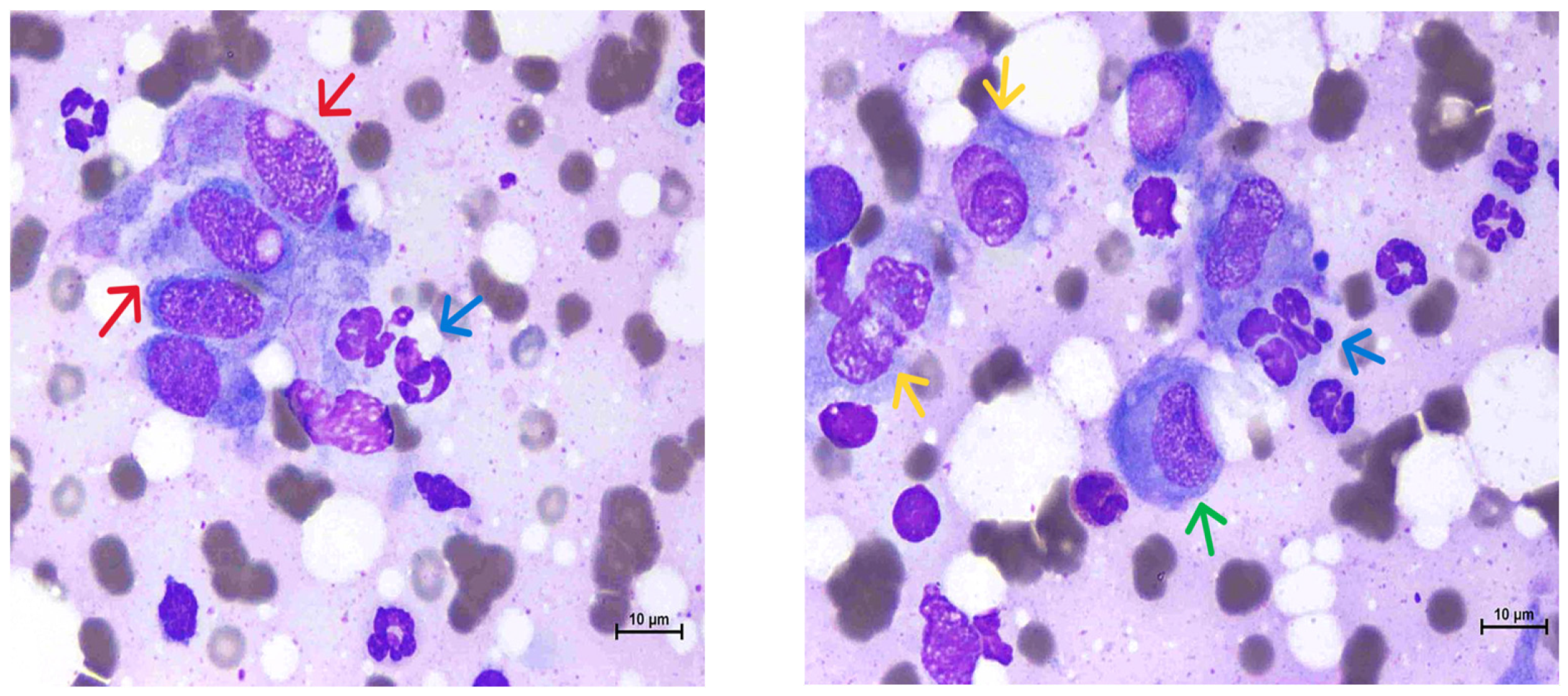
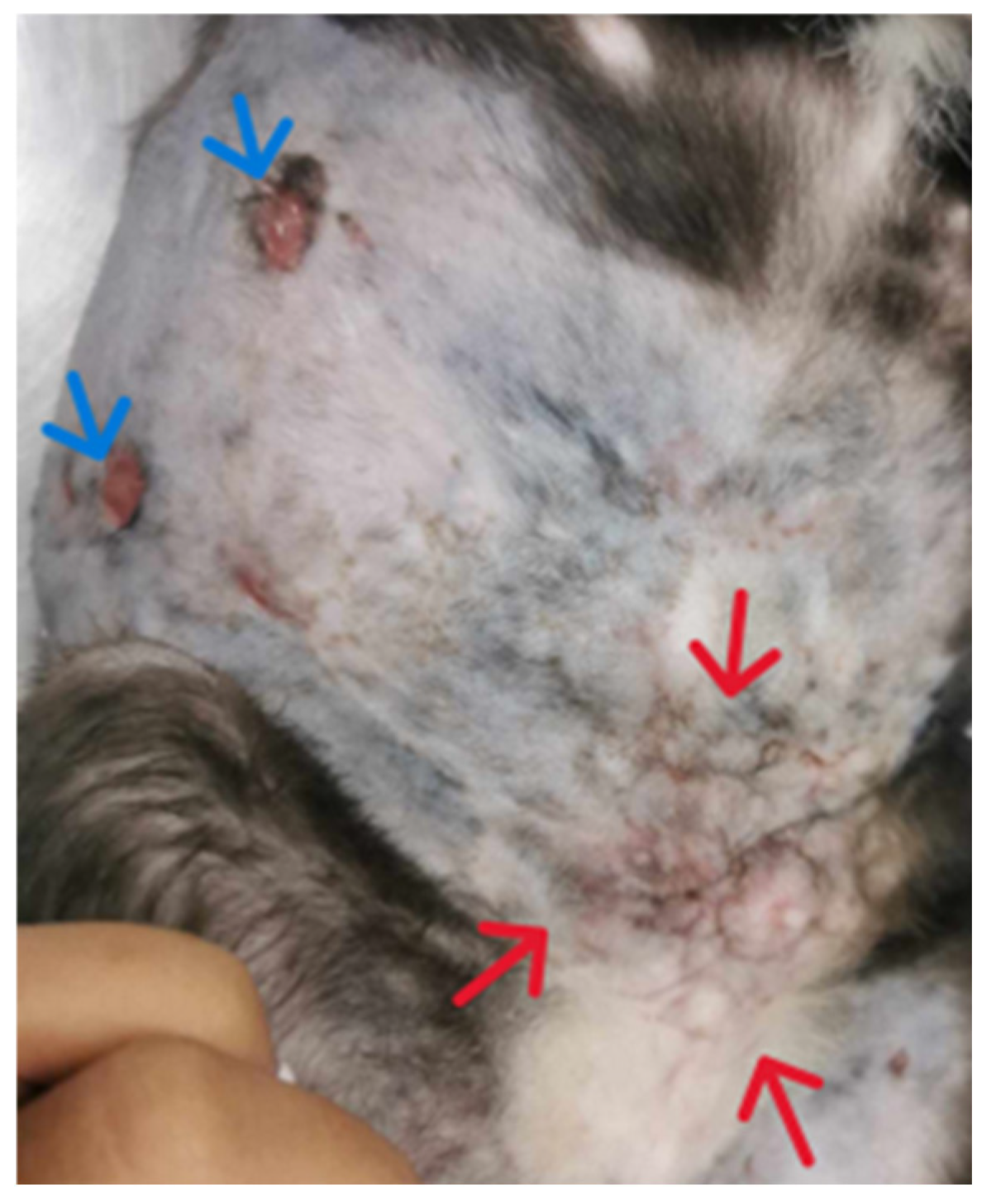
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gunn-Moore, D.A.; McFarland, S.E.; Brewer, J.I.; Crawshaw, T.R.; Clifton-Hadley, R.S.; Kovalik, M.; Shaw, D.J. Mycobacterial disease in cats in Great Britain: I. Culture results, geographical distribution and clinical presentation of 339 cases. J. Feline Med. Surg. 2011, 13, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Lloret, A.; Hartmann, K.; Pennisi, M.G.; Gruffydd-Jones, T.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Hosie, M.J.; et al. Mycobacterioses in Cats: ABCD guidelines on prevention and management. J. Feline Med. Surg. 2013, 15, 591–597. [Google Scholar] [PubMed]
- Krajewska-Wędzina, M.; Dąbrowska, A.; Augustynowicz-Kopeć, E.; Weiner, M.; Szulowski, K. Nontuberculous mycobacterial skin disease in cat; diagnosis and treatment—Case report. Ann. Agric. Environ. Med. 2019, 26, 511–513. [Google Scholar]
- Peterhans, S.; Grest, P.; Friedel, U.; Roosje, P.; Ghielmetti, G. Highly resistant Mycobacterium abscessus subsp. abscessus infection in a Swiss cat. Vet. Rec. Case Rep. 2021, 9, e153. [Google Scholar]
- Gunn-Moore, D.A. Feline mycobacterial infections. Vet. J. 2014, 201, 230–238. [Google Scholar] [PubMed]
- Vishkautsan, P.; Reagan, K.L.; Keel, M.K.; Sykes, J.E. Mycobacterial panniculitis caused by Mycobacterium thermoresistibile in a cat. J. Feline Med. Surg. Open Rep. 2016, 2, 2055116916672786. [Google Scholar]
- Beccati, M.; Peano, A.; Gallo, M.G. Pyogranulomatous panniculitis caused by Mycobacterium alvei in a cat. J. Small Anim. Pract. 2007, 48, 664. [Google Scholar]
- Sykes, J.E. Cutaneous Mycobacterioses of Cats and Dogs; Veterinary Clinics of North America—Small Animal Practice; W.B. Saunders: Philadelphia, PA, USA, 2025. [Google Scholar]
- Han, H.S.; Gunn-Moore, D. First report of Mycobacteria avium complex (Mycobacteria intracellulare) in a cat from Southeast Asia. J. Feline Med. Surg. Open Rep. 2023, 9, 20551169231194311. [Google Scholar] [CrossRef] [PubMed]
- Didkowska, A.; Żmuda, P.; Orłowska, B.; Anusz, K. Mycobacterial infections in cats (Felis catus) as a potential threat to humans—A review 2014–2023. In Medycyna Weterynaryjna; Polskie Towarzystwo Nauk Weterynaryjnych: Warsaw, Poland, 2023; Volume 79. [Google Scholar] [CrossRef]
- Gunn-Moore, D.A.; McFarland, S.E.; Schock, A.; Brewer, J.I.; Crawshaw, T.R.; Clifton-Hadley, R.S.; Shaw, D.J. Mycobacterial disease in a population of 339 cats in Great Britain: II. Histopathology of 225 cases, and treatment and outcome of 184 cases. J. Feline Med. Surg. 2011, 13, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Patel, R. MALDI-TOF MS for the diagnosis of infectious diseases. In Clinical Chemistry; American Association for Clinical Chemistry Inc.: Washington, DC, USA, 2015; Volume 61, pp. 100–111. [Google Scholar]
- Fernández-Esgueva, M.; Fernández-Simon, R.; Monforte-Cirac, M.L.; López-Calleja, A.I.; Fortuño, B.; Viñuelas-Bayon, J. Use of MALDI-TOF MS (Bruker Daltonics) for identification of Mycobacterium species isolated directly from liquid medium. Enfermedades Infecc. Microbiol. Clin. 2021, 39, 241–243. [Google Scholar]
- Alcaide, F.; Amlerová, J.; Bou, G.; Ceyssens, P.J.; Coll, P.; Corcoran, D.; Fangous, M.S.; González-Álvarez, I.; Gorton, R.; Greub, G.; et al. How to: Identify non-tuberculous Mycobacterium species using MALDI-TOF mass spectrometry. In Clinical Microbiology and Infection; Elsevier: Amsterdam, The Netherlands, 2018; Volume 24, pp. 599–603. [Google Scholar]
- Mortier, T.; Wieme, A.D.; Vandamme, P.; Waegeman, W. Bacterial species identification using MALDI-TOF mass spectrometry and machine learning techniques: A large-scale benchmarking study. Comput. Struct. Biotechnol. J. 2021, 19, 6157–6168. [Google Scholar] [PubMed]
- Alsaad, K.M.; Al-Saasd, K.M. A Clinical Case of Panniculitis in a Cat (Case Report). IOSR J. Agric. Vet. Sci. 2020, 13, 27–30. Available online: www.iosrjournals.org (accessed on 21 October 2024).
- Ong, L.T. Epidemiology of nontuberculous mycobacteria infection in Asia: A narrative review. Indian J. Tuberc. 2024. [Google Scholar] [CrossRef]
- Jassies-van der Lee, A.; Houwers, D.J.; Meertens, N.; van der Zanden, A.G.M.; Willemse, T. Localised pyogranulomatous dermatitis due to Mycobacterium abscessus in a cat: A case report. Vet. J. 2009, 179, 304–306. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Dogra, S.; De, D.; Saikia, U.N. Orofacial Granulomatosis Responding to Weekly Azithromycin Pulse Therapy. JAMA Dermatol. 2015, 151, 219–220. [Google Scholar] [CrossRef] [PubMed]
- Banjanac, M.; Munić Kos, V.; Nujić, K.; Vrančić, M.; Belamarić, D.; Crnković, S.; Hlevnjak, M.; Haber, V.E. Anti-inflammatory mechanism of action of azithromycin in LPS-stimulated J774A.1 cells. Pharmacol. Res. 2012, 66, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Triviño, F.; Pérez-López, I.; Ruíz-Villaverde, R. Doxycycline, an antibiotic or an anti-inflammatory agent? The Most Common uses in dermatology. Actas Dermo-Sifiliográficas (Engl. Ed.) 2020, 111, 561–566. [Google Scholar] [CrossRef]
- Jeha, G.M.; Luckett, K.O.; Kole, L. Actinic granuloma responding to doxycycline. JAAD Case Rep. 2020, 6, 1132–1134. [Google Scholar] [CrossRef] [PubMed]

| Interpretation | |||
|---|---|---|---|
| Antimicrobial | S | I | R |
| Amikacin | ✓ | ||
| Azithromycin | ✓ | ||
| Imipenem | ✓ | ||
| Tetracycline | ✓ | ||
| Doxycycline | ✓ | ||
| Erythromycin | ✓ | ||
| Amoxicillin–clavulanic acid | ✓ | ||
| Cefixime | ✓ | ||
| Cefovecin | ✓ | ||
| Ceftriaxone | ✓ | ||
| Cephalexin | ✓ | ||
| Chloramphenicol | ✓ | ||
| Clindamycin | ✓ | ||
| Gentamicin | ✓ | ||
| Meropenem | ✓ | ||
| Metronidazole | ✓ | ||
| Oxacillin | ✓ | ||
| Sulphamethoxazole/trimetroprim | ✓ | ||
| Ciprofloxacin | ✓ | ||
| Enrofloxacin | ✓ | ||
| Levofloxacin | ✓ | ||
| Marbofloxacin | ✓ | ||
| Pradofloxacin | ✓ | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuenngam, T.; Chermprapai, S. First Case Report of Successful Treatment of Mycobacterium abscessus Infection in a Cat in Thailand. Animals 2025, 15, 925. https://doi.org/10.3390/ani15070925
Chuenngam T, Chermprapai S. First Case Report of Successful Treatment of Mycobacterium abscessus Infection in a Cat in Thailand. Animals. 2025; 15(7):925. https://doi.org/10.3390/ani15070925
Chicago/Turabian StyleChuenngam, Thapanee, and Suttiwee Chermprapai. 2025. "First Case Report of Successful Treatment of Mycobacterium abscessus Infection in a Cat in Thailand" Animals 15, no. 7: 925. https://doi.org/10.3390/ani15070925
APA StyleChuenngam, T., & Chermprapai, S. (2025). First Case Report of Successful Treatment of Mycobacterium abscessus Infection in a Cat in Thailand. Animals, 15(7), 925. https://doi.org/10.3390/ani15070925






