Repurposing Vancomycin as a Potential Antiviral Agent Against PEDV via nsp13 Helicase Inhibition
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus and Cells
2.2. Homology Modeling
2.3. Docking-Based Virtual Screening
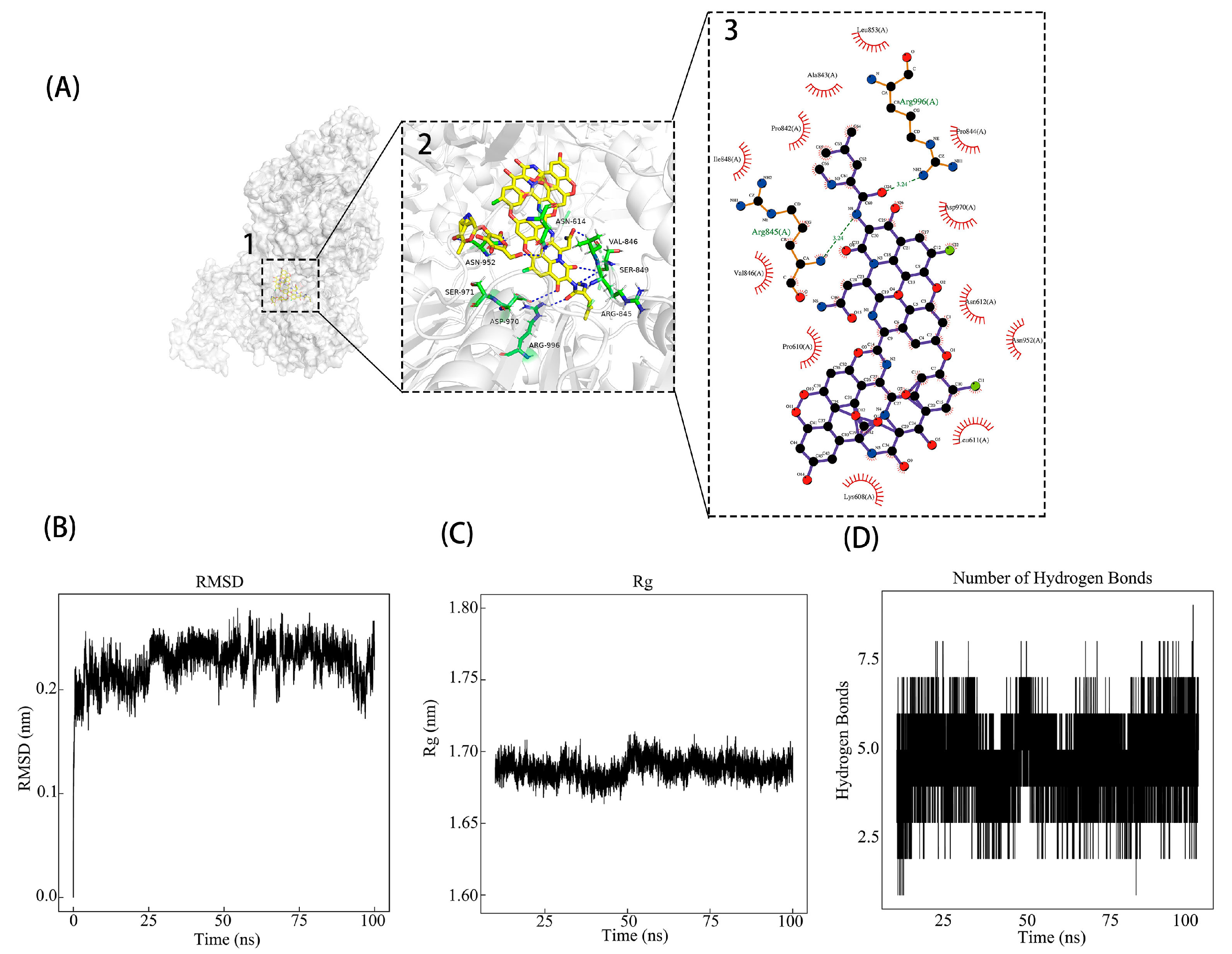
2.4. Molecular Dynamics (MD)
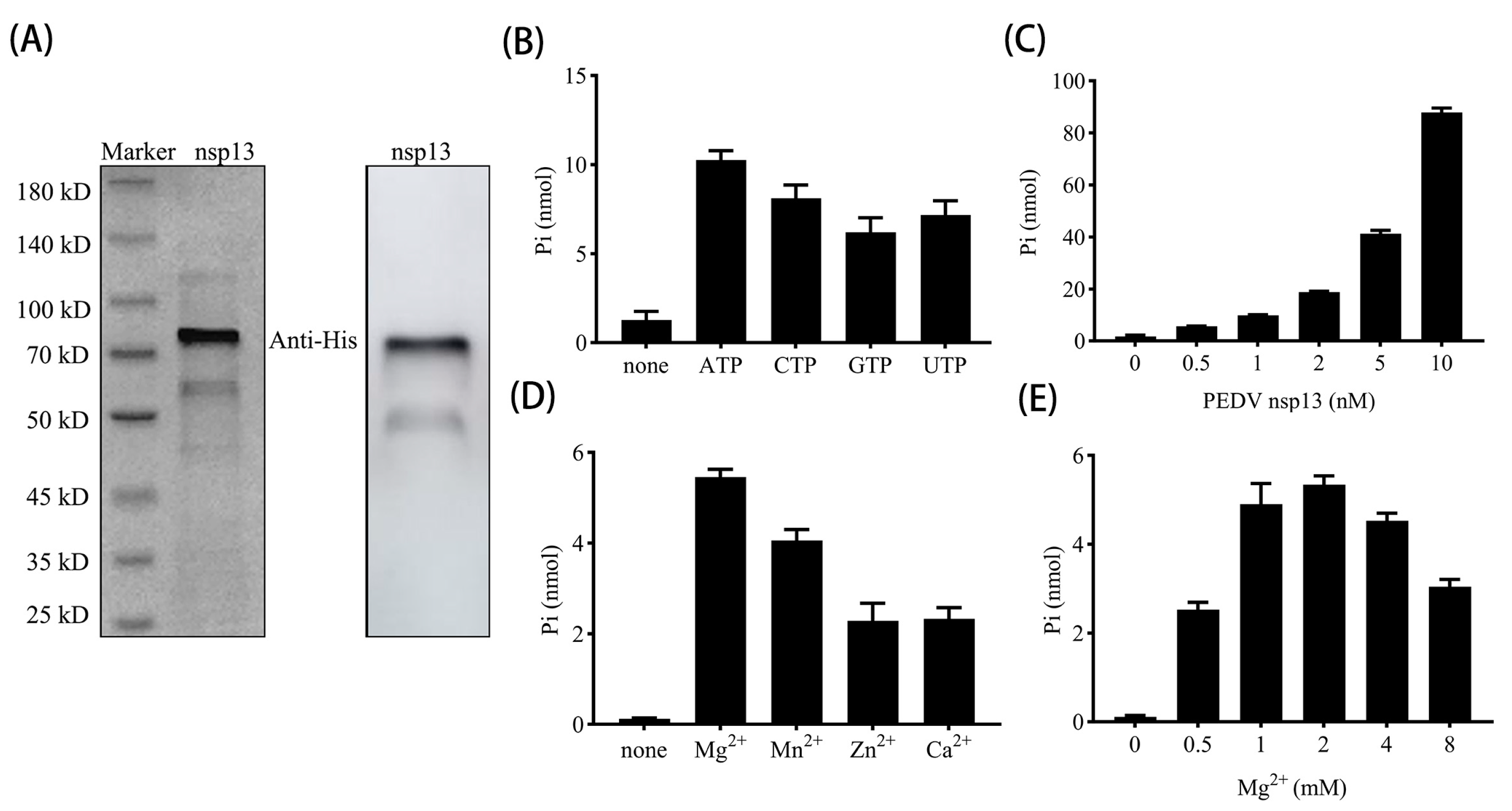
2.5. Expression and Purification of Recombinant Protein
2.6. NTPase Assay
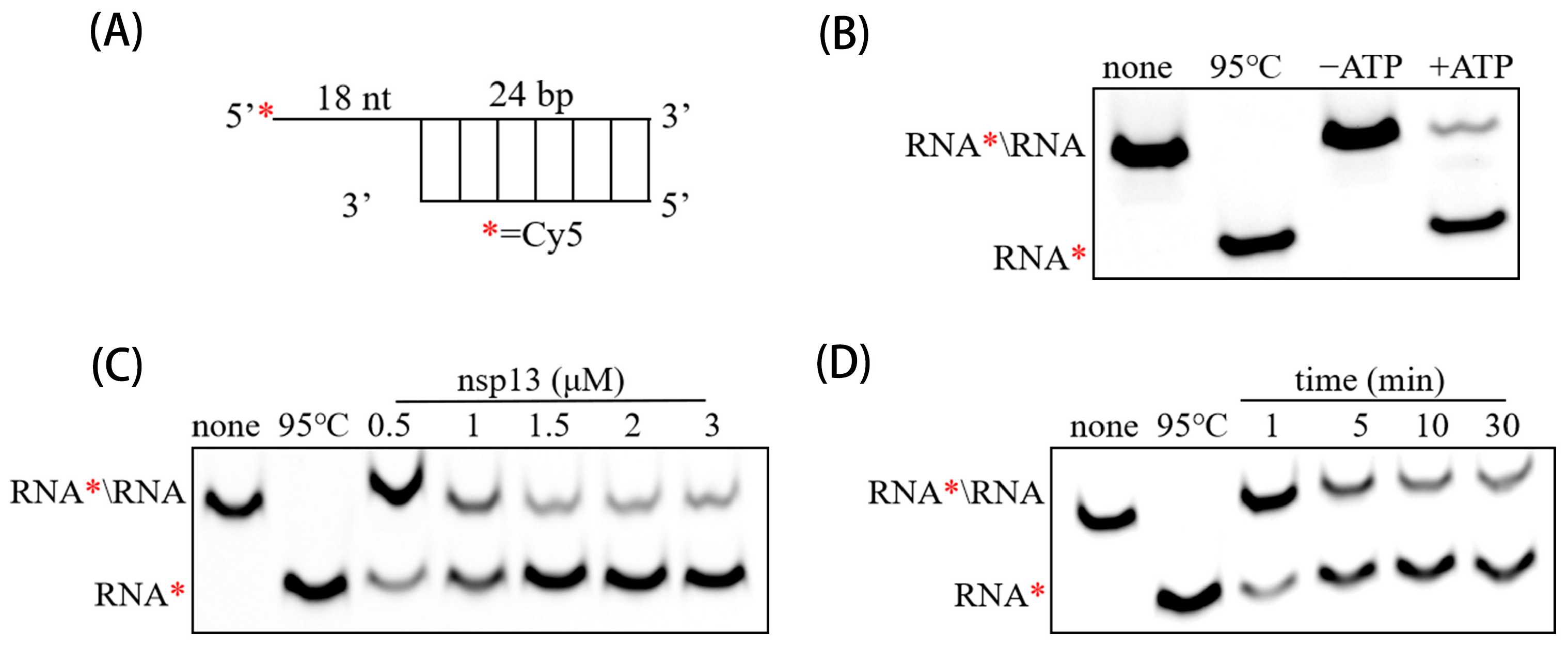
2.7. Preparation of RNA Duplex Substrate
2.8. RNA Duplex Unwinding Assay
2.9. Cytotoxicity Assay
2.10. Drug Inhibition of PEDV
2.11. Quantitative Real-Time PCR
2.12. Virus Titration
2.13. Western Blot
2.14. Immunofluorescence Assay
2.15. Statistical Analysis
3. Results
3.1. Identifying Potential Inhibitors of PEDV nsp13 Through Virtual Screening
3.2. PEDV nsp13 Exhibits ATP Hydrolysis Activity
3.3. PEDV nsp13 Unwinds RNA Duplex in an ATP-Dependent Manner
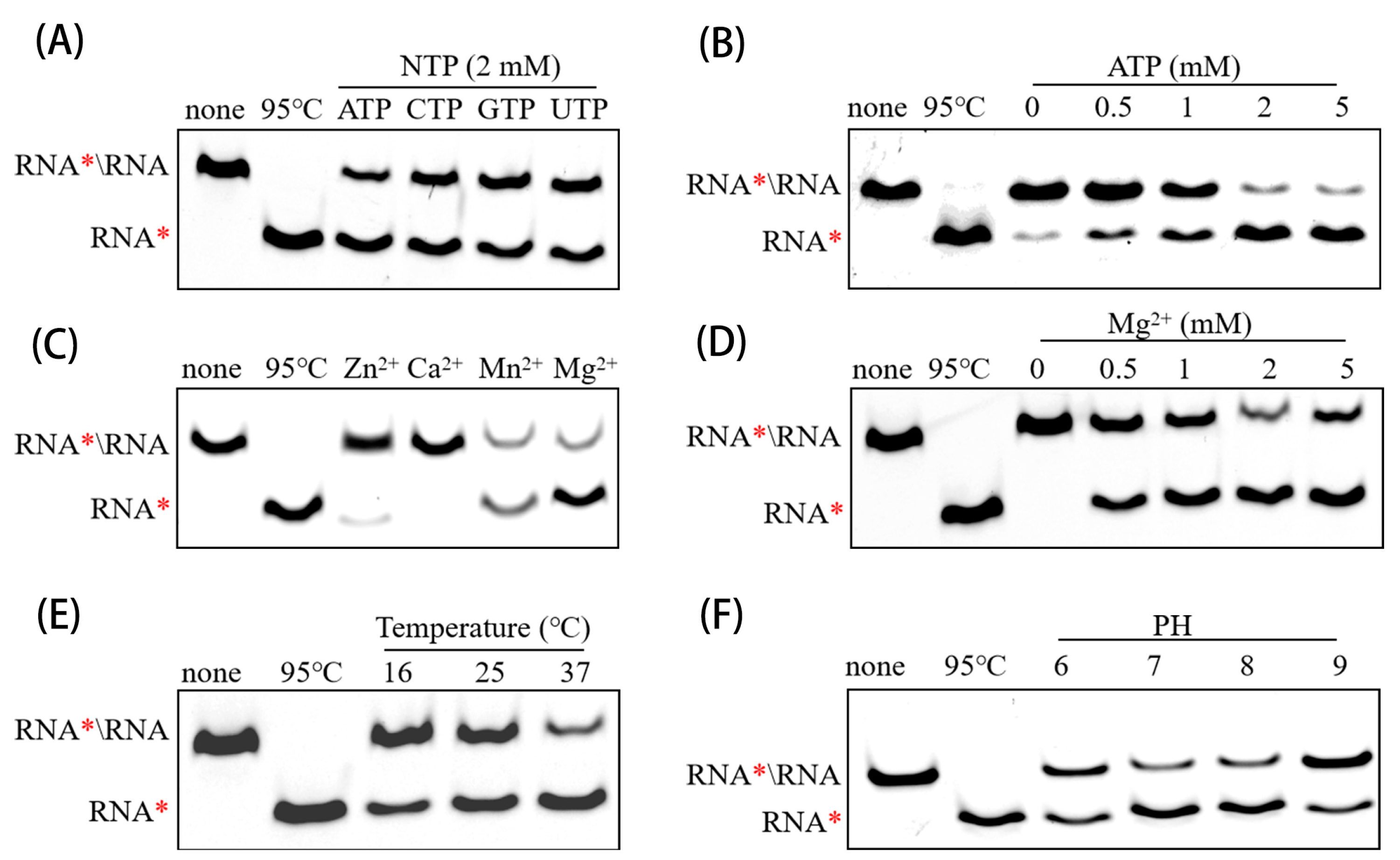
3.4. Determining Optimal Biochemical Conditions for RNA Helicase Activity of PEDV nsp13
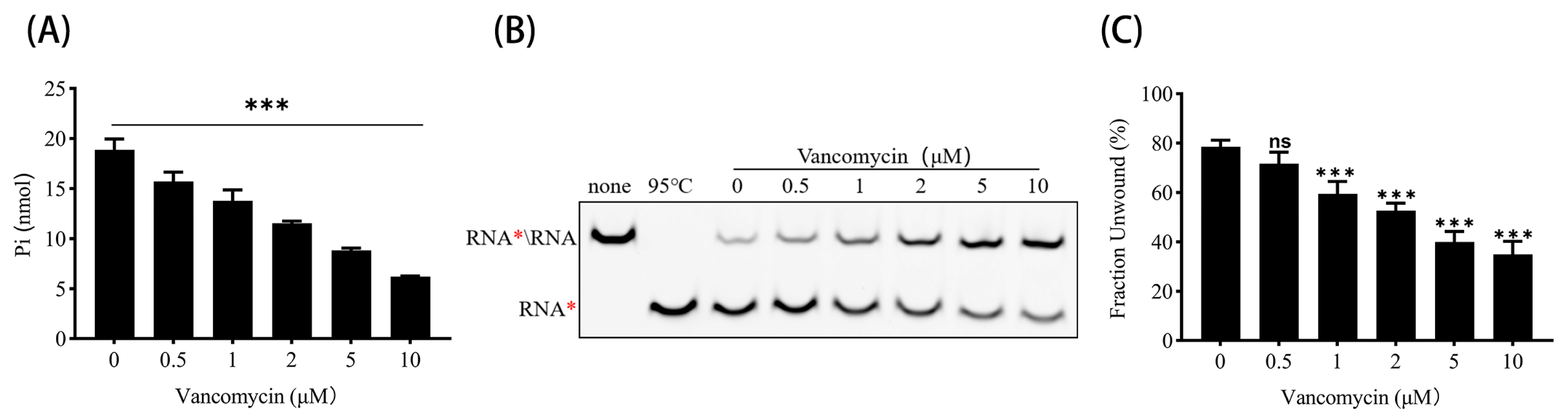
3.5. Vancomycin Inhibits ATPase and RNA Helicase Activities of PEDV nsp13
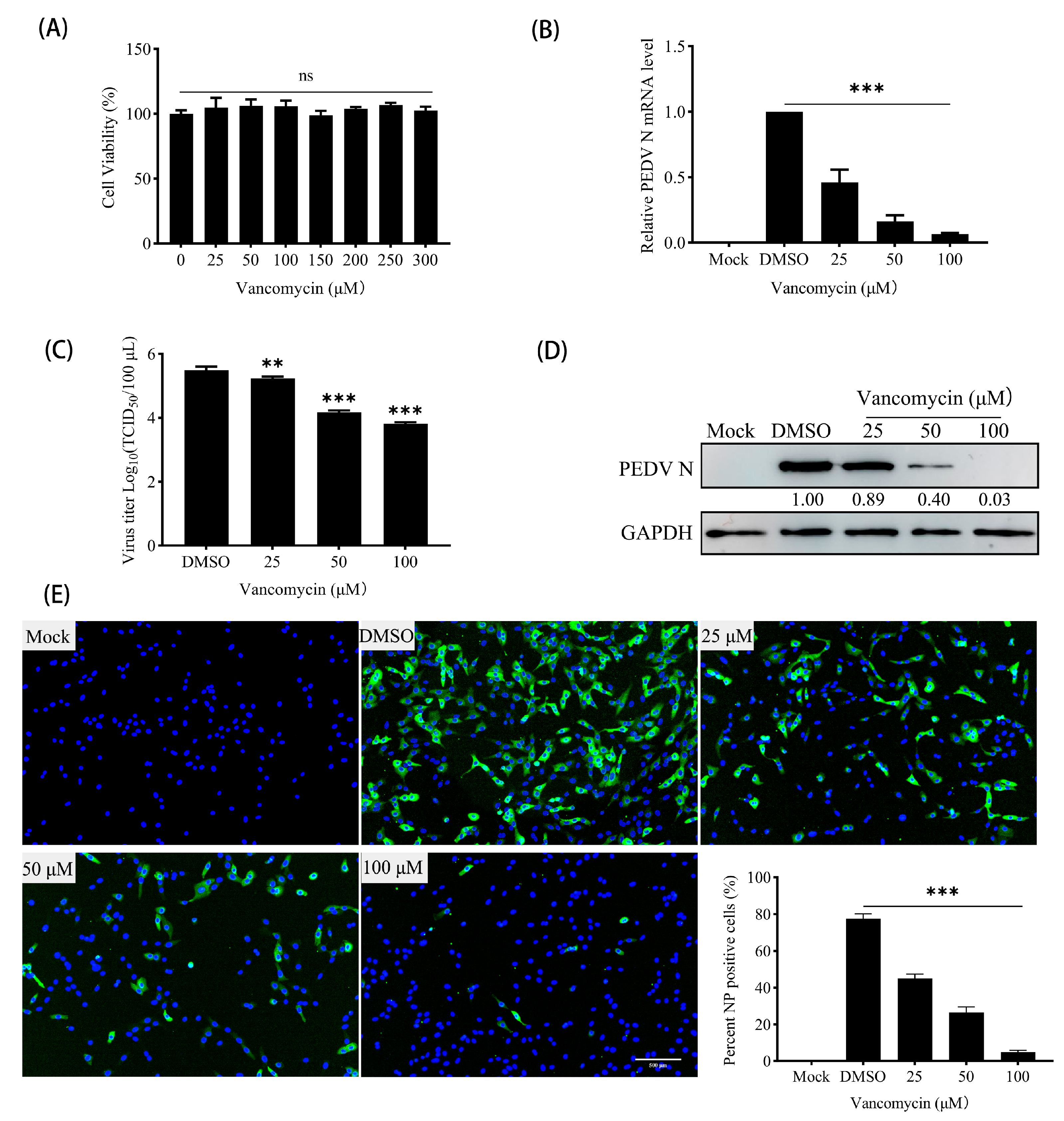
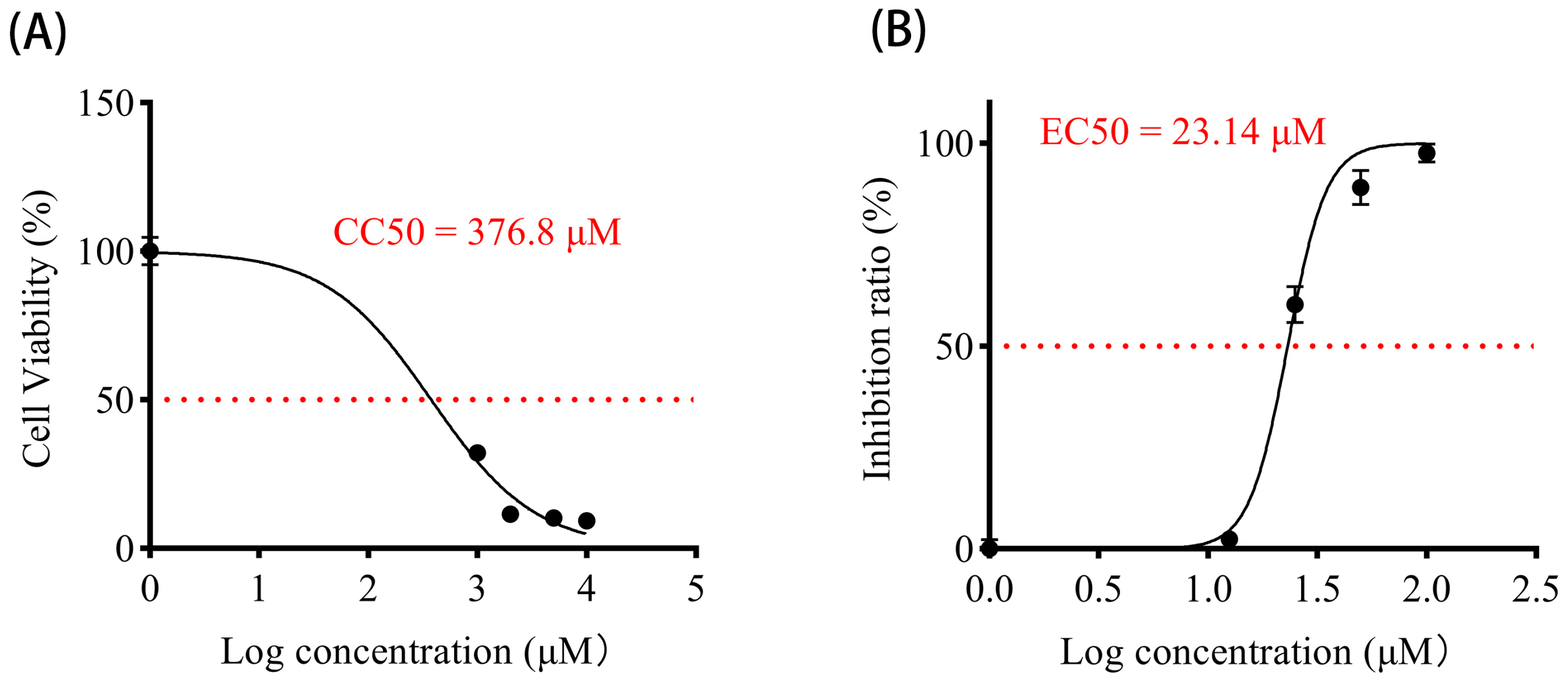
3.6. Vancomycin Exhibits Significant Antiviral Activity Against PEDV
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pensaert, M.B.; de Bouck, P. A new coronavirus-like particle associated with diarrhea in swine. Arch. Virol. 1978, 58, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zou, C.; Peng, O.; Ashraf, U.; Xu, Q.; Gong, L.; Fan, B.; Zhang, Y.; Xu, Z.; Xue, C.; et al. Global Dynamics of Porcine Enteric Coronavirus PEDV Epidemiology, Evolution, and Transmission. Mol. Biol. Evol. 2023, 40, msad052. [Google Scholar] [CrossRef]
- Ferrara, G.; Nocera, F.P.; Longobardi, C.; Ciarcia, R.; Fioretti, A.; Damiano, S.; Iovane, G.; Pagnini, U.; Montagnaro, S. Retrospective Serosurvey of Three Porcine Coronaviruses among the Wild Boar (Sus scrofa) Population in the Campania Region of Italy. J. Wildl. Dis. 2022, 58, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, G.; D’Anza, E.; Rossi, A.; Improda, E.; Iovane, V.; Pagnini, U.; Iovane, G.; Montagnaro, S. A Serological Investigation of Porcine Reproductive and Respiratory Syndrome and Three Coronaviruses in the Campania Region, Southern Italy. Viruses 2023, 15, 300. [Google Scholar] [CrossRef]
- Gerdts, V.; Zakhartchouk, A. Vaccines for porcine epidemic diarrhea virus and other swine coronaviruses. Vet. Microbiol. 2017, 206, 45–51. [Google Scholar] [CrossRef]
- Wang, Q.; Vlasova, A.N.; Kenney, S.P.; Saif, L.J. Emerging and re-emerging coronaviruses in pigs. Curr. Opin. Virol. 2019, 34, 39–49. [Google Scholar] [CrossRef]
- Lu, L.; Su, S.; Yang, H.; Jiang, S. Antivirals with common targets against highly pathogenic viruses. Cell 2021, 184, 1604–1620. [Google Scholar] [CrossRef]
- Shu, T.; Huang, M.; Wu, D.; Ren, Y.; Zhang, X.; Han, Y.; Mu, J.; Wang, R.; Qiu, Y.; Zhang, D.Y.; et al. SARS-Coronavirus-2 Nsp13 Possesses NTPase and RNA Helicase Activities That Can Be Inhibited by Bismuth Salts. Virol. Sin. 2020, 35, 321–329. [Google Scholar] [CrossRef]
- Ren, J.; Ding, Z.; Fang, P.; Xiao, S.; Fang, L. ATPase and helicase activities of porcine epidemic diarrhea virus nsp13. Vet. Microbiol. 2021, 257, 109074. [Google Scholar] [CrossRef]
- Spratt, A.N.; Gallazzi, F.; Quinn, T.P.; Lorson, C.L.; Sönnerborg, A.; Singh, K. Coronavirus helicases: Attractive and unique targets of antiviral drug-development and therapeutic patents. Expert Opin. Ther. Pat. 2021, 31, 339–350. [Google Scholar] [CrossRef]
- Hao, W.; Wojdyla, J.A.; Zhao, R.; Han, R.; Das, R.; Zlatev, I.; Manoharan, M.; Wang, M.; Cui, S. Crystal structure of Middle East respiratory syndrome coronavirus helicase. PLoS Pathog. 2017, 13, e1006474. [Google Scholar] [CrossRef]
- Jia, Z.; Yan, L.; Ren, Z.; Wu, L.; Wang, J.; Guo, J.; Zheng, L.; Ming, Z.; Zhang, L.; Lou, Z.; et al. Delicate structural coordination of the Severe Acute Respiratory Syndrome coronavirus Nsp13 upon ATP hydrolysis. Nucleic Acids Res. 2019, 47, 6538–6550. [Google Scholar] [CrossRef]
- Chen, J.; Malone, B.; Llewellyn, E.; Grasso, M.; Shelton, P.M.M.; Olinares, P.D.B.; Maruthi, K.; Eng, E.T.; Vatandaslar, H.; Chait, B.T.; et al. Structural Basis for Helicase-Polymerase Coupling in the SARS-CoV-2 Replication-Transcription Complex. Cell 2020, 182, b1560–b1573.e13. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, Y.; Ge, J.; Zheng, L.; Gao, Y.; Wang, T.; Jia, Z.; Wang, H.; Huang, Y.; Li, M.; et al. Architecture of a SARS-CoV-2 mini replication and transcription complex. Nat. Commun. 2020, 11, 5874. [Google Scholar] [CrossRef]
- Yan, L.; Ge, J.; Zheng, L.; Zhang, Y.; Gao, Y.; Wang, T.; Huang, Y.; Yang, Y.; Gao, S.; Li, M.; et al. Cryo-EM Structure of an Extended SARS-CoV-2 Replication and Transcription Complex Reveals an Intermediate State in Cap Synthesis. Cell 2021, 184, 184–193.e10. [Google Scholar] [CrossRef] [PubMed]
- Adedeji, A.O.; Lazarus, H. Biochemical Characterization of Middle East Respiratory Syndrome Coronavirus Helicase. Msphere 2016, 1, e00235-16. [Google Scholar] [CrossRef]
- Frick, D.N.; Virdi, R.S.; Vuksanovic, N.; Dahal, N.; Silvaggi, N.R. Molecular Basis for ADP-Ribose Binding to the Mac1 Domain of SARS-CoV-2 nsp3. Biochemistry 2020, 59, 2608–2615. [Google Scholar] [CrossRef] [PubMed]
- Adedeji, A.O.; Singh, K.; Calcaterra, N.E.; DeDiego, M.L.; Enjuanes, L.; Weiss, S.; Sarafianos, S.G. Severe acute respiratory syndrome coronavirus replication inhibitor that interferes with the nucleic acid unwinding of the viral helicase. Antimicrob. Agents Chemother. 2012, 56, 4718–4728. [Google Scholar] [CrossRef]
- Adedeji, A.O.; Singh, K.; Kassim, A.; Coleman, C.M.; Elliott, R.; Weiss, S.R.; Frieman, M.B.; Sarafianos, S.G. Evaluation of SSYA10-001 as a replication inhibitor of severe acute respiratory syndrome, mouse hepatitis, and Middle East respiratory syndrome coronaviruses. Antimicrob. Agents Chemother. 2012, 58, 4894–4898. [Google Scholar] [CrossRef]
- Zeng, J.; Weissmann, F.; Bertolin, A.P.; Posse, V.; Canal, B.; Ulferts, R.; Wu, M.; Harvey, R.; Hussain, S.; Milligan, J.C.; et al. Identifying SARS-CoV-2 antiviral compounds by screening for small molecule inhibitors of nsp13 helicase. Biochem. J. 2021, 478, 2405–2423. [Google Scholar] [CrossRef]
- Nandi, R.; Bhowmik, D.; Srivastava, R.; Prakash, A.; Kumar, D. Discovering potential inhibitors against SARS-CoV-2 by targeting Nsp13 Helicase. J. Biomol. Struct. Dyn. 2022, 40, 12062–12074. [Google Scholar] [CrossRef]
- El Hassab, M.A.; Eldehna, W.M.; Al-Rashood, S.T.; Alharbi, A.; Eskandrani, R.O.; Alkahtani, H.M.; Elkaeed, E.B.; Abou-Seri, S.M. Multi-stage structure-based virtual screening approach towards identification of potential SARS-CoV-2 NSP13 helicase inhibitors. J. Enzym. Inhib. Med. Chem. 2022, 37, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wang, F.; Jiao, W.; Zhang, M.; Xing, G.; Feng, H.; Sun, X.; Hu, M.; Zhang, G. Virtual Screening-Based Peptides Targeting Spike Protein to Inhibit Porcine Epidemic Diarrhea Virus (PEDV) Infection. Viruses 2023, 15, 381. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Yin, B.; Xing, X.; Li, Z.; Zhang, J.; Feng, S.; Li, L.; Zhao, F.; Yang, X.; Yu, S.; et al. Octyl gallate targeting the 3C-like protease exhibits highly efficient antiviral activity against swine enteric coronavirus PEDV. Vet. Microbiol. 2023, 281, 109743. [Google Scholar] [CrossRef]
- Huang, Z.X.; Zhou, S.T.; Yang, Z.B.; Wang, Z. Molnupiravir Inhibits Porcine Epidemic Diarrhea Virus Infection in vitro. Viruses 2023, 15, 1317. [Google Scholar] [CrossRef]
- Wang, P.; Bai, J.; Liu, X.; Wang, M.; Wang, X.; Jiang, P. Tomatidine inhibits porcine epidemic diarrhea virus replication by targeting 3CL protease. Vet. Res. 2020, 51, 136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, Z.; Wang, M.; Lan, M.; Zhang, K.; Jiang, P.; Li, Y.; Bai, J.; Wang, X. Cholesterol 25-hydroxylase negatively regulates porcine intestinal coronavirus replication by the production of 25-hydroxycholesterol. Vet. Microbiol. 2019, 231, 129–138. [Google Scholar] [CrossRef]
- Rastelli, G.; Del Rio, A.; Degliesposti, G.; Sgobba, M. Fast and accurate predictions of binding free energies using MM-PBSA and MM-GBSA. J. Comput. Chem. 2010, 31, 797–810. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.M.; Wang, W.; Song, Z.G.; Hu, Y.; Tao, Z.W.; Tian, J.H.; Pei, Y.Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- Adedeji, A.O.; Marchand, B.; Te Velthuis, A.J.; Snijder, E.J.; Weiss, S.; Eoff, R.L.; Singh, K.; Sarafianos, S.G. Mechanism of nucleic acid unwinding by SARS-CoV helicase. PLoS ONE 2012, 7, e36521. [Google Scholar] [CrossRef]
- Lu, L.; Peng, Y.; Yao, H.; Wang, Y.; Li, J.; Yang, Y.; Lin, Z. Punicalagin as an allosteric NSP13 helicase inhibitor potently suppresses SARS-CoV-2 replication in vitro. Antivir. Res. 2022, 206, 105389. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, X.; Liu, X.; Sun, M.; Liang, X.; Bai, J.; Jiang, P. Natural Compound ZINC12899676 Reduces Porcine Epidemic Diarrhea Virus Replication by Inhibiting the Viral NTPase Activity. Front. Pharmacol. 2022, 13, 879733. [Google Scholar] [CrossRef]
- Kuzikov, M.; Reinshagen, J.; Wycisk, K.; Corona, A.; Esposito, F.; Malune, P.; Manelfi, C.; Iaconis, D.; Beccari, A.; Tramontano, E.; et al. Drug repurposing screen to identify inhibitors of the RNA polymerase (nsp12) and helicase (nsp13) from SARS-CoV-2 replication and transcription complex. Virus Res. 2024, 343, 199356. [Google Scholar] [CrossRef] [PubMed]
- Mehyar, N.; Mashhour, A.; Islam, I.; Alhadrami, H.A.; Tolah, A.M.; Alghanem, B.; Alkhaldi, S.; Somaie, B.A.; Al Ghobain, M.; Alobaida, Y.; et al. Discovery of Zafirlukast as a novel SARS-CoV-2 helicase inhibitor using in silico modelling and a FRET-based assay. SAR QSAR Environ. Res. 2021, 32, 963–983. [Google Scholar] [CrossRef] [PubMed]
- Corona, A.; Madia, V.N.; De Santis, R.; Manelfi, C.; Emmolo, R.; Ialongo, D.; Patacchini, E.; Messore, A.; Amatore, D.; Faggioni, G.; et al. Diketo acid inhibitors of nsp13 of SARS-CoV-2 block viral replication. Antivir. Res. 2023, 217, 105697. [Google Scholar] [CrossRef]
- Soper, N.; Yardumian, I.; Chen, E.; Yang, C.; Ciervo, S.; Oom, A.L.; Desvignes, L.; Mulligan, M.J.; Zhang, Y.; Lupoli, T.J. A Repurposed Drug Interferes with Nucleic Acid to Inhibit the Dual Activities of Coronavirus Nsp13. ACS Chem. Biol. 2024, 19, 1593–1603. [Google Scholar] [CrossRef]
- Mergenhagen, K.A.; Borton, A.R. Vancomycin nephrotoxicity: A review. J. Pharm. Pract. 2014, 27, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Humanes, B.; Jado, J.C.; Camaño, S.; López-Parra, V.; Torres, A.M.; Álvarez-Sala, L.A.; Cercenado, E.; Tejedor, A.; Lázaro, A. Protective Effects of Cilastatin against Vancomycin-Induced Nephrotoxicity. BioMed Res. Int. 2015, 2015, 704382. [Google Scholar] [CrossRef]
- Courvalin, P. Vancomycin resistance in gram-positive cocci. Clin. Infect. Dis. 2006, 42 (Suppl. S1), S25–S34. [Google Scholar] [CrossRef]
| Name | 5′−3′ | Label | kDa |
|---|---|---|---|
| RNA1 | CAUACAGUACAGGGAUCACCUCAGUUCGACUAUCGAGUAAUC | 5′-Cy5 | 13.86 |
| RNA2 | GAUUACUCGAUAGUCGAACUGAGG | 7.92 | |
| RNA3 | CCUCAGUUCGACUAUCGAGUAAUC | 7.92 |
| Name | 5′-3′ |
|---|---|
| PEDV N-Forward | TTCTTGTTTCACAGGTGGATG |
| PEDV N-Reverse | GCTGCTGCGTGGTTTCA |
| GAPDH-Forward | CCTTCCGTGTCCCTACTGCCAAC |
| GAPDH-Reverse | GACGCCTGCTTCACCACCTTCT |
| Compound | Binding Energy (kcal/mol) |
|---|---|
| Linaclotide | −18.1 |
| Vancomycin | −16.3 |
| Quinupristin | −14.8 |
| Deslanoside | −14.0 |
| Gamithromycin | −13.0 |
| Thiostrepton | −12.4 |
| Amcinonide | −11.7 |
| BMS-927711 | −11.4 |
| Glecaprevir | −11.2 |
| Lanreotide | −11.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Yu, M.; Guo, J.; Qiu, J.; Liu, F.; Shan, Y. Repurposing Vancomycin as a Potential Antiviral Agent Against PEDV via nsp13 Helicase Inhibition. Animals 2025, 15, 923. https://doi.org/10.3390/ani15070923
Chen Q, Yu M, Guo J, Qiu J, Liu F, Shan Y. Repurposing Vancomycin as a Potential Antiviral Agent Against PEDV via nsp13 Helicase Inhibition. Animals. 2025; 15(7):923. https://doi.org/10.3390/ani15070923
Chicago/Turabian StyleChen, Qiao, Mengqi Yu, Jiajing Guo, Jingqi Qiu, Fei Liu, and Yanke Shan. 2025. "Repurposing Vancomycin as a Potential Antiviral Agent Against PEDV via nsp13 Helicase Inhibition" Animals 15, no. 7: 923. https://doi.org/10.3390/ani15070923
APA StyleChen, Q., Yu, M., Guo, J., Qiu, J., Liu, F., & Shan, Y. (2025). Repurposing Vancomycin as a Potential Antiviral Agent Against PEDV via nsp13 Helicase Inhibition. Animals, 15(7), 923. https://doi.org/10.3390/ani15070923





