Preliminary Exploration of MAGE-B1, -B4, -B5, and -B10 mRNA Expression in Canine Mammary Tumors in Dogs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Samples
2.2. Primary Cell Culture
2.3. 5-aza-CdR and TSA Treatment of Cells
2.4. Total RNA Extraction of Tissue Specimen
2.5. Total RNA Extraction of Cell Culture
2.6. cDNA Synthesis
2.7. qPCR Methods
2.7.1. Primer Design
2.7.2. Primer Selection
2.7.3. Standard Curve and Regression Analysis for qPCR Conditions
2.7.4. Sensitivity Evaluation of Real-Time PCR Method
2.7.5. qPCR Conditions
2.8. Data Analysis
3. Results
3.1. Primer Design, Specificity, and Sensitivity
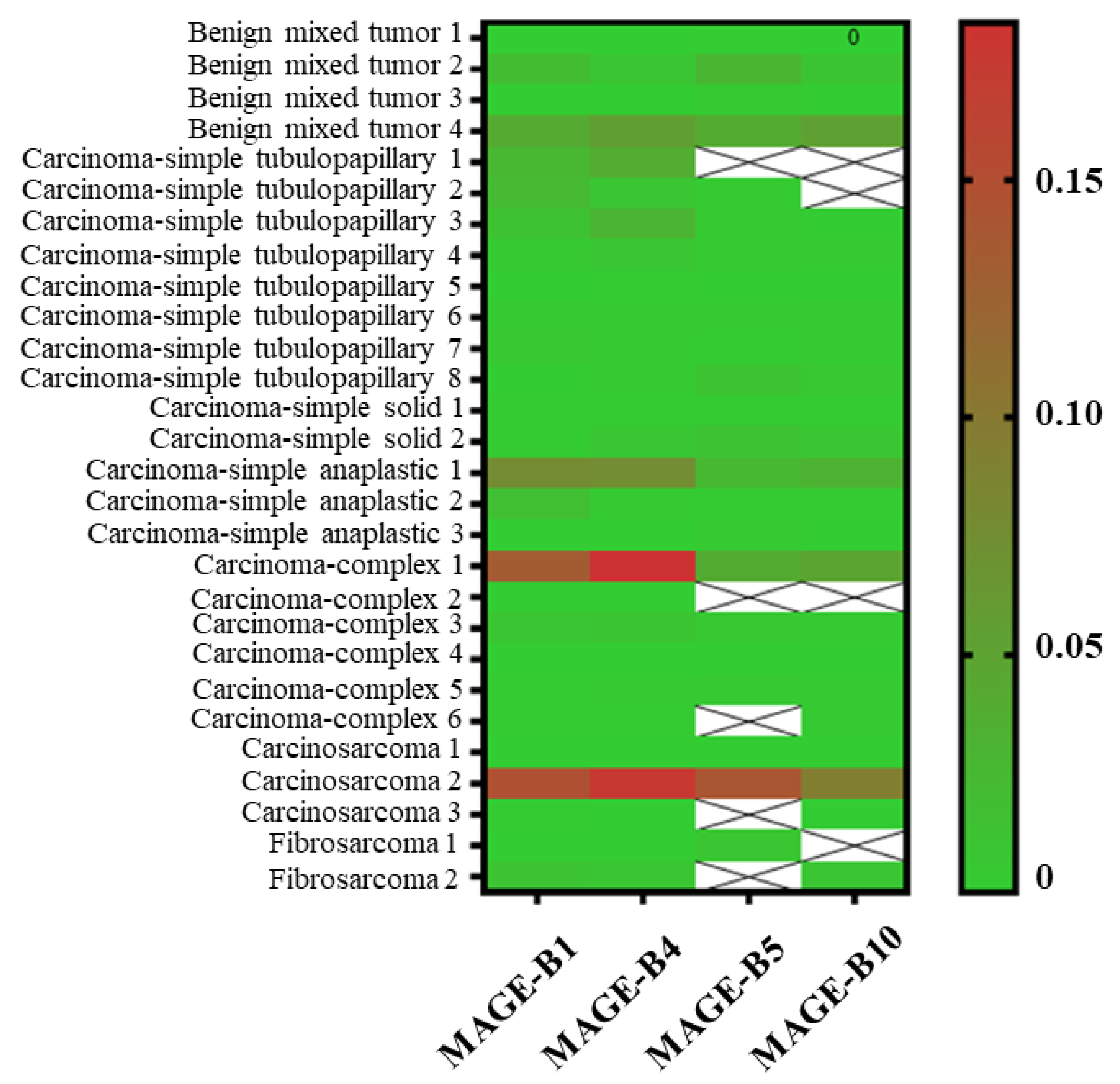
3.2. The Proportion of MAGE-B1, -B4, -B5, and -B10 mRNA Expression in CMTs
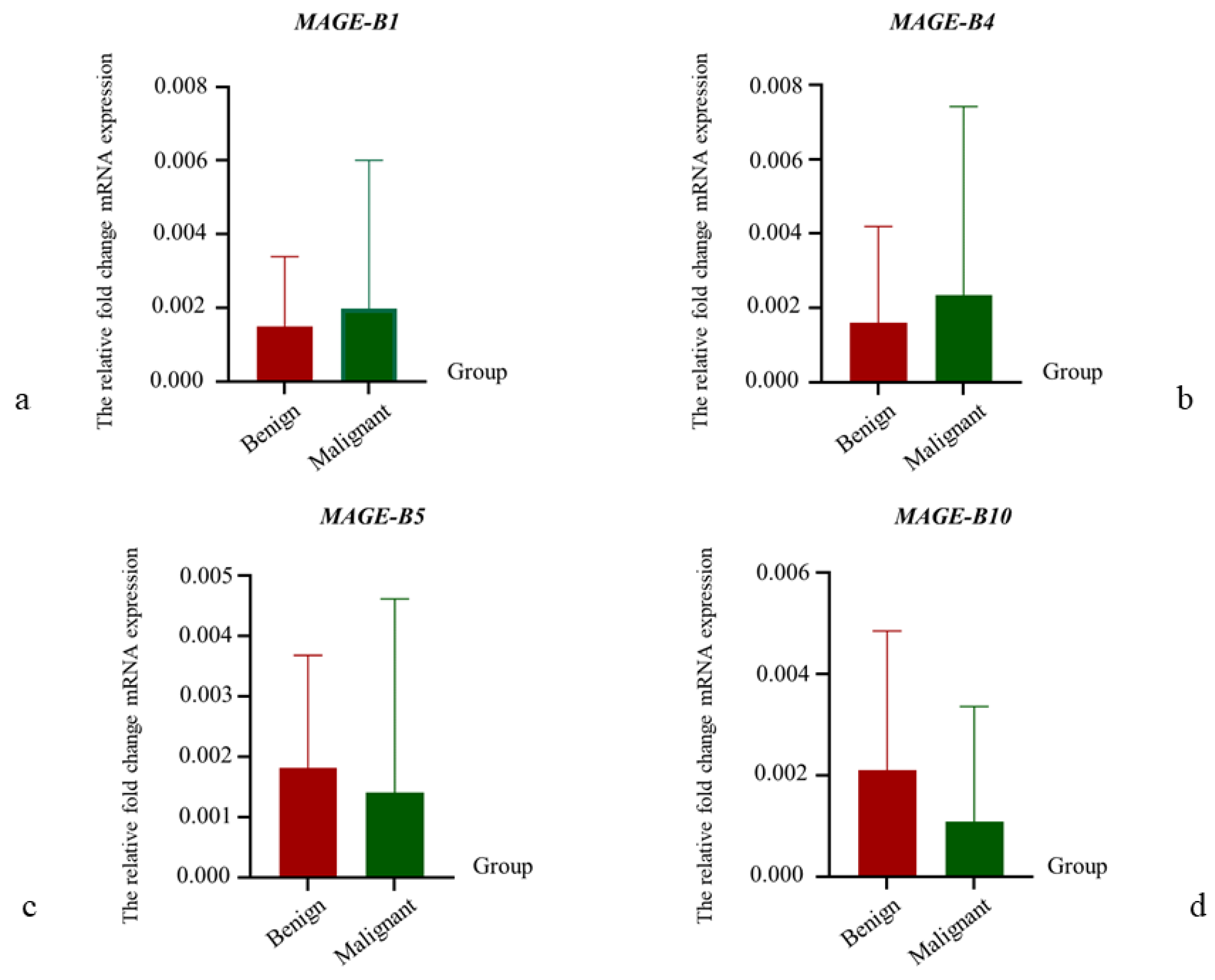
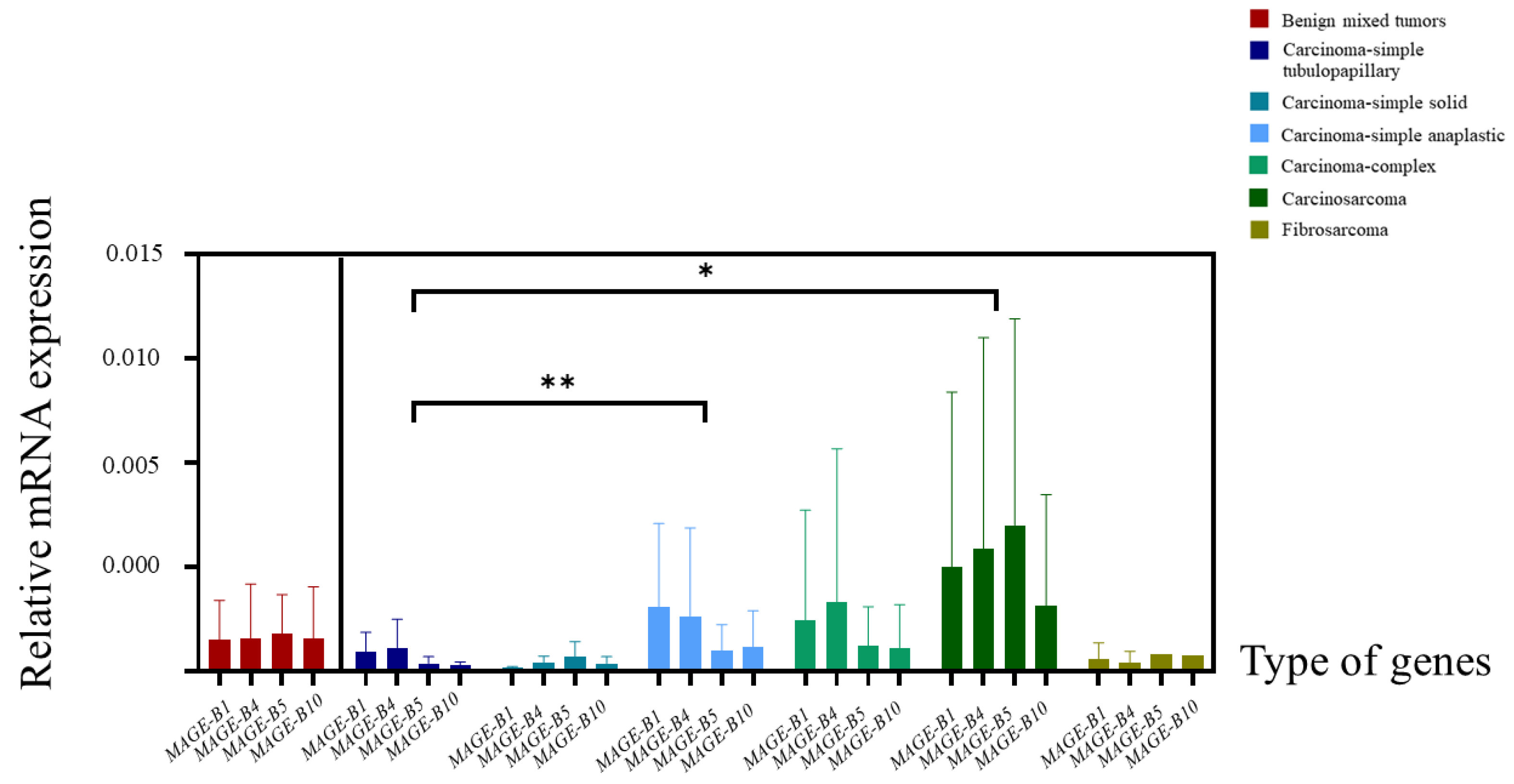
3.3. The Relative mRNA Expression of MAGE-B1, -B4, -B5, and -B10 in CMT Tissues
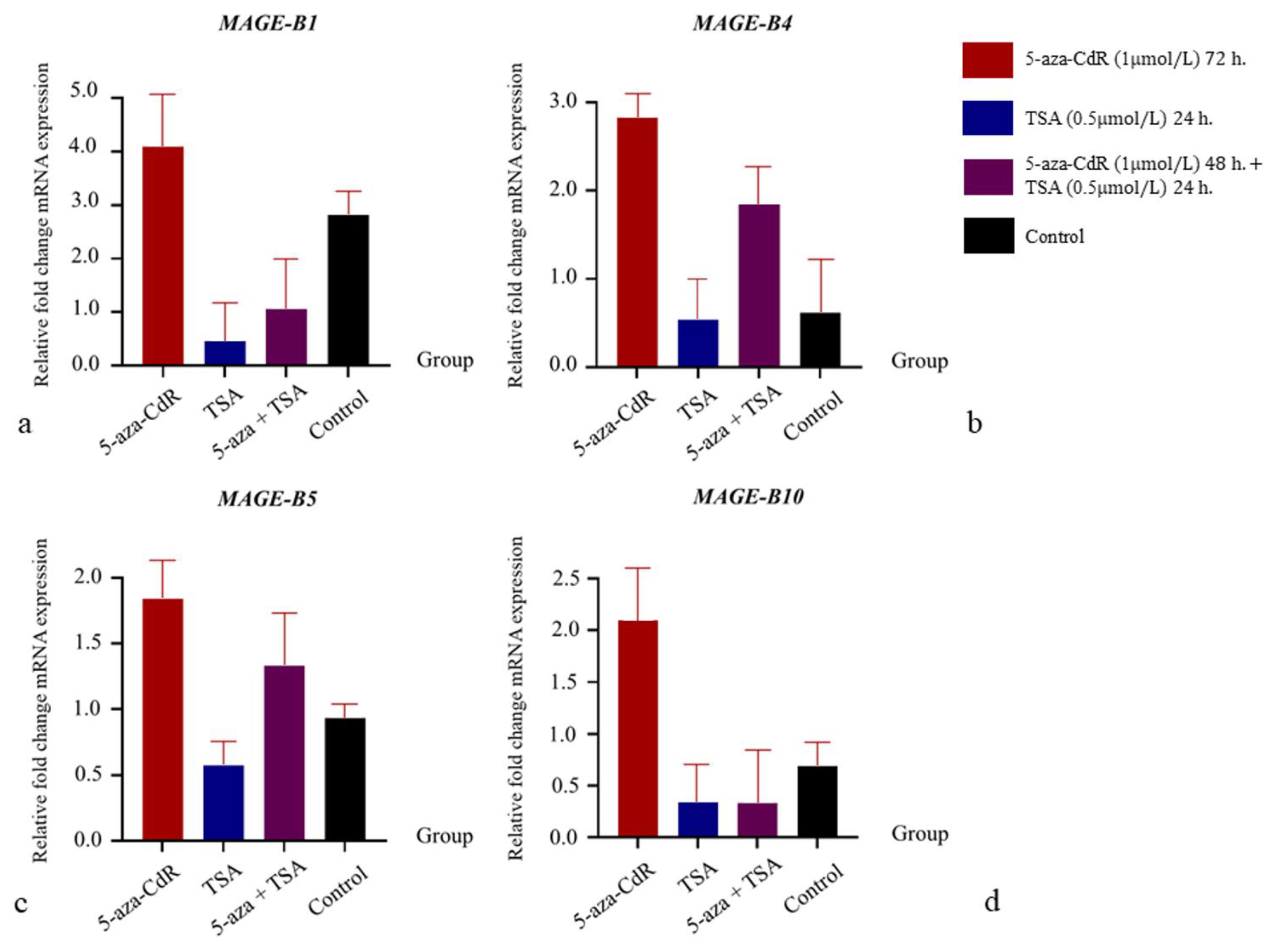
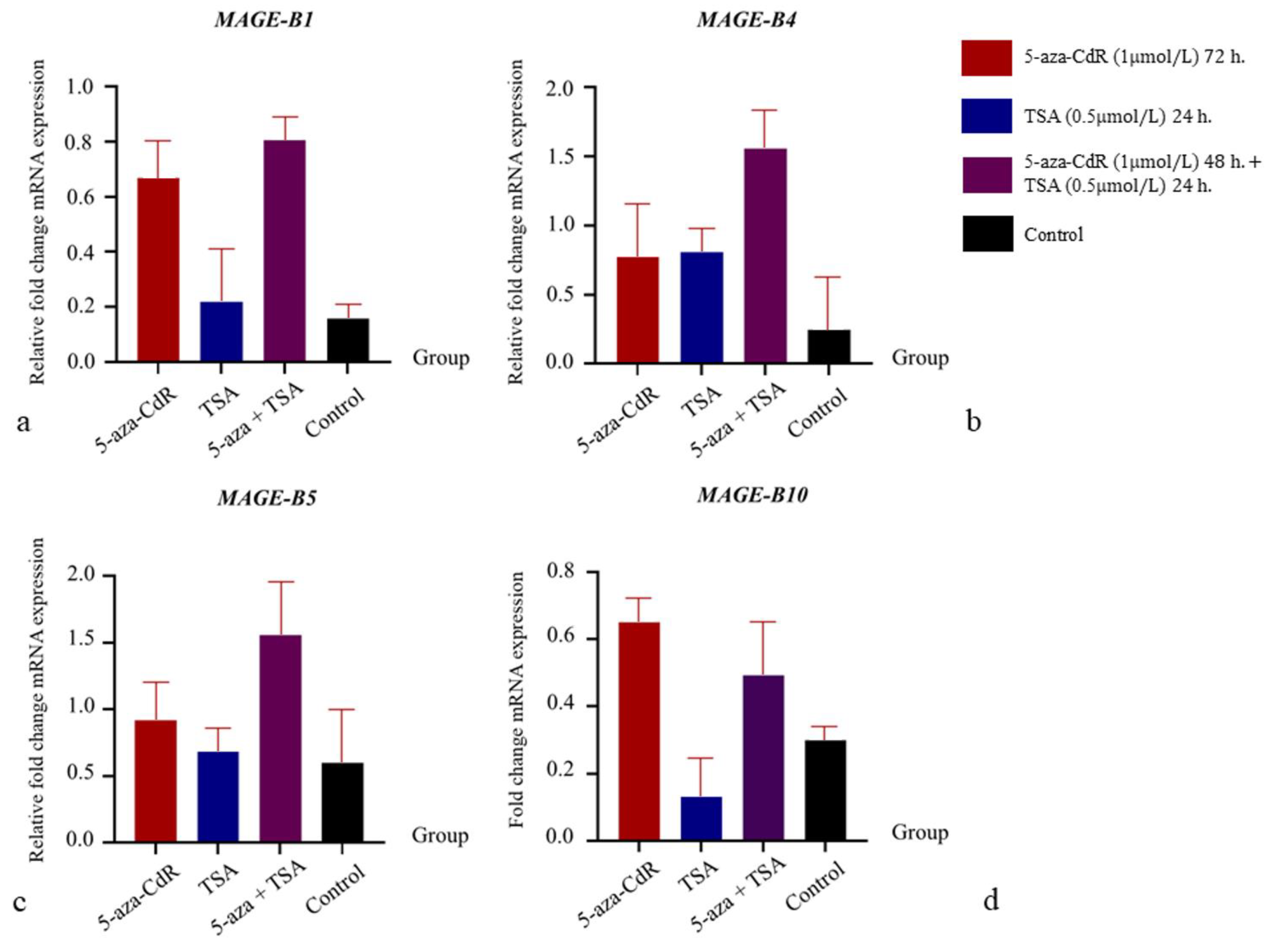
3.4. The Influence of 5-aza-CdR and TSA on mRNA Expression Relations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Misdorp, W. Tumors of the mammary gland. In Tumors in Domestic Animals, 1st ed.; Meuten, D.J., Ed.; Blackwell Press: Oxford, UK, 2002; pp. 575–606. [Google Scholar]
- Salas, Y.; Márquez, A.; Diaz, D.; Romero, L. Epidemiological study of mammary tumors in female dogs diagnosed during the period 2002–2012: A growing animal health problem. PLoS ONE 2015, 10, e0127381. [Google Scholar]
- Srisawat, W.; Pringproa, K.; Prachasilchai, W.; Thongtharb, A.; Sthitmatee, N. Epidemiology and classification for canine and feline mammary gland tumors: A histopathological survey of 437 mammary gland tumor biopsies performed in a secondary care hospital in Chiang Mai, Thailand from 2012 to 2019. PeerJ 2024, 12, e17077. [Google Scholar] [PubMed]
- Lana, S.E.; Rutteman, G.R.; Withrow, S.J. Tumors of the mammary gland. In Small Animal Clinical Oncology, 1st ed.; Withrow, S.J., Vail, D.M., Eds.; Elsevier Press: St. Louis, MO, USA, 2007; pp. 619–636. [Google Scholar]
- Saba, C.F.; Rogers, K.S.; Newman, S.J.; Mauldin, G.E.; Vail, D.M. Mammary gland tumors in male dogs. J. Vet. Intern. Med. 2007, 21, 1056–1059. [Google Scholar]
- Vascellari, M.; Capello, K.; Carminato, A.; Zanardello, C.; Baioni, E.; Mutinelli, F. Incidence of mammary tumors in the canine population living in the Veneto region (Northeastern Italy): Risk factors and similarities to human breast cancer. Prev. Vet. Med. 2016, 126, 183–189. [Google Scholar]
- Marconato, L.; Romanelli, G.; Stefanello, D.; Giacaoboni, C.; Bonfanti, U.; Bettini, G.; Finotello, R.; Verganti, S.; Valenti, P.; Ciaramella, L.; et al. Prognostic factors for dogs with mammary inflammatory carcinoma: 43 cases (2003–2008). J. Am. Vet. Med. Assoc. 2009, 235, 972–976. [Google Scholar]
- Valdivia, G.; Alonso-Diez, Á.; Pérez-Alenza, D.; Peña, L. From Conventional to Precision Therapy in Canine Mammary Cancer: A Comprehensive Review. Front. Vet. Sci. 2021, 8, 623800. [Google Scholar] [CrossRef]
- Vazquez, E.; Lipovka, Y.; Cervantes-Arias, A.; Garibay-Escobar, A.; Haby, M.M.; Queiroga, F.L.; Velazquez, C. Canine Mammary Cancer: State of the Art and Future Perspectives. Animals 2023, 13, 3147. [Google Scholar] [CrossRef]
- Sorenmo, K. Canine mammary gland tumors. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 573–596. [Google Scholar]
- Beauvais, W.; Cardwell, J.M.; Brodbelt, D.C. The effect of neutering on the risk of mammary tumours in dogs–a systematic review. J. Small Anim. Pract. 2012, 53, 314–322. [Google Scholar]
- Kristiansen, V.M.; Peña, L.; Díez Córdova, L.; Illera, J.C.; Skjerve, E.; Breen, A.M.; Cofone, M.A.; Langeland, M.; Teige, J.; Goldschmidt, M.; et al. Effect of ovariohysterectomy at the time of tumor removal in dogs with mammary carcinomas: A randomized controlled trial. J. Vet. Intern. Med. 2016, 30, 230–241. [Google Scholar]
- Karayannopoulou, M.; Kaldrymidou, E.; Constantinidis, T.C.; Dessiris, A. Adjuvant post-operative chemotherapy in bitches with mammary cancer. J. Vet. Med. A. 2001, 48, 85–96. [Google Scholar]
- Simon, D.; Schoenrock, D.; Baumgärtner, W.; Nolte, I. Postoperative adjuvant treatment of invasive malignant mammary gland tumors in dogs with doxorubicin and docetaxel. J. Vet. Intern. Med. 2006, 20, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Marconato, L.; Lorenzo, R.M.; Abramo, F.; Ratto, A.; Zini, E. Adjuvant gemcitabine after surgical removal of aggressive malignant mammary tumours in dogs. Vet. Comp. Oncol. 2008, 6, 90–101. [Google Scholar] [PubMed]
- Lavalle, G.E.; De Campos, C.B.; Bertagnolli, A.C.; Cassali, G.D. Canine malignant mammary gland neoplasms with advanced clinical staging treated with carboplatin and cyclooxygenase inhibitors. In Vivo 2012, 26, 375–379. [Google Scholar]
- Nosalova, N.; Huniadi, M.; Horňáková, L.; Valenčáková, A.; Horňák, S.; Nagoos, K.; Vozar, J.; Cizkova, D. Canine mammary tumors: Classification, biomarkers, traditional and personalized Therapies. Int. J. Mol. Sci. 2024, 25, 2891. [Google Scholar] [CrossRef]
- Martínez-Lostao, L.; Anel, A.; Pardo, J. How do cytotoxic lymphocytes kill cancer cells? Clin. Cancer Res. 2015, 21, 5047–5056. [Google Scholar]
- van der Bruggen, P.; Traversari, C.; Chomez, P.; Lurquin, C.; De Plaen, E.; Van den Eynde, B.; Knuth, A.; Boon, T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 1991, 254, 1643–1647. [Google Scholar]
- Brasseur, F.; Rimoldi, D.; Liénard, D.; Lethé, B.; Carrel, S.; Arienti, F.; Suter, L.; Vanwijck, R.; Bourlond, A.; Humblet, Y.; et al. Expression of MAGE genes in primary and metastatic cutaneous melanoma. Int. J. Cancer 1995, 63, 375–380. [Google Scholar]
- Mori, M.; Inoue, H.; Mimori, K.; Shibuta, K.; Baba, K.; Nakashima, H.; Haraguchi, M.; Tsuji, K.; Ueo, H.; Barnard, G.F.; et al. Expression of MAGE genes in human colorectal carcinoma. Ann. Surg. 1996, 224, 183–188. [Google Scholar]
- Tajima, K.; Obata, Y.; Tamaki, H.; Yoshida, M.; Chen, Y.T.; Scanlan, M.J.; Old, L.J.; Kuwano, H.; Takahashi, T.; Takahashi, T.; et al. Expression of cancer/testis (CT) antigens in lung cancer. Lung Cancer 2003, 42, 23–33. [Google Scholar]
- Simpson, A.J.; Caballero, O.L.; Jungbluth, A.; Chen, Y.T.; Old, L.J. Cancer/testis antigens, gametogenesis and cancer. Nat. Rev. Cancer 2005, 5, 615–625. [Google Scholar] [PubMed]
- Barrow, C.; Browning, J.; MacGregor, D.; Davis, I.D.; Sturrock, S.; Jungbluth, A.A.; Cebon, J. Tumor antigen expression in melanoma varies according to antigen and stage. Clin. Cancer Res. 2006, 12, 764–771. [Google Scholar] [PubMed]
- Chomez, P.; De Backer, O.; Bertrand, M.; De Plaen, E.; Boon, T.; Lucas, S. An overview of the MAGE gene family with the identification of all human members of the family. Cancer Res. 2001, 61, 5544–5551. [Google Scholar] [PubMed]
- Lee, A.K.; Potts, P.R. A comprehensive guide to the MAGE family of ubiquitin ligases. J. Mol. Biol. 2017, 429, 1114–1142. [Google Scholar]
- Gillespie, A.M.; Coleman, R.E. The potential of melanoma antigen expression in cancer therapy. Cancer Treat. Rev. 1999, 25, 219–227. [Google Scholar]
- Barker, P.A.; Salehi, A. The MAGE proteins: Emerging roles in cell cycle progression, apoptosis, and neurogenetic disease. J. Neurosci. Res. 2002, 67, 705–712. [Google Scholar]
- Colemon, A.; Harri, T.M.; Ramanathan, S. DNA hypomethylation drives changes in MAGE-A gene expression resulting in alteration of proliferative status of cells. Genes Environ. 2020, 42, 24. [Google Scholar]
- Lurquin, C.; De Smet, C.; Brasseur, F.; Muscatelli, F.; Martelange, V.; De Plaen, E.; Brasseur, R.; Monaco, A.P.; Boon, T. Two members of the humanmagebgene family located in Xp21. 3 are expressed in tumors of various histological origins. Genomics 1997, 46, 397–408. [Google Scholar]
- Nagashima, H.; Sadanaga, N.; Mashino, K.; Yamashita, K.; Inoue, H.; Mori, M.; Sugimachi, K. Expression of MAGE-B genes in esophageal squamous cell carcinoma. Jpn. J. Cancer Res. 2001, 92, 167–173. [Google Scholar]
- Tsai, J.R.; Chong, I.W.; Chen, Y.H.; Yang, M.J.; Sheu, C.C.; Chang, H.C.; Hwang, J.J.; Hung, J.Y.; Lin, S.R. Differential expression profile of MAGE family in non-small-cell lung cancer. Lung Cancer 2007, 56, 185–192. [Google Scholar]
- Chung, F.Y.; Cheng, T.L.; Chang, H.J.; Chiu, H.H.; Huang, M.Y.; Chang, M.S.; Chen, C.C.; Yang, M.J.; Wang, J.Y.; Lin, S.R. Differential gene expression profile of MAGE family in taiwanese patients with colorectal cancer. J. Surg. Oncol. 2010, 102, 148–153. [Google Scholar] [PubMed]
- Sypniewska, R.K.; Hoflack, L.; Tarango, M.; Gauntt, S.; Leal, B.Z.; Reddick, R.L.; Gravekamp, C. Prevention of metastases with a Mage-b DNA vaccine in a mouse breast tumor model: Potential for breast cancer therapy. Breast Cancer Res. Treat. 2005, 91, 19–28. [Google Scholar] [PubMed]
- Gravekamp, C.; Leal, B.; Denny, A.; Bahar, R.; Lampkin, S.; Castro, F.; Kim, S.H.; Moore, D.; Reddick, R. In vivo responses to vaccination with Mage-b, GM-CSF and thioglycollate in a highly metastatic mouse breast tumor model, 4T1. Cancer Immunol. Immunother. 2008, 57, 1067–1077. [Google Scholar] [PubMed]
- Castro, F.; Leal, B.; Denny, A.; Bahar, R.; Lampkin, S.; Reddick, R.; Gravekamp, C. Vaccination with Mage-b DNA induces CD8 T-cell responses at young but not old age in mice with metastatic breast cancer. Br. J. Cancer 2009, 101, 1329–1337. [Google Scholar]
- Kim, S.H.; Castro, F.; Gonzalez, D.; Maciag, P.C.; Paterson, Y.; Gravekamp, C. Mage-b vaccine delivered by recombinant Listeria monocytogenes is highly effective against breast cancer metastases. Br. J. Cancer 2008, 99, 741–749. [Google Scholar]
- Kim, S.H.; Castro, F.; Paterson, Y.; Gravekamp, C. High efficacy of a Listeria-based vaccine against metastatic breast cancer reveals a dual mode of action. Cancer Res. 2009, 69, 5860–5866. [Google Scholar]
- Singh, M.; Ramos, I.; Asafu-Adjei, D.; Quispe-Tintaya, W.; Chandra, D.; Jahangir, A.; Zang, X.; Aggarwal, B.B.; Gravekamp, C. Curcumin improves the therapeutic efficacy of Listeria-Mage-b vaccine in correlation with improved T-cell responses in blood of a triple-negative breast cancer model 4T1. Cancer Med. 2013, 2, 571–582. [Google Scholar]
- Klose, R.J.; Bird, A.P. Genomic DNA methylation: The mark and its mediators. Trends Biochem. Sci. 2006, 31, 89–97. [Google Scholar]
- Cameron, E.E.; Bachman, K.E.; Myöhänen, S.; Herman, J.G.; Baylin, S.B. Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat. Genet. 1999, 21, 103–107. [Google Scholar]
- Picard, V.; Bergeron, A.; LaRue, H.; Fradet, Y. MAGE-A9 mRNA and protein expression in bladder cancer. Int. J. Cancer 2007, 120, 2170–2177. [Google Scholar]
- Adair, S.J.; Hogan, K.T. Treatment of ovarian cancer cell lines with 5-aza-2’-deoxycytidine upregulates the expression of cancer-testis antigens and class I major histocompatibility complex-encoded molecules. Cancer Immunol. Immunother. 2009, 58, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.Y.; Sang, M.X.; Geng, C.Z.; Liu, W.H.; Lü, W.H.; Xu, Y.Y.; Shan, B.E. Expressions of MAGE-A9 and MAGE-A11 in breast cancer and their expression mechanism. Arch. Med. Res. 2014, 45, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Sang, M.; Zhao, L.; Hou, R.; Shan, B. The expression of MAGE-C1 and MAGE-C2 in breast cancer and their clinical significance. Am. J. Surg. 2016, 211, 142–151. [Google Scholar] [CrossRef]
- Goldschmidt, M.; Peña, L.; Rasotto, R.; Zappulli, V. Classification and grading of canine mammary tumors. Vet. Pathol. 2011, 48, 117–131. [Google Scholar] [CrossRef]
- Fhaikrue, I.; Srisawat, W.; Nambooppha, B.; Pringproa, K.; Thongtharb, A.; Prachasilchai, W.; Sthitmatee, N. Identification of potential canine mammary tumour cell biomarkers using proteomic approach: Differences in protein profiles among tumour and normal mammary epithelial cells by two-dimensional electrophoresis-based mass spectrometry. Vet. Comp. Oncol. 2020, 18, 787–795. [Google Scholar] [CrossRef]
- Wischnewski, F.; Pantel, K.; Schwarzenbach, H. Promoter demethylation and histone acetylation mediate gene expression of MAGE-A1, -A2, -A3, and -A12 in human cancer cells. Mol. Cancer Res. 2006, 4, 339–349. [Google Scholar] [CrossRef]
- Skrypina, N.A.; Timofeeva, A.V.; Khaspekov, G.L.; Savochkina, L.P.; Beabealashvilli, R.S. Total RNA suitable for molecular biology analysis. J. Biotechnol. 2003, 105, 1–9. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Weon, J.L.; Potts, P.R. The MAGE protein family and cancer. Curr. Opin. Cell Biol. 2015, 37, 1–8. [Google Scholar] [CrossRef]
- Lucas, S.; De Plaen, E.; Boon, T. MAGE-B5, MAGE-B6, MAGE-C2, and MAGE-C3: Four new members of the MAGE family with tumor-specific expression. Int. J. Cancer 2000, 87, 55–60. [Google Scholar] [CrossRef]
- Fleige, S.; Pfaf, M.W. RNA integrity and the effect on the real-time qRT-PCR performance. Mol. Aspects Med. 2006, 27, 126–139. [Google Scholar] [PubMed]
- Rutledge, R.G.; Stewart, D. Critical evaluation of methods used to determine amplification efficiency refutes the exponential character of real-time PCR. BMC Mol. Biol. 2008, 9, 1–2. [Google Scholar]
- Jaillon, O.; Bouhouche, K.; Gout, J.F.; Aury, J.M.; Noel, B.; Saudemont, B.; Nowacki, M.; Serrano, V.; Porcel, B.M.; Ségurens, B.; et al. Translational control of intron splicing in eukaryotes. Nature 2008, 451, 359–362. [Google Scholar] [PubMed]
- Almutairi, M.H.; Alotaibi, M.M.; Alonaizan, R.; Almutairi, B.O. Expression profile of MAGE-B1 gene and its hypomethylation activation in colon cancer. BioMed Res. Int. 2022, 2022, 6066567. [Google Scholar]
- Almatrafi, A.M.; Alamery, S.; Almutairi, M.H. Expression pattern analysis of the MAGE family genes in breast cancer patients and hypomethylation activation in the MCF-7 cells. Heliyon 2024, 10, e34506. [Google Scholar] [CrossRef]
- Chia-Ho, C.; Yang, M.J.; Wang, J.Y.; Lin, S.R. Study of the MAGE family in carcinogenesis and clinical application of human colorectal cancer. Clin. Cancer Res. 2008, 14, B4. [Google Scholar]
- Yang, B.; O‘Herrin, S.; Wu, J.; Reagan-Shaw, S.; Ma, Y.; Nihal, M.; Longley, B.J. Select cancer testes antigens of the MAGE-A,-B, and-C families are expressed in mast cell lines and promote cell viability in vitro and in vivo. J. Investig. Dermatol. 2007, 127, 267–275. [Google Scholar]
- Yang, B.; O‘Herrin, S.M.; Wu, J.; Reagan-Shaw, S.; Ma, Y.; Bhat, K.M.; Gravekamp, C.; Setaluri, V.; Peters, N.; Hoffmann, F.M.; et al. MAGE-A, mMage-b, and MAGE-C proteins form complexes with KAP1 and suppress p53-dependent apoptosis in MAGE-positive cell lines. Cancer Res. 2007, 67, 9954–9962. [Google Scholar] [CrossRef]
- Rasotto, R.; Berlato, D.; Goldschmidt, M.H.; Zappulli, V. Prognostic significance of canine mammary tumor histologic subtypes: An observational cohort study of 229 cases. Vet. Pathol. 2017, 54, 571–578. [Google Scholar] [CrossRef]
- Jones, P.A.; Baylin, S.B. The fundamental role of epigenetic events in cancer. Nat. Rev. Genet. 2002, 3, 415–428. [Google Scholar]
- Coral, S.; Sigalotti, L.; Altomonte, M.; Engelsberg, A.; Colizzi, F.; Cattarossi, L.; Maraskovsky, E.; Jager, E.; Seliger, B.; Maio, M. 5-aza-2′-Deoxycytidine-induced Expression of Functional Cancer Testis Antigens in Human Renal Cell Carcinoma: Immunotherapeutic Implications. Clin. Cancer Res. 2002, 8, 2690–2695. [Google Scholar] [PubMed]
- Weinert, B.T.; Krishnadath, K.K.; Milano, F.; Pedersen, A.W.; Claesson, M.H.; Zocca, M. Real-time PCR analysis of genes encoding tumor antigens in esophageal tumors and a cancer vaccine. Cancer Immun. 2009, 9, 1–11. [Google Scholar]
- Momparler, R.L. Epigenetic therapy of cancer with 5-aza-2’-deoxycytidine (decitabine). Semin. Oncol. 2005, 32, 443–451. [Google Scholar] [PubMed]
- Kim, K.H.; Choi, J.S.; Kim, I.J.; Ku, J.L.; Park, J.G. Promoter hypomethylation and reactivation of MAGE-A1 and MAGE-A3 genes in colorectal cancer cell lines and cancer tissues. World J. Gastroenterol. 2006, 12, 5651–5657. [Google Scholar]
- Vigushin, D.M.; Ali, S.; Pace, P.E.; Mirsaidi, N.; Ito, K.; Adcock, I.; Coombes, R.C. Trichostatin A is a histone deacetylase inhibitor with potent antitumor activity against breast cancer in vivo. Clin. Cancer Res. 2001, 7, 971–976. [Google Scholar]
- Lian, Y.; Meng, L.; Ding, P.; Sang, M. Epigenetic regulation of MAGE family in human cancer progression-DNA methylation, histone modification, and non-coding RNAs. Clin. Epigenet. 2018, 10, 115. [Google Scholar]
- Jang, E.R.; Lim, S.; Lee, E.S.; Jeong, G.; Kim, T.; Bang, Y.; Lee, J. The histone deacetylase inhibitor trichostatin A sensitizes estrogen receptor α-negative breast cancer cells to tamoxifen. Oncogene 2004, 23, 1724–1736. [Google Scholar]
| No. | Genes | Specific Primers | Size (bp) | NCBI Database | Efficiency | R2 |
|---|---|---|---|---|---|---|
| 1 | GAPDH | 5′-GTCATCATCTCTGCTCCTTCTG-3′ | 90 | NM_001003142 | 0.90 | 0.99 |
| 5′-GCTGACAATCTTGAGGGAGTT-3′ | ||||||
| 2 | MAGE-B1 | 5′-GCCTCTTCTGAGTGTGATCTTC-3′ | 104 | XM_005641221 | 0.92 | 0.98 |
| 5′-CTACGTGCTTCCTTCCTTCATAG-3′ | ||||||
| 3 | MAGE-B4 | 5′-CATGAGCACCCTAAACCTC-3′ | 108 | XM_005641220 | 0.93 | 0.97 |
| 5′-TTGCCATTCAAGAAGATCACAC-3′ | ||||||
| 4 | MAGE-B5 | 5′-TGAGATCCTTAAGCAAGCCT-3′ | 119 | XM_014111462 | 0.88 | 0.99 |
| 5′-CATTGTTGGGTAGCTTCACT-3′ | ||||||
| 5 | MAGE-B10 | 5′-GCTGGGTCATATAGCGTTTC-3′ | 108 | NM_001003116 | 0.98 | 0.97 |
| 5′-CACTTGGTTGTCAGCACTTTC-3′ |
| Type of Tumors (Number of Samples) | Age (Mean ± S.D.) | Proportion of MAGE-B mRNA Expression (%) | |||||
|---|---|---|---|---|---|---|---|
| MAGE-B1 | MAGE-B4 | MAGE-B5 | MAGE-B10 | ||||
| Benign (4) | 10.67 ± 1.92 | 4/4 (100) | 4/4 (100) | 4/4 (100) | 4/4 (100) | ||
| Malignant (24) | 10.21 ± 2.15 | 24/24 (100) | 24/24 (100) | 79.2 (19/24) | 17/24 (70.8) | ||
| Epithelial neoplasms (19) | 10.04 ± 2.08 | 19/19 (100) | 19/19 (100) | 16/19 (84.2) | 16/19 (84.2) | ||
| Carcinoma-simple (13) | 9.08 ± 1.88 | 13/13 (100) | 13/13 (100) | 12/13 (58.3) | 10/13 (76.9) | ||
| Carcinoma-complex (6) | 9.73 ± 1.07 | 6/6 (100) | 6/6 (100) | 4/6 (71.4) | 6/6 (100) | ||
| Carcinosarcoma (3) | 11.67 ± 2.51 | 3/3 (100) | 3/3 (100) | 2/3 (75.0) | 3/3 (100) | ||
| Mesenchymal neoplasms (2) | 12.50 ± 3.53 | 2/2 (100) | 2/2 (100) | 1/2 (50) | 1/2 (50) | ||
| Total CMT (28) | 10.39 ± 2.13 | 28/28 (100) | 28/28 (100) | 23/28 (82.1) | 24/28 (85.7) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srisawat, W.; Koonyosying, P.; Muenthaisong, A.; Sangkakam, K.; Varinrak, T.; Sthitmatee, N. Preliminary Exploration of MAGE-B1, -B4, -B5, and -B10 mRNA Expression in Canine Mammary Tumors in Dogs. Animals 2025, 15, 910. https://doi.org/10.3390/ani15070910
Srisawat W, Koonyosying P, Muenthaisong A, Sangkakam K, Varinrak T, Sthitmatee N. Preliminary Exploration of MAGE-B1, -B4, -B5, and -B10 mRNA Expression in Canine Mammary Tumors in Dogs. Animals. 2025; 15(7):910. https://doi.org/10.3390/ani15070910
Chicago/Turabian StyleSrisawat, Wanwisa, Pongpisid Koonyosying, Anucha Muenthaisong, Kanokwan Sangkakam, Thanya Varinrak, and Nattawooti Sthitmatee. 2025. "Preliminary Exploration of MAGE-B1, -B4, -B5, and -B10 mRNA Expression in Canine Mammary Tumors in Dogs" Animals 15, no. 7: 910. https://doi.org/10.3390/ani15070910
APA StyleSrisawat, W., Koonyosying, P., Muenthaisong, A., Sangkakam, K., Varinrak, T., & Sthitmatee, N. (2025). Preliminary Exploration of MAGE-B1, -B4, -B5, and -B10 mRNA Expression in Canine Mammary Tumors in Dogs. Animals, 15(7), 910. https://doi.org/10.3390/ani15070910








