Ecological Connectivity for Reptiles in Agroecosystems: A Case Study with Olive Groves in Liguria (Northwestern Italy)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
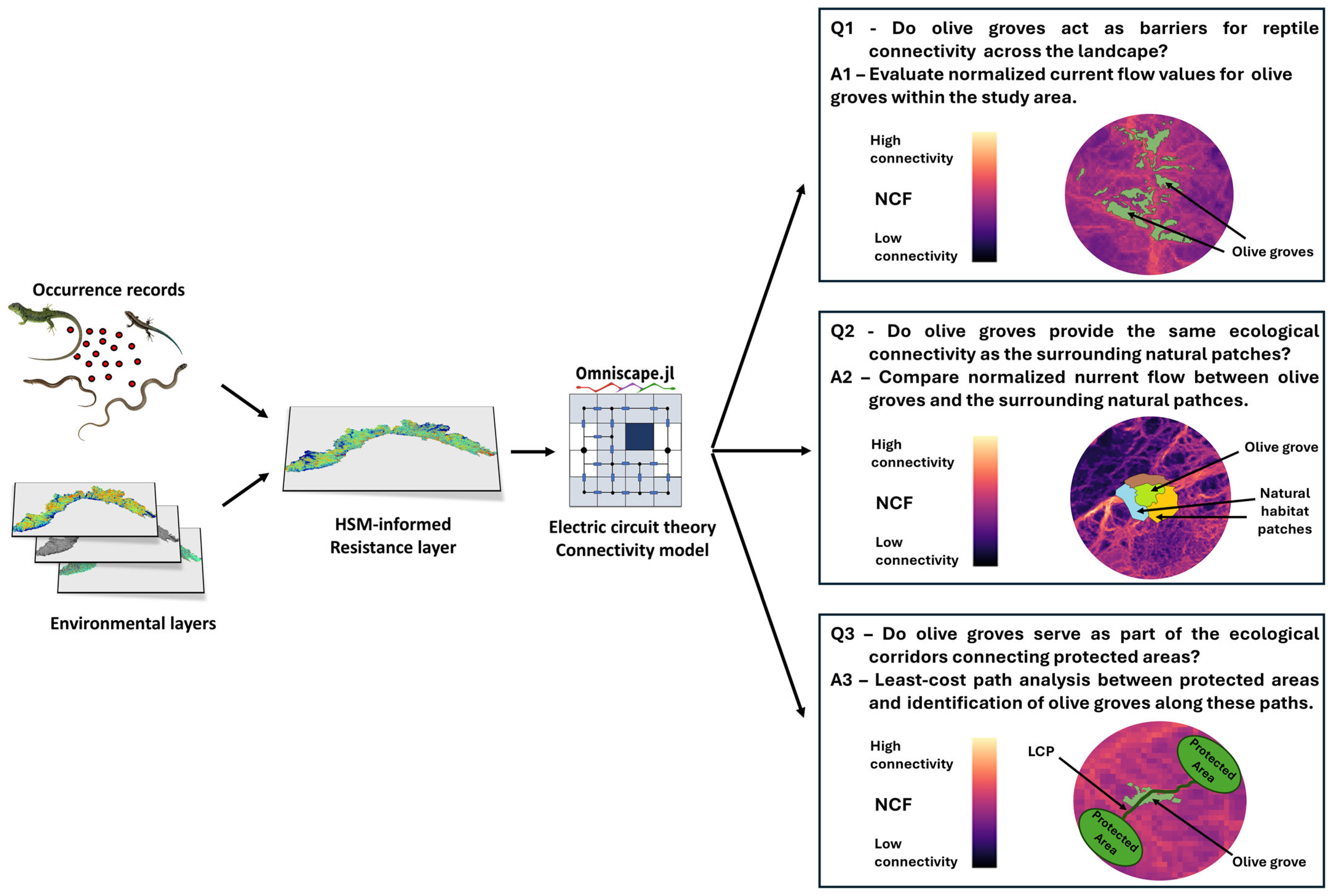
2.1. Study Rationale and Framework
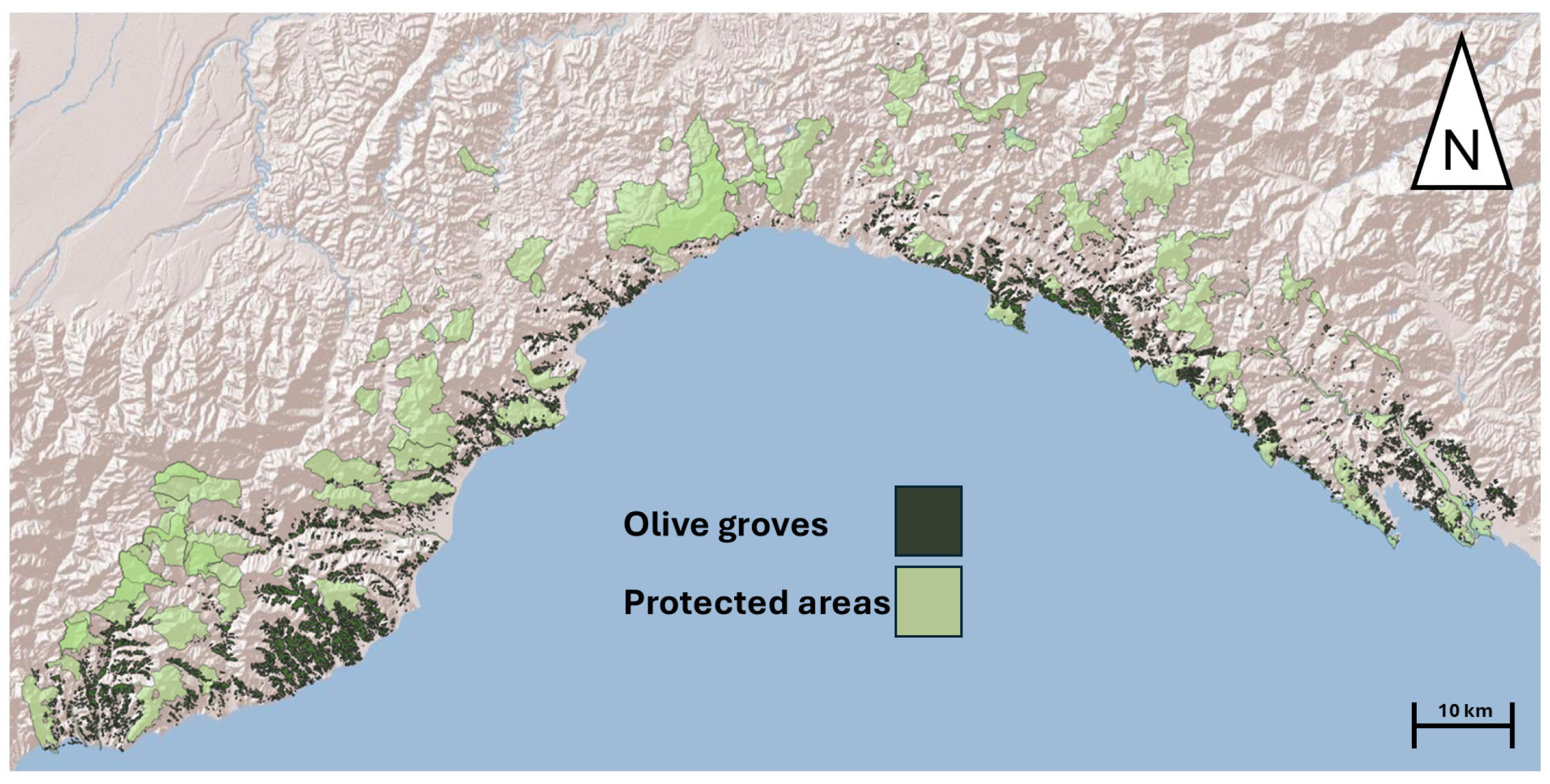
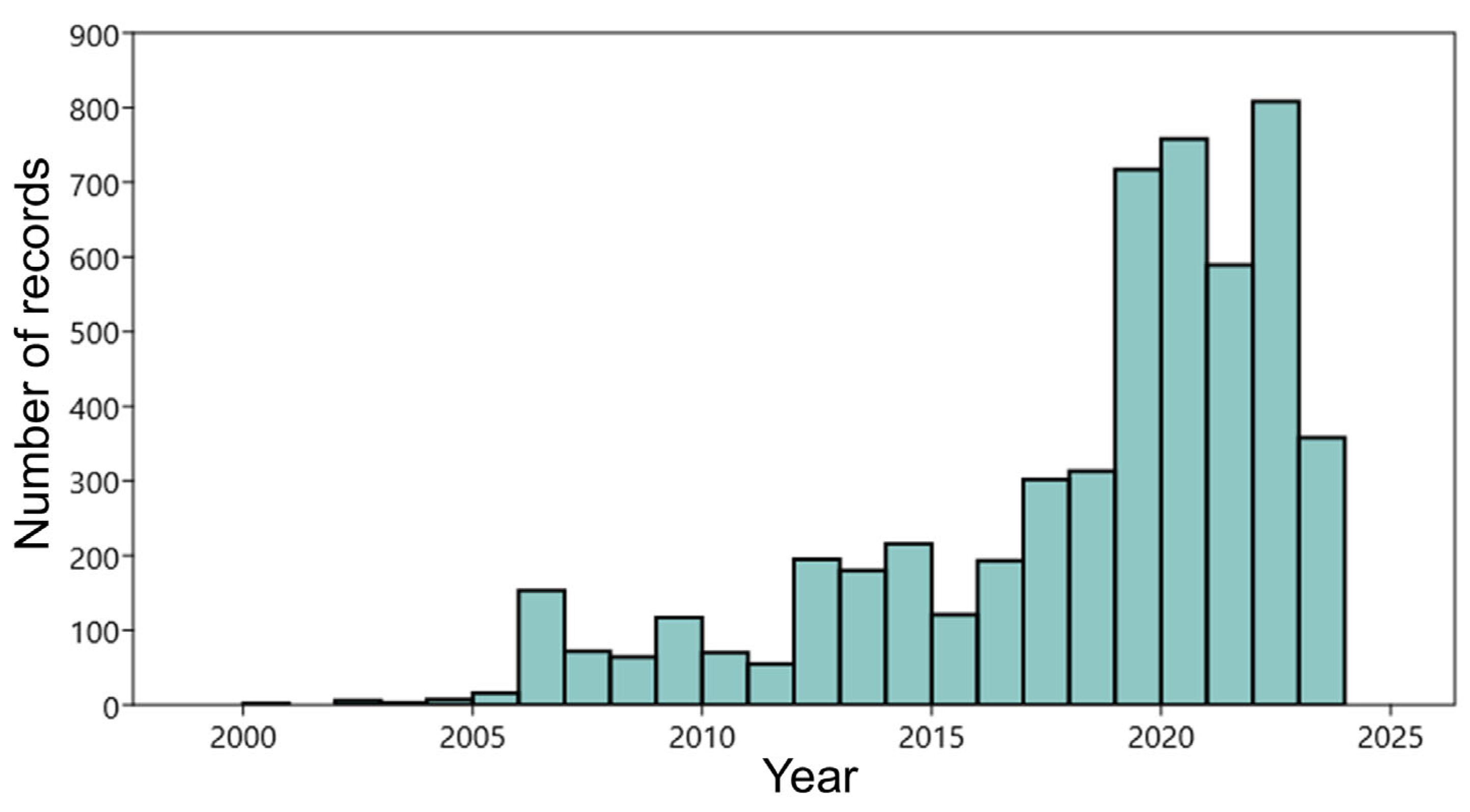
2.2. Study Area and Reptiles’ Occurrence Data
2.3. Building Landscape-Scale Connectivity Maps for Reptiles
2.4. Q1: Do Olive Groves Act as Barriers for Reptile Connectivity Across the Landscape?
2.5. Q2: When Embedded Within Natural Habitats, Do Olive Groves Provide the Same Connectivity as the Surrounding Natural Patches?
2.6. Q3: Do Olive Groves Serve as a Part of the Ecological Corridors Connecting Protected Areas?
3. Results
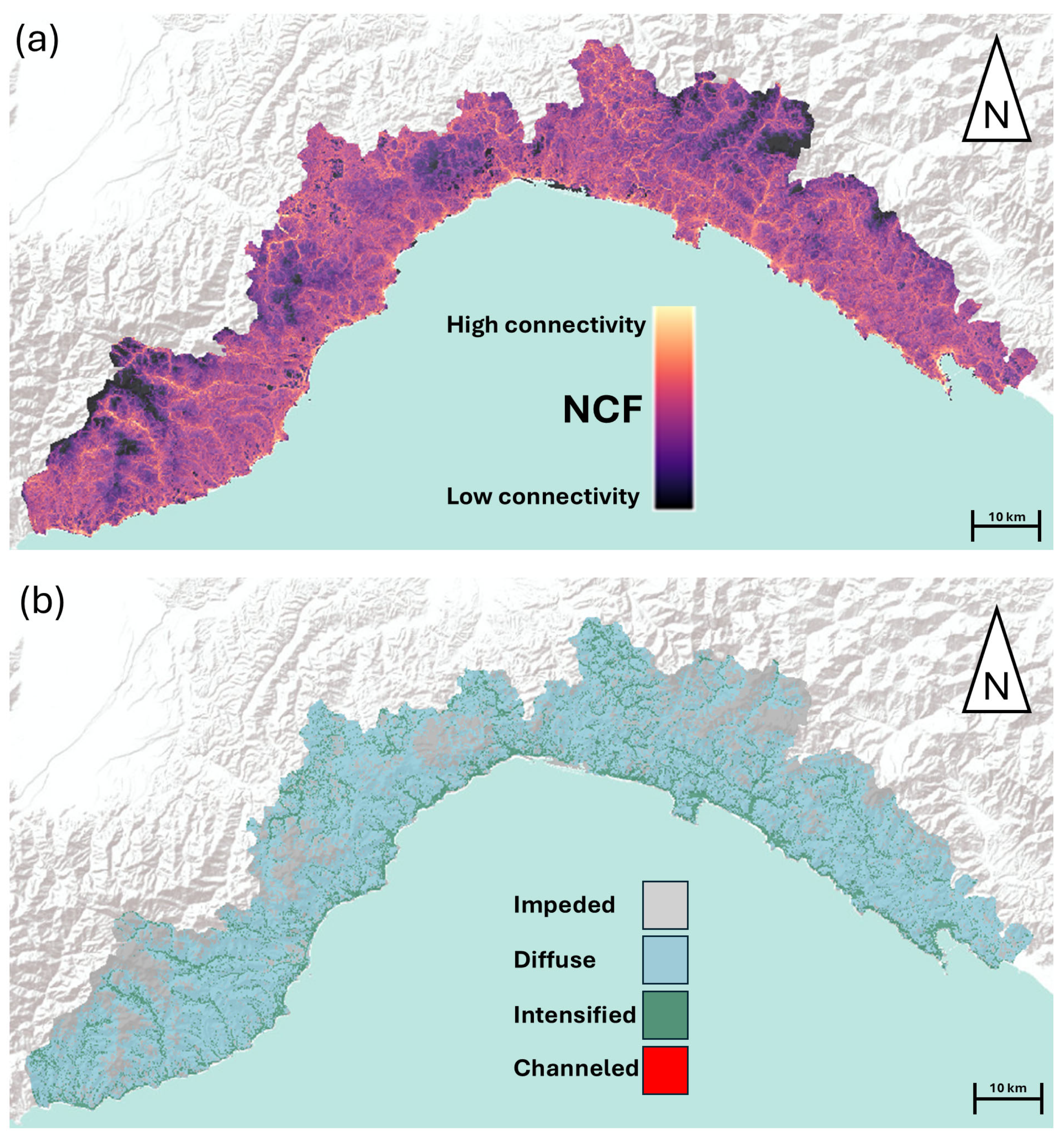
3.1. Landscape Scale Connectivity Maps for Reptiles
3.2. Q1: Do Olive Groves Act as Barriers for Reptile Connectivity Across the Landscape?
3.3. Q2: When Embedded Within Natural Habitats, Do Olive Groves Provide the Same Connectivity as the Surrounding Natural Patches?
3.4. Q3: Do Olive Groves Serve as a Part of the Ecological Corridors Connecting Protected Areas?
4. Discussion
4.1. Q1: Do Olive Groves Act as Barriers for Reptile Connectivity Across the Landscape?
4.2. Q2: When Embedded Within Natural Habitats, Do Olive Groves Provide the Same Connectivity as the Surrounding Natural Patches?
4.3. Q3: Do Olive Groves Serve as a Part of the Ecological Corridors Connecting Protected Areas?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global Consequences of Land Use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef]
- Newbold, T.; Hudson, L.N.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Börger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global Effects of Land Use on Local Terrestrial Biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef]
- Romano, A.; Bernabò, I.; Rosa, G.; Salvidio, S.; Costa, A. Artificial Paradises: Man-Made Sites for the Conservation of Amphibians in a Changing Climate. Biol. Conserv. 2023, 286, 110309. [Google Scholar] [CrossRef]
- Dickson, B.G.; Albano, C.M.; Anantharaman, R.; Beier, P.; Fargione, J.; Graves, T.A.; Gray, M.E.; Hall, K.R.; Lawler, J.J.; Leonard, P.B.; et al. Circuit-theory Applications to Connectivity Science and Conservation. Conserv. Biol. 2019, 33, 239–249. [Google Scholar] [CrossRef]
- McRae, B.H.; Dickson, B.G.; Keitt, T.H.; Shah, V.B. Using circuit theory to model connectivity in ecology, evolution, and conservation. Ecology 2008, 89, 2712–2724. [Google Scholar] [CrossRef]
- Tucker, M.A.; Böhning-Gaese, K.; Fagan, W.F.; Fryxell, J.M.; Van Moorter, B.; Alberts, S.C.; Ali, A.H.; Allen, A.M.; Attias, N.; Avgar, T.; et al. Moving in the Anthropocene: Global Reductions in Terrestrial Mammalian Movements. Science 2018, 359, 466–469. [Google Scholar] [CrossRef]
- Blondel, J. The ‘Design’ of Mediterranean Landscapes: A Millennial Story of Humans and Ecological Systems during the Historic Period. Hum. Ecol. 2006, 34, 713–729. [Google Scholar] [CrossRef]
- Benton, T.G.; Vickery, J.A.; Wilson, J.D. Farmland Biodiversity: Is Habitat Heterogeneity the Key? Trends. Ecol. Evol. 2003, 18, 182–188. [Google Scholar] [CrossRef]
- Manenti, R. Dry Stone Walls Favour Biodiversity: A Case-Study from the Appennines. Biodivers. Conserv. 2014, 23, 1879–1893. [Google Scholar] [CrossRef]
- Romano, A.; Rosa, G.; Salvidio, S.; Novaga, R.; Costa, A. How Landscape and Biotic Interactions Shape a Mediterranean Reptile Community. Landsc. Ecol. 2022, 37, 2915–2927. [Google Scholar] [CrossRef]
- Collier, M.J. Field Boundary Stone Walls as Exemplars of ‘Novel’ Ecosystems. Landsc. Res. 2013, 38, 141–150. [Google Scholar] [CrossRef]
- Salvidio, S.; Costa, A.; Crovetto, F. Individual Trophic Specialisation in the Alpine Newt Increases with Increasing Resource Diversity. Ann. Zool. Fenn. 2019, 56, 17. [Google Scholar] [CrossRef]
- Rey, P.J.; Manzaneda, A.J.; Valera, F.; Alcántara, J.M.; Tarifa, R.; Isla, J.; Molina-Pardo, J.L.; Calvo, G.; Salido, T.; Gutiérrez, J.E.; et al. Landscape-Moderated Biodiversity Effects of Ground Herb Cover in Olive Groves: Implications for Regional Biodiversity Conservation. Agric. Ecosyst. Environ. 2019, 277, 61–73. [Google Scholar] [CrossRef]
- Guiller, G.; Legentilhomme, J.; Boissinot, A.; Blouin-Demers, G.; Barbraud, C.; Lourdais, O. Response of Farmland Reptiles to Agricultural Intensification: Collapse of the Common Adder Vipera Berus and the Western Green Lizard Lacerta Bilineata in a Hedgerow Landscape. Anim. Conserv. 2022, 25, 849–864. [Google Scholar] [CrossRef]
- Taylor, P.D.; Fahrig, L.; Henein, K.; Merriam, G. Connectivity Is a Vital Element of Landscape Structure. Oikos 1993, 68, 571. [Google Scholar] [CrossRef]
- With, K.A.; Gardner, R.H.; Turner, M.G. Landscape Connectivity and Population Distributions in Heterogeneous Environments. Oikos 1997, 78, 151. [Google Scholar] [CrossRef]
- Bailey, S. Increasing Connectivity in Fragmented Landscapes: An Investigation of Evidence for Biodiversity Gain in Woodlands. Forest. Ecol. Manage. 2007, 238, 7–23. [Google Scholar] [CrossRef]
- Grande, T.O.; Aguiar, L.M.S.; Machado, R.B. Heating a Biodiversity Hotspot: Connectivity Is More Important than Remaining Habitat. Landsc. Ecol. 2020, 35, 639–657. [Google Scholar] [CrossRef]
- Plieninger, T.; Hui, C.; Gaertner, M.; Huntsinger, L. The Impact of Land Abandonment on Species Richness and Abundance in the Mediterranean Basin: A Meta-Analysis. PLoS ONE 2014, 9, e98355. [Google Scholar] [CrossRef]
- Dennis, R.L.H.; Dapporto, L.; Dover, J.W.; Shreeve, T.G. Corridors and Barriers in Biodiversity Conservation: A Novel Resource-Based Habitat Perspective for Butterflies. Biodivers. Conserv. 2013, 22, 2709–2734. [Google Scholar] [CrossRef]
- Falcucci, A.; Maiorano, L.; Boitani, L. Changes in Land-Use/Land-Cover Patterns in Italy and Their Implications for Biodiversity Conservation. Landsc. Ecol. 2007, 22, 617–631. [Google Scholar] [CrossRef]
- Costanza, J.K.; Terando, A.J. Landscape Connectivity Planning for Adaptation to Future Climate and Land-Use Change. Curr. Landsc. Ecol. Rep. 2019, 4, 1–13. [Google Scholar] [CrossRef]
- Gibbon, J.W.; Scott, D.E.; Ryan, T.J.; Buhlmann, K.A.; Tuberville, T.D.; Metts, B.S.; Greene, J.L.; Mills, T.; Leiden, Y.; Poppy, S.; et al. The Global Decline of Reptiles, Déjà Vu Amphibians. BioScience 2000, 50, 653. [Google Scholar] [CrossRef]
- Cox, N.; Young, B.E.; Bowles, P.; Fernandez, M.; Marin, J.; Rapacciuolo, G.; Böhm, M.; Brooks, T.M.; Hedges, S.B.; Hilton-Taylor, C.; et al. A Global Reptile Assessment Highlights Shared Conservation Needs of Tetrapods. Nature 2022, 605, 285–290. [Google Scholar] [CrossRef]
- Dudley, N.; Alexander, S. Agriculture and Biodiversity: A Review. Biodiversity 2017, 18, 45–49. [Google Scholar] [CrossRef]
- Doherty, T.S.; Balouch, S.; Bell, K.; Burns, T.J.; Feldman, A.; Fist, C.; Garvey, T.F.; Jessop, T.S.; Meiri, S.; Driscoll, D.A. Reptile Responses to Anthropogenic Habitat Modification: A Global Meta-analysis. Global. Ecol. Biogeogr. 2020, 29, 1265–1279. [Google Scholar] [CrossRef]
- Llorca, A.B. Arboreal Behavior of Psammodromus Algirus (Squamata: Lacertidae) in Olive Groves. Herp. Conserv. Biol. 2023, 18, 155–160. [Google Scholar]
- Duarte, J.; Farfán, M.Á. Ocellated Lizard Predation Patterns on Red-Legged Partridge Nests in Olive Groves. J. Vert. Biol. 2023, 72. [Google Scholar] [CrossRef]
- Kazes, K.; Rotem, G.; Ziv, Y. Effects of Vineyards and Olive Plantations on Reptiles in a Mediterranean Agroecosystem. Herpetologica 2020, 76, 414–422. [Google Scholar] [CrossRef]
- Băncilă, R.I.; Lattuada, M.; Sillero, N. Distribution of Amphibians and Reptiles in Agricultural Landscape across Europe. Landsc. Ecol. 2023, 38, 861–874. [Google Scholar] [CrossRef]
- Carpio, A.J.; Oteros, J.; Tortosa, F.S.; Guerrero-Casado, J. Land Use and Biodiversity Patterns of the Herpetofauna: The Role of Olive Groves. Acta. Oecol. 2016, 70, 103–111. [Google Scholar] [CrossRef]
- Piraccini, R.; Cammarano, M.; Costa, A.; Basile, M.; Posillico, M.; Boitani, L.; Bascietto, M.; Matteucci, G.; De Cinti, B.; Romano, A. Habitat Trees and Salamanders: Conservation and Management Implications in Temperate Forests. Forest. Ecol. Manage. 2017, 384, 17–25. [Google Scholar] [CrossRef]
- Romano, A.; Costa, A.; Salvidio, S.; Menegon, M.; Garollo, E.; Tabarelli De Fatis, K.; Miserocchi, D.; Matteucci, G.; Pedrini, P. Forest Management and Conservation of an Elusive Amphibian in the Alps: Habitat Selection by the Golden Alpine Salamander Reveals the Importance of Fine Woody Debris. Forest. Ecol. Manage. 2018, 424, 338–344. [Google Scholar] [CrossRef]
- Sacchini, A.; Ferraris, F.; Faccini, F.; Firpo, M. Environmental Climatic Maps of Liguria (Italy). J. Maps 2012, 8, 199–207. [Google Scholar] [CrossRef]
- Blondel, J. The Mediterranean Region: Biolodical Diversity in Space and Time; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Margules, C.R.; Pressey, R.L. Systematic Conservation Planning. Nature 2000, 405, 243–253. [Google Scholar] [CrossRef]
- Adriaensen, F.; Chardon, J.P.; De Blust, G.; Swinnen, E.; Villalba, S.; Gulinck, H.; Matthysen, E. The Application of ‘Least-Cost’ Modelling as a Functional Landscape Model. Landsc. Urban Plan. 2003, 64, 233–247. [Google Scholar] [CrossRef]
- Beier, P.; Majka, D.R.; Spencer, W.D. Forks in the Road: Choices in Procedures for Designing Wildland Linkages. Conserv. Biol. 2008, 22, 836–851. [Google Scholar] [CrossRef]
- Mangiacotti, M.; Flego, M.; Oneto, F.; Ottonello, D.; Cottalasso, R.; Ferraro, G.; Sacchi, R. Climate and Land Use Change through the Eyes of Two Endemic Amphibians: Temporal Trajectories of Suitability and Connectivity Reveal Differential Responses. Biol. Conserv. 2025, 302, 110971. [Google Scholar] [CrossRef]
- Balestrieri, A.; Mori, E.; Menchetti, M.; Ruiz-González, A.; Milanesi, P. Far from the Madding Crowd: Tolerance toward Human Disturbance Shapes Distribution and Connectivity Patterns of Closely Related Martes Spp. Popul. Ecol. 2019, 61, 289–299. [Google Scholar] [CrossRef]
- Pitman, R.T.; Fattebert, J.; Williams, S.T.; Williams, K.S.; Hill, R.A.; Hunter, L.T.B.; Robinson, H.; Power, J.; Swanepoel, L.; Slotow, R.; et al. Cats, Connectivity and Conservation: Incorporating Data Sets and Integrating Scales for Wildlife Management. J. Appl. Ecol. 2017, 54, 1687–1698. [Google Scholar] [CrossRef]
- Costa, A.; Dondero, L.; Allaria, G.; Morales Sanchez, B.N.; Rosa, G.; Salvidio, S.; Grasselli, E. Modelling the Amphibian Chytrid Fungus Spread by Connectivity Analysis: Towards a National Monitoring Network in Italy. Biodivers. Conserv. 2021, 30, 2807–2825. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Elith*, J.; Graham*, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel Methods Improve Prediction of Species’ Distributions from Occurrence Data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef]
- Chevalier, M.; Zarzo-Arias, A.; Guélat, J.; Mateo, R.G.; Guisan, A. Accounting for Niche Truncation to Improve Spatial and Temporal Predictions of Species Distributions. Front. Ecol. Evol. 2022, 10, 944116. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A Practical Guide to MaxEnt for Modeling Species’ Distributions: What It Does, and Why Inputs and Settings Matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- McRae, B.H.; Popper, K.; Jones, A.; Schindel, M.; Buttrick, S.; Hall, K.; Unnasch, B.; Platt, J. Conserving Nature’s Stage: Mapping Omnidirectional Connectivity for Resilient Terrestrial Landscapes in the Pacific Northwest; The Nature Conservancy: Portland, OR, USA, 2016. [Google Scholar]
- Liu, C.; Berry, P.M.; Dawson, T.P.; Pearson, R.G. Selecting Thresholds of Occurrence in the Prediction of Species Distributions. Ecography 2005, 28, 385–393. [Google Scholar] [CrossRef]
- Landau, V.; Shah, V.; Anantharaman, R.; Hall, K. Omniscape.Jl: Software to Compute Omnidirectional Landscape Connectivity. J. Open Source Softw. 2021, 6, 2829. [Google Scholar] [CrossRef]
- Brennan, A.; Beytell, P.; Aschenborn, O.; Du Preez, P.; Funston, P.J.; Hanssen, L.; Kilian, J.W.; Stuart-Hill, G.; Taylor, R.D.; Naidoo, R. Characterizing Multispecies Connectivity across a Transfrontier Conservation Landscape. J. Appl. Ecol. 2020, 57, 1700–1710. [Google Scholar] [CrossRef]
- Belote, R.T.; Dietz, M.S.; McRae, B.H.; Theobald, D.M.; McClure, M.L.; Irwin, G.H.; McKinley, P.S.; Gage, J.A.; Aplet, G.H. Identifying Corridors among Large Protected Areas in the United States. PLoS ONE 2016, 11, e0154223. [Google Scholar] [CrossRef]
- Romano, A.; Salvidio, S.; Mongillo, D.; Olivari, S. Importance of a Traditional Irrigation System in Amphibian Conservation in the Cinque Terre National Park (NW Italy). J. Nat. Conserv. 2014, 22, 445–452. [Google Scholar] [CrossRef]
- Rosa, G.; Salvidio, S.; Costa, A. The Role of Familiarity in Shelter Site Fidelity: Insights from a Mesocosm Experiment with a Plethodontid Salamander. Ethol. Ecol. Evol. 2024, 36, 616–626. [Google Scholar] [CrossRef]
- Gaigher, R.; Pryke, J.S.; Samways, M.J. Habitat Complementarity and Butterfly Traits Are Essential Considerations When Mitigating the Effects of Exotic Plantation Forestry. Biodivers. Conserv. 2021, 30, 4089–4109. [Google Scholar] [CrossRef]
- Mandelik, Y.; Winfree, R.; Neeson, T.; Kremen, C. Complementary Habitat Use by Wild Bees in Agro-Natural Landscapes. Ecol. Appl. 2012, 22, 1535–1546. [Google Scholar]
- Sirami, C.; Nespoulous, A.; Cheylan, J.-P.; Marty, P.; Hvenegaard, G.T.; Geniez, P.; Schatz, B.; Martin, J.-L. Long-Term Anthropogenic and Ecological Dynamics of a Mediterranean Landscape: Impacts on Multiple Taxa. Landsc. Urban. Plan. 2010, 96, 214–223. [Google Scholar] [CrossRef]
- Daskalova, G.N.; Kamp, J. Abandoning Land Transforms Biodiversity. Science 2023, 380, 581–583. [Google Scholar] [CrossRef]
| Family | Species | IUCN Category | Taxon Considered in the Analyses | Number of Records |
|---|---|---|---|---|
| Gekkonidae | Euleptes europaea Genè, 1839 | NT | Euleptes europaea | 53 |
| Hemidactylus turcicus (Linnaeus, 1758) | LC | Hemidactylus turcicus | 46 | |
| Tarentola mauritanica (Linnaeus, 1758) | LC | Tarentola mauritanica | 218 | |
| Scincidae | Chalcides chalcides (Linnaeus, 1758) | LC | Chalcides sp. | 98 |
| Chalcides striatus (Cuvier, 1829) | LC | |||
| Lacertidae | Lacerta bilineata Daudin, 1802 | LC | Lacerta bilineata | 1048 |
| Podarcis muralis Laurenti, 1768 | LC | Podarcis sp. | 2422 | |
| Podarcis siculus Rafinesque, 1810 | LC | |||
| Timon lepidus Daudin, 1802 | LC | Timon lepidus | 158 | |
| Anguidae | Anguis veronensis Pollini, 1818 | LC | Anguis veronensis | 126 |
| Colubridae | Coronella austriaca Laurenti, 1768 | LC | Coronella sp. | 107 |
| Coronella girondica Daudin, 1803 | LC | |||
| Hierophis viridiflavus (Lacépède, 1789) | LC | Hierophis viridiflavus | 294 | |
| Malpolon monspessulanus (Hermann, 1804) | LC | Malpolon monspessulanus | 183 | |
| Natrix helvetica (Linnaeus, 1758) | LC | Natrix sp. | 281 | |
| Natrix maura (Linnaeus, 1758) | LC | |||
| Natrix tessellata (Laurenti, 1768) | LC | |||
| Zamenis longissimus (Laurenti, 1768) | LC | Zamenis longissimus | 102 | |
| Viperidae | Vipera aspis (Linnaeus, 1758) | VU | Vipera aspis | 75 |
| Variable | Description | Origin of the Layer |
|---|---|---|
| Elevation a.s.l. | Meters above sea level | Derived from Digital Elevation Model (DEM) |
| TPI (Topographic Position Index) | An index giving information on the position of each cell within a slope. Assumes zero values when a cell lays on a topographic flat, negative values for valleys, and positive values for ridges. | Calculated from DEM using software SAGA (System for Automated Geoscientific Analyses, V 9.7.2) |
| Diffuse insolation | Index of diffuse insolation, expressing the amount of incoming solar radiation for each cell | Calculated from DEM using software SAGA for seasonal period of activity of reptiles (April–Sept) |
| Grasslands | Presence or absence of grasslands for each 100 m cell for the 2018 reference year | Provided by European Global Monitoring for Environment and Security Programme (COPERNICUS) |
| TCD (Tree Cover Density) | Percent density representing tree cover for each 100 m cell for the 2018 reference year | Provided by European Global Monitoring for Environment and Security Programme (COPERNICUS) |
| SWF (Small Woody Features) | Presence or absence of small woody vegetation for each 100 m cell for the 2018 reference year | Provided by European Global Monitoring for Environment and Security Programme (COPERNICUS) |
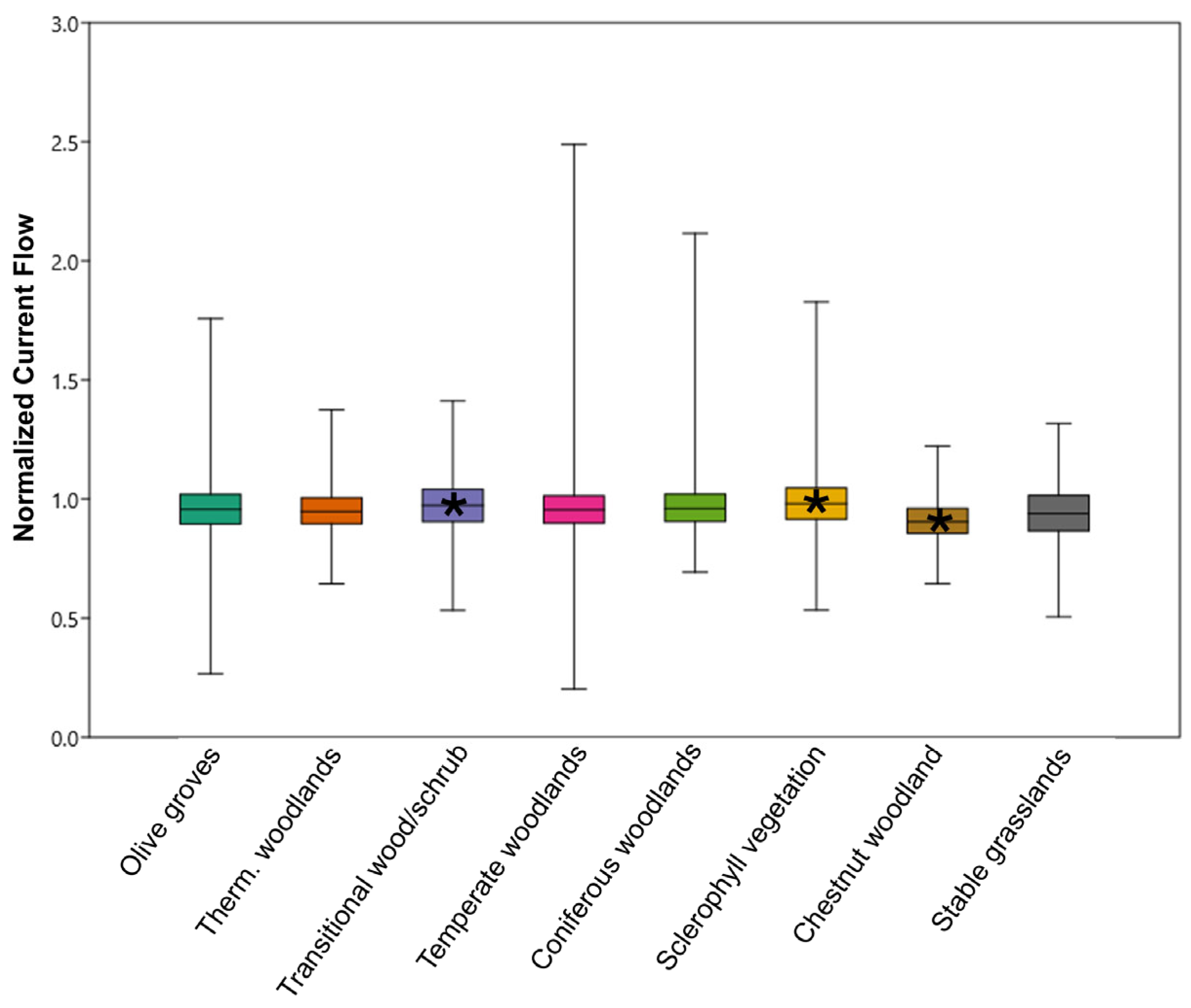
| Patch Type | N of Polygons | % Coverage Area | Average NCF (SD) |
|---|---|---|---|
| Olive Groves | 4212 | - | 0.96 (0.12) |
| Thermophilic mixed woodlands | 1419 | 11.54 | 0.96 (0.07) |
| Transitional woodlands/shrubs | 1059 | 11.37 | 0.97 (0.06) |
| Temperate mixed woodlands | 852 | 9.18 | 0.96 (0.08) |
| Coniferous woodlands | 717 | 5.84 | 0.97 (0.07) |
| Sclerophyllous vegetation | 419 | 3.04 | 0.99 (0.08) |
| Chestnut woodlands | 324 | 2.64 | 0.91 (0.08) |
| Stable grasslands | 267 | 2.17 | 0.94 (0.06) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, A.; Oneto, F.; Rosa, G.; Actis Dato, G.; Ottonello, D. Ecological Connectivity for Reptiles in Agroecosystems: A Case Study with Olive Groves in Liguria (Northwestern Italy). Animals 2025, 15, 909. https://doi.org/10.3390/ani15070909
Costa A, Oneto F, Rosa G, Actis Dato G, Ottonello D. Ecological Connectivity for Reptiles in Agroecosystems: A Case Study with Olive Groves in Liguria (Northwestern Italy). Animals. 2025; 15(7):909. https://doi.org/10.3390/ani15070909
Chicago/Turabian StyleCosta, Andrea, Fabrizio Oneto, Giacomo Rosa, Giacomo Actis Dato, and Dario Ottonello. 2025. "Ecological Connectivity for Reptiles in Agroecosystems: A Case Study with Olive Groves in Liguria (Northwestern Italy)" Animals 15, no. 7: 909. https://doi.org/10.3390/ani15070909
APA StyleCosta, A., Oneto, F., Rosa, G., Actis Dato, G., & Ottonello, D. (2025). Ecological Connectivity for Reptiles in Agroecosystems: A Case Study with Olive Groves in Liguria (Northwestern Italy). Animals, 15(7), 909. https://doi.org/10.3390/ani15070909







