Studying the Impact of the DDB2 T338M Missense Mutation on the Development of Equine Squamous Cell Carcinoma and Sarcoid
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Database Search
2.2. Sample Collection
2.3. Genotyping
2.4. DNA Uracil-DNA Glycosylase (UDG) Treatment
2.5. Statistical Analysis
3. Results
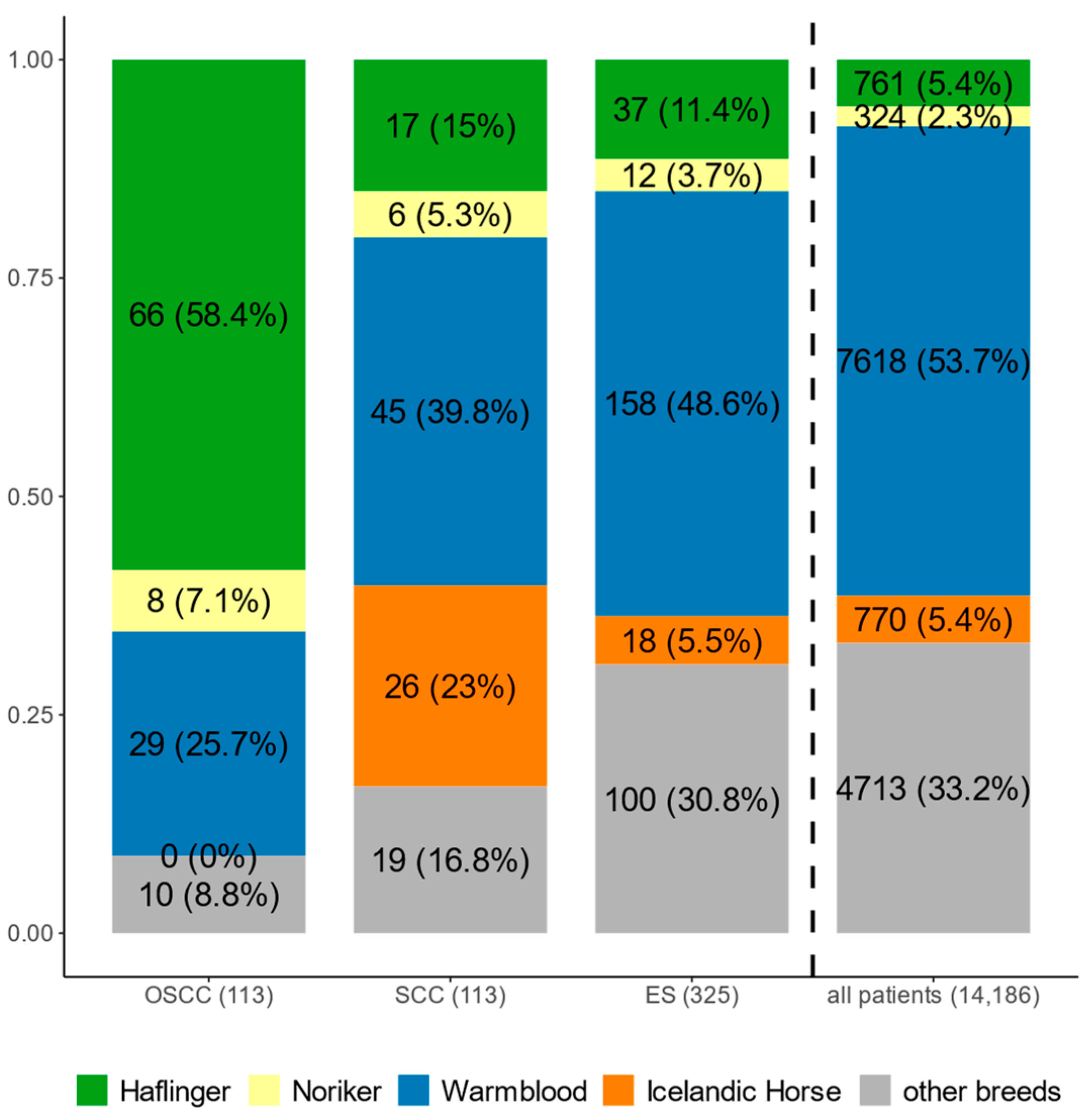
3.1. Database Search on Patients Presented to the Clinic
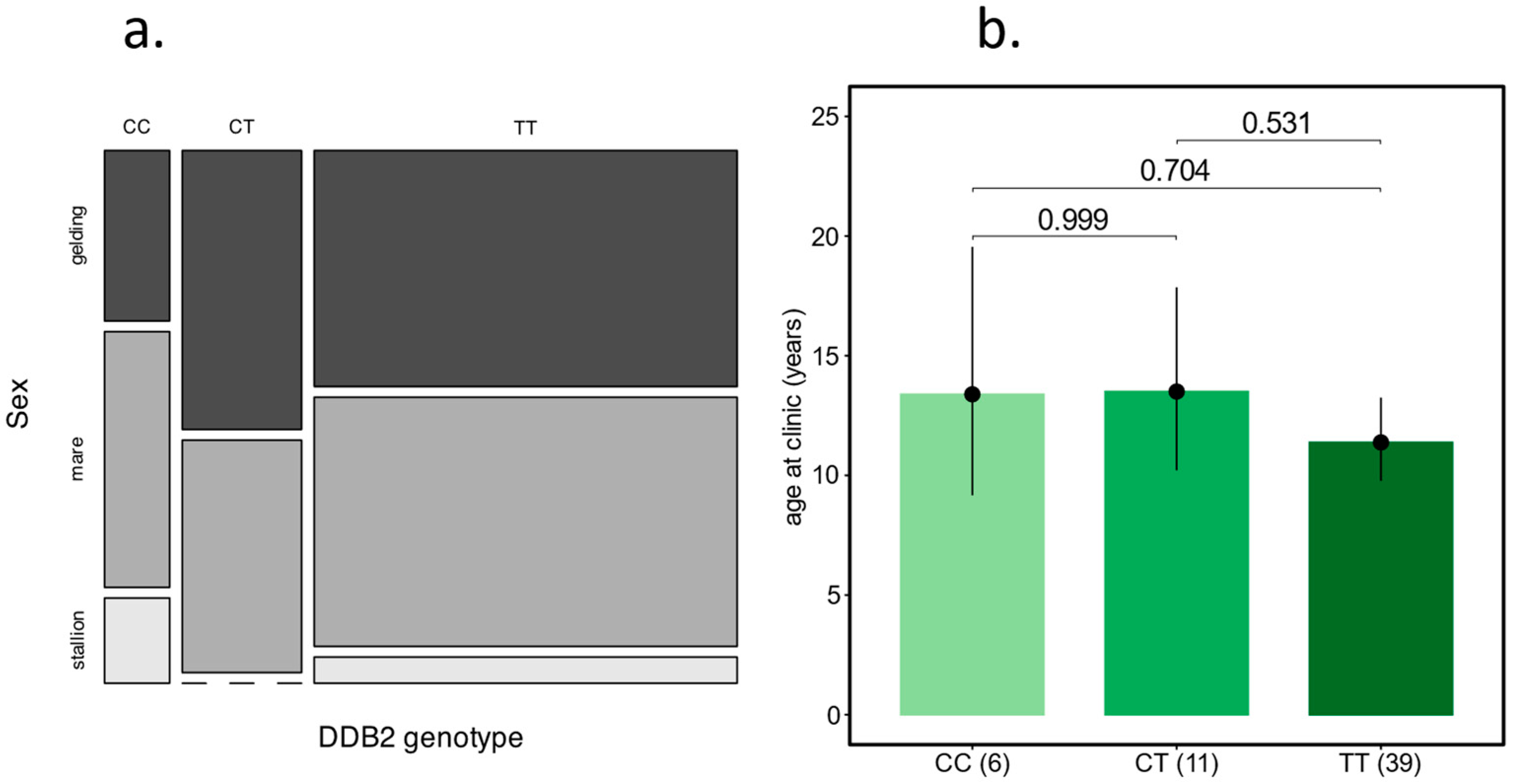
3.2. DDB2 Risk Allele Status in Horses Diagnosed with OSCC, SCC and ES
Influence of the DDB2 Risk Allele on the Development of OSCC in Haflingers
3.3. DDB2 Risk Allele in SCCs at Localisations Other than the Eye
3.4. DDB2 Risk Allele in ESs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sundberg, J.P.; Burnstein, T.; Page, E.H.; Kirkham, W.W.; Robinson, F.R. Neoplasms of Equidae. J. Am. Vet. Med. Assoc. 1977, 170, 150–152. [Google Scholar]
- Scott, D.W.; Miller, W.H.J. Squamous cell carcinoma. In Equine Dermatology; Scott, D.W., Miller, W.H.J., Eds.; Saunders Elsevier: St. Louis, MO, USA, 2003; pp. 707–712. [Google Scholar]
- Strafuss, A.C. Squamous cell carcinoma in horses. J. Am. Vet. Med. Assoc. 1976, 168, 61–62. [Google Scholar] [PubMed]
- Dugan, S.J.; Curtis, C.R.; Roberts, S.M.; Severin, G.A. Epidemiologic study of ocular/adnexal squamous cell carcinoma in horses. J. Am. Vet. Med. Assoc. 1991, 198, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Lassaline, M.; Cranford, T.L.; Latimer, C.A.; Bellone, R.R. Limbal squamous cell carcinoma in Haflinger horses. Vet. Ophthalmol. 2015, 18, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Bosch, G.; Klein, W.R. Superficial keratectomy and cryosurgery as therapy for limbal neoplasms in 13 horses. Vet. Ophthalmol. 2005, 8, 241–246. [Google Scholar] [CrossRef]
- King, T.C.; Priehs, D.R.; Gum, G.G.; Miller, T.R. Therapeutic management of ocular squamous cell carcinoma in the horse: 43 cases (1979–1989). Equine Vet. J. 1991, 23, 449–452. [Google Scholar] [CrossRef]
- Dugan, S.J. Ocular neoplasia. Vet. Clin. N. Am. Equine Pract. 1992, 8, 609–626. [Google Scholar] [CrossRef]
- Mosunic, C.B.; Moore, P.A.; Carmicheal, K.P.; Chandler, M.J.; Vidyashankar, A.; Zhao, Y.; Roberts, R.E.; Dietrich, U.M. Effects of treatment with and without adjuvant radiation therapy on recurrence of ocular and adnexal squamous cell carcinoma in horses: 157 cases (1985–2002). J. Am. Vet. Med. Assoc. 2004, 225, 1733–1738. [Google Scholar] [CrossRef]
- Kaps, S.; Richter, M.; Philipp, M.; Bart, M.; Eule, C.; Spiess, B.M. Primary invasive ocular squamous cell carcinoma in a horse. Vet. Ophthalmol. 2005, 8, 193–197. [Google Scholar] [CrossRef]
- Michau, T.M.; Davidson, M.G.; Gilger, B.C. Carbon dioxide laser photoablation adjunctive therapy following superficial lamellar keratectomy and bulbar conjunctivectomy for the treatment of corneolimbal squamous cell carcinoma in horses: A review of 24 cases. Vet. Ophthalmol. 2012, 15, 245–253. [Google Scholar] [CrossRef]
- Bellone, R.R.; Liu, J.; Petersen, J.L.; Mack, M.; Singer-Berk, M.; Drögemüller, C.; Malvick, J.; Wallner, B.; Brem, G.; Penedo, M.C.; et al. A missense mutation in damage-specific DNA binding protein 2 is a genetic risk factor for limbal squamous cell carcinoma in horses. Int. J. Cancer 2017, 141, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Newkirk, K.M.; Hendrix, D.V.H.; Anis, E.A.; Rohrbach, B.W.; Ehrhart, E.J.; Lyons, J.A.; Kania, S.A. Detection of papillomavirus in equine periocular and penile squamous cell carcinoma. J. Vet. Diagn. Investig. 2014, 26, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, P.A.; Wobeser, B.; Martin, L.E.R.; Dennis, M.M.; Duncan, C.G. Cutaneous neoplastic lesions of equids in the central United States and Canada: 3351 biopsy specimens from 3272 equids (2000–2010). J. Am. Vet. Med. Assoc. 2013, 242, 99–104. [Google Scholar] [CrossRef]
- Kafarnik, C.; Rawlings, M.; Dubielzig, R.R. Corneal stromal invasive squamous cell carcinoma: A retrospective morphological description in 10 horses. Vet. Ophthalmol. 2009, 12, 6–12. [Google Scholar] [CrossRef]
- Singer-Berk, M.H.; Knickelbein, K.E.; Lounsberry, Z.T.; Crausaz, M.; Vig, S.; Joshi, N.; Britton, M.; Settles, M.L.; Reilly, C.M.; Bentley, E.; et al. Additional Evidence for DDB2 T338M as a Genetic Risk Factor for Ocular Squamous Cell Carcinoma in Horses. Int. J. Genom. 2019, 2019, 3610965. [Google Scholar] [CrossRef]
- Dugan, S.J.; Roberts, S.M.; Curtis, C.R.; Severin, G.A. Prognostic factors and survival of horses with ocular/adnexal squamous cell carcinoma: 147 cases (1978–1988). J. Am. Vet. Med. Assoc. 1991, 198, 298–303. [Google Scholar] [CrossRef]
- Schwink, K. Factors influencing morbidity and outcome of equine ocular squamous cell carcinoma. Equine Vet. J. 1987, 19, 198–200. [Google Scholar] [CrossRef]
- Plummer, C.E.; Smith, S.; Andrew, S.E.; Lassaline, M.E.; Gelatt, K.N.; Brooks, D.E.; Kallberg, M.E.; Ollivier, F.J. Combined keratectomy, strontium-90 irradiation and permanent bulbar conjunctival grafts for corneolimbal squamous cell carcinomas in horses (1990–2002): 38 horses. Vet. Ophthalmol. 2007, 10, 37–42. [Google Scholar] [CrossRef]
- Clode, A.B.; Miller, C.; McMullen, R.J.J.; Gilger, B.C. A retrospective comparison of surgical removal and subsequent CO2 laser ablation versus topical administration of mitomycin C as therapy for equine corneolimbal squamous cell carcinoma. Vet. Ophthalmol. 2012, 15, 254–262. [Google Scholar] [CrossRef]
- Scase, T.; Brandt, S.; Kainzbauer, C.; Sykora, S.; Bijmholt, S.; Hughes, K.; Sharpe, S.; Foote, A. Equus caballus papillomavirus-2 (EcPV-2): An infectious cause for equine genital cancer? Equine Vet. J. 2010, 42, 738–745. [Google Scholar] [CrossRef]
- Knight, C.G.; Munday, J.S.; Peters, J.; Dunowska, M. Equine penile squamous cell carcinomas are associated with the presence of equine papillomavirus type 2 DNA sequences. Vet. Pathol. 2011, 48, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.W.; Affolter, V.K.; Gaynor, A.M.; Cruz, F.N.J.D.; Pesavento, P.A. Equine Genital Squamous Cell Carcinoma: In Situ Hybridization Identifies a Distinct Subset Containing Equus caballus Papillomavirus 2. Vet. Pathol. 2015, 52, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Sykora, S.; Brandt, S. Papillomavirus infection and squamous cell carcinoma in horses. Vet. J. 2017, 223, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Chambers, G.; Ellsmore, V.A.; O’Brien, P.M.; Reid, S.W.J.; Love, S.; Campo, M.S.; Nasir, L. Association of bovine papillomavirus with the equine sarcoid. J. Gen. Virol. 2003, 84, 1055–1062. [Google Scholar] [CrossRef]
- Nasir, L.; Campo, M.S. Bovine papillomaviruses: Their role in the aetiology of cutaneous tumours of bovids and equids. Vet. Dermatol. 2008, 19, 243–254. [Google Scholar] [CrossRef]
- Christen, G.; Gerber, V.; Dolf, G.; Burger, D.; Koch, C. Inheritance of equine sarcoid disease in Franches-Montagnes horses. Vet. J. 2014, 199, 68–71. [Google Scholar] [CrossRef]
- Jandova, V.; Klukowska-Rötzler, J.; Dolf, G.; Janda, J.; Roosje, P.; Marti, E.; Koch, C.; Gerber, V.; Swinburne, J. Whole genome scan identifies several chromosomal regions linked to equine sarcoids. Schweiz. Arch. Tierheilkd. 2012, 154, 19–25. [Google Scholar] [CrossRef]
- Staiger, E.A.; Tseng, C.T.; Miller, D.; Cassano, J.M.; Nasir, L.; Garrick, D.; Brooks, S.A.; Antczak, D.F. Host genetic influence on papillomavirus-induced tumors in the horse. Int. J. Cancer 2016, 139, 784–792. [Google Scholar] [CrossRef]
- Tuomisto, L.; Virtanen, J.; Kegler, K.; Levanov, L.; Sukura, A.; Sironen, T.; Kareskoski, M. Equus caballus papillomavirus type 2 (EcPV2)-associated benign penile lesions and squamous cell carcinomas. Vet. Med. Sci. 2024, 10, e1342. [Google Scholar] [CrossRef]
- Chen, L.; Bellone, R.R.; Wang, Y.; Singer-Berk, M.; Sugasawa, K.; Ford, J.M.; Artandi, S.E. A novel DDB2 mutation causes defective recognition of UV-induced DNA damages and prevalent equine squamous cell carcinoma. DNA Repair 2021, 97, 103022. [Google Scholar] [CrossRef]
- Crausaz, M.; Launois, T.; Smith-Fleming, K.; McCoy, A.M.; Knickelbein, K.E.; Bellone, R.R. DDB2 Genetic Risk Factor for Ocular Squamous Cell Carcinoma Identified in Three Additional Horse Breeds. Genes 2020, 11, 1460. [Google Scholar] [CrossRef] [PubMed]
- Knickelbein, K.E.; Lassaline, M.E.; Singer-Berk, M.; Reilly, C.M.; Clode, A.B.; Famula, T.R.; Michau, T.M.; Bellone, R.R. A missense mutation in damage-specific DNA binding protein 2 is a genetic risk factor for ocular squamous cell carcinoma in Belgian horses. Equine Vet. J. 2020, 52, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Bellone, R.R. Genetic Testing as a Tool to Identify Horses with or at Risk for Ocular Disorders. Vet. Clin. North Am.-Equine Pract. 2017, 33, 627–645. [Google Scholar] [CrossRef] [PubMed]
- Durward-Akhurst, S.A.; Marlowe, J.L.; Schaefer, R.J.; Springer, K.; Grantham, B.; Carey, W.K.; Bellone, R.R.; Mickelson, J.R.; McCue, M.E. Predicted genetic burden and frequency of phenotype-associated variants in the horse. Sci. Rep. 2024, 14, 8396. [Google Scholar] [CrossRef]
- Schäfer, J.; May, A.; Wittenberg, J.; Hahn, K.; Graubner, C.; Gerber, V.; Drögemüller, C.; Unger, L. DDB2-assoziiertes Vorkommen von Plattenepithelkarzinomen beim Haflinger: Risikominimierung durch Genotypisierung. Schweiz. Arch. Tierheilkd. 2023, 165, 707–715. [Google Scholar]
- Broström, H.; Fahlbrink, E.; Dubath, M.L.; Lazary, S. Association between equine leucocyte antigens (ELA) and equine sarcoid tumors in the population of Swedish halfbreds and some of their families. Vet. Immunol. Immunopathol. 1988, 19, 215–223. [Google Scholar] [CrossRef]
- Lazary, S.; Gerber, H.; Glatt, P.A.; Straub, R. Equine leucocyte antigens in sarcoid-affected horses. Equine Vet. J. 1985, 17, 283–286. [Google Scholar] [CrossRef]
- Lazary, S.; Marti, E.; Szalai, G.; Gaillard, C.; Gerber, H. Studies on the frequency and associations of equine leucocyte antigens in sarcoid and summer dermatitis. Anim. Genet. 1994, 25 (Suppl. 1), 75–80. [Google Scholar] [CrossRef]
- Meredith, D.; Elser, A.H.; Wolf, B.; Soma, L.R.; Donawick, W.J.; Lazary, S. Equine leukocyte antigens: Relationships with sarcoid tumors and laminitis in two pure breeds. Immunogenetics 1986, 23, 221–225. [Google Scholar] [CrossRef]
- Wobeser, B.K.; Davies, J.L.; Hill, J.E.; Jackson, M.L.; Kidney, B.A.; Mayer, M.N.; Townsend, H.G.G.; Allen, A.L. Epidemiology of equine sarcoids in horses in western Canada. Can. Vet. J. = La Rev. Vet. Can. 2010, 51, 1103–1108. [Google Scholar]
- Angelos, J.; Oppenheim, Y.; Rebhun, W.; Mohammed, H.; Antczak, D.F. Evaluation of breed as a risk factor for sarcoid and uveitis in horses. Anim. Genet. 1988, 19, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.O.; Rebhun, W.C.; Antczak, D.F. Factors associated with the risk of developing sarcoid tumours in horses. Equine Vet. J. 1992, 24, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Mele, M.; Gerber, V.; Straub, R.; Gaillard, C.; Jallon, L.; Burger, D. Prevalence of hereditary diseases in three-year-old horses of the Freiberger breed. Schweiz. Arch. Tierheilkd. 2007, 149, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Sakai, K.; Uehara, N.; Hoshi, Y.; Mori, A.; Koyama, H.; Sato, M.; Saito, K.; Osaki, Y.; Nishio, K.; et al. Genetic variants of cancer-associated genes analyzed using next-generation sequencing in small sporadic vestibular schwannomas. Oncol. Lett. 2023, 25, 121. [Google Scholar] [CrossRef]
- Beecher, M.; Kumar, N.; Jang, S.; Rapić-Otrin, V.; Van Houten, B. Expanding molecular roles of UV-DDB: Shining light on genome stability and cancer. DNA Repair 2020, 94, 102860. [Google Scholar] [CrossRef]
- An, M.; Chen, C.; Xiang, J.; Li, Y.; Qiu, P.; Tang, Y.; Liu, X.; Gu, Y.; Qin, N.; He, Y.; et al. Systematic identification of pathogenic variants of non-small cell lung cancer in the promoters of DNA-damage repair genes. EBioMedicine 2024, 110, 105480. [Google Scholar] [CrossRef]
- Do, H.; Dobrovic, A. Sequence artifacts in DNA from formalin-fixed tissues: Causes and strategies for minimization. Clin. Chem. 2015, 61, 64–71. [Google Scholar] [CrossRef]
- Wong, S.Q.; Li, J.; Tan, A.Y.-C.; Vedururu, R.; Pang, J.-M.B.; Do, H.; Ellul, J.; Doig, K.; Bell, A.; MacArthur, G.A.; et al. Sequence artefacts in a prospective series of formalin-fixed tumours tested for mutations in hotspot regions by massively parallel sequencing. BMC Med. Genom. 2014, 7, 23. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, version 4.2.2; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Wickham, H.; François, R.; Henry, L.; Müller, K. dplyr: A Grammar of Data Manipulation. Available online: https://github.com/tidyverse/dplyr (accessed on 20 November 2024).
- Tukey, J.W. Comparing individual means in the analysis of variance. Biometrics 1949, 5, 99–114. [Google Scholar]
- Lenth, R. emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.11.0. Available online: https://rvlenth.github.io/emmeans/ (accessed on 5 January 2024).
- Neuwirth, E. RColorBrewer: ColorBrewer Palettes. R package version 1.1-2. Available online: https://cran.r-project.org/web/packages/RColorBrewer/index.html (accessed on 20 August 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots. R package version 0.6.0.999. Available online: https://github.com/kassambara/ggpubr (accessed on 20 August 2024).
- Druml, T.; Sauer, K.; Elsbacher, J.; Grilz-Seger, G.; Brem, G. Analyse des Genpools, der genetischen Diversität und der Inzuchtverhältnisse der österreichischen Haflingerpopulation. Züchtungskunde 2016, 88, 379–394. [Google Scholar]
- Kalyta, K.; Stelmaszczyk, W.; Szczęśniak, D.; Kotuła, L.; Dobosz, P.; Mroczek, M. The Spectrum of the Heterozygous Effect in Biallelic Mendelian Diseases—The Symptomatic Heterozygote Issue. Genes 2023, 14, 1562. [Google Scholar] [CrossRef] [PubMed]
- Knickelbein, K.E.; Lassaline, M.; Bellone, R.R. Limbal squamous cell carcinoma in a Rocky Mountain Horse: Case report and investigation of genetic contribution. Vet. Ophthalmol. 2019, 22, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Grilz-Seger, G.; Mesarič, M.; Cotman, M.; Neuditschko, M.; Druml, T.; Brem, G. Runs of Homozygosity and Population History of Three Horse Breeds With Small Population Size. J. Equine Vet. Sci. 2018, 71, 27–34. [Google Scholar] [CrossRef]
- Schröder, U.; Licka, T.F.; Zsoldos, R.; Hahn, C.N.; MacIntyre, N.; Schwendenwein, I.; Schwarz, B.; Van Den Hoven, R. Effect of Diet on Haflinger Horses with GYS1 Mutation (Polysaccharide Storage Myopathy Type 1). J. Equine Vet. Sci. 2015, 35, 598–605. [Google Scholar] [CrossRef]
- Druml, T.; Grilz-Seger, G.; Brem, G. Effect of the glycogen synthase 1 (GYS1) mutation on performance traits in 169 Noriker draft horse stallions-A retrospective study. Arch. Anim. Breed. 2016, 59, 453–459. [Google Scholar] [CrossRef]
- Grilz-Seger, G.; Druml, T.; Neuditschko, M.; Mesarič, M.; Cotman, M.; Brem, G. Analysis of ROH patterns in the Noriker horse breed reveals signatures of selection for coat color and body size. Anim. Genet. 2019, 50, 334–346. [Google Scholar] [CrossRef]
- Van Den Top, J.G.B.; Ensink, J.M.; Gröne, A.; Klein, W.R.; Barneveld, A.; Van Weeren, P.R. Penile and preputial tumours in the horse: Literature review and proposal of a standardised approach. Equine Vet. J. 2010, 42, 746–757. [Google Scholar] [CrossRef]
- Grilz-Seger, G.; Neuditschko, M.; Ricard, A.; Velie, B.D.; Lindgren, G.; Mesarič, M.; Cotman, M.; Horna, M.; Dobretsberger, M.; Brem, G.; et al. Genome-wide homozygosity patterns and evidence for selection in a set of european and near eastern horse breeds. Genes 2019, 10, 491. [Google Scholar] [CrossRef]


| DDB2 Genotype Prior FFPE Treatment | Number of Samples/Genotype After UDG Treatment Confirmed | Mean (min/max) Years in Paraffin |
|---|---|---|
| CC | 16/16 | 9.43 (2/17) |
| CT | 12/12 | 8.75 (3/17) |
| TT | 33/33 | 9.03 (1/19) |
| Group | Genotype | Haflinger | Noriker | Warmblood | Icelandic Horse |
|---|---|---|---|---|---|
| OSCC | CC | 6 | 1 | 14 | |
| CT | 11 | 2 | - | n.e. | |
| TT | 39 | 4 | - | ||
| SCC head and neck | CC | 4 | 1 | 10 | 4 |
| CT | 2 | - | 1 | - | |
| TT | 1 | - | - | - | |
| SCC genital | CC | 5 | 15 | 9 | |
| CT | - | n.e. | - | - | |
| TT | - | - | - | ||
| ES | CC | 10 | 2 | ||
| CT | 1 | 2 | n.e. | n.e. | |
| TT | - | - | |||
| Tumour free | CC | 38 | |||
| CT | 19 | n.e. | n.e. | n.e. | |
| TT | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quatember, H.; Nell, B.; Richter, B.; Rigler, D.; Dolezal, M.; Sykora, S.; Wallner, B. Studying the Impact of the DDB2 T338M Missense Mutation on the Development of Equine Squamous Cell Carcinoma and Sarcoid. Animals 2025, 15, 911. https://doi.org/10.3390/ani15070911
Quatember H, Nell B, Richter B, Rigler D, Dolezal M, Sykora S, Wallner B. Studying the Impact of the DDB2 T338M Missense Mutation on the Development of Equine Squamous Cell Carcinoma and Sarcoid. Animals. 2025; 15(7):911. https://doi.org/10.3390/ani15070911
Chicago/Turabian StyleQuatember, Hannah, Barbara Nell, Barbara Richter, Doris Rigler, Marlies Dolezal, Sabine Sykora, and Barbara Wallner. 2025. "Studying the Impact of the DDB2 T338M Missense Mutation on the Development of Equine Squamous Cell Carcinoma and Sarcoid" Animals 15, no. 7: 911. https://doi.org/10.3390/ani15070911
APA StyleQuatember, H., Nell, B., Richter, B., Rigler, D., Dolezal, M., Sykora, S., & Wallner, B. (2025). Studying the Impact of the DDB2 T338M Missense Mutation on the Development of Equine Squamous Cell Carcinoma and Sarcoid. Animals, 15(7), 911. https://doi.org/10.3390/ani15070911







