The Impact of Allicin on the Growth of Clostridium spp. in the Digestive Track of Quails
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collections
2.2. Cultures
2.3. Detection of the Ntnh Gene Using Real-Time PCR
2.4. Clostridium Perfringens Detection
2.5. Detection of Clostridium Strains Using Amplification and Sanger Sequencing of 16S rDNA
2.6. Electrophoresis of PCR Products
2.7. Statistical Analysis
- The addition of allicin fed to quails does not significantly affect the level of Clostridium spp. isolated from the digestive tracts.
- The addition of allicin fed to quails reduces the level of Clostridium spp. isolated from the digestive tracts.
3. Results
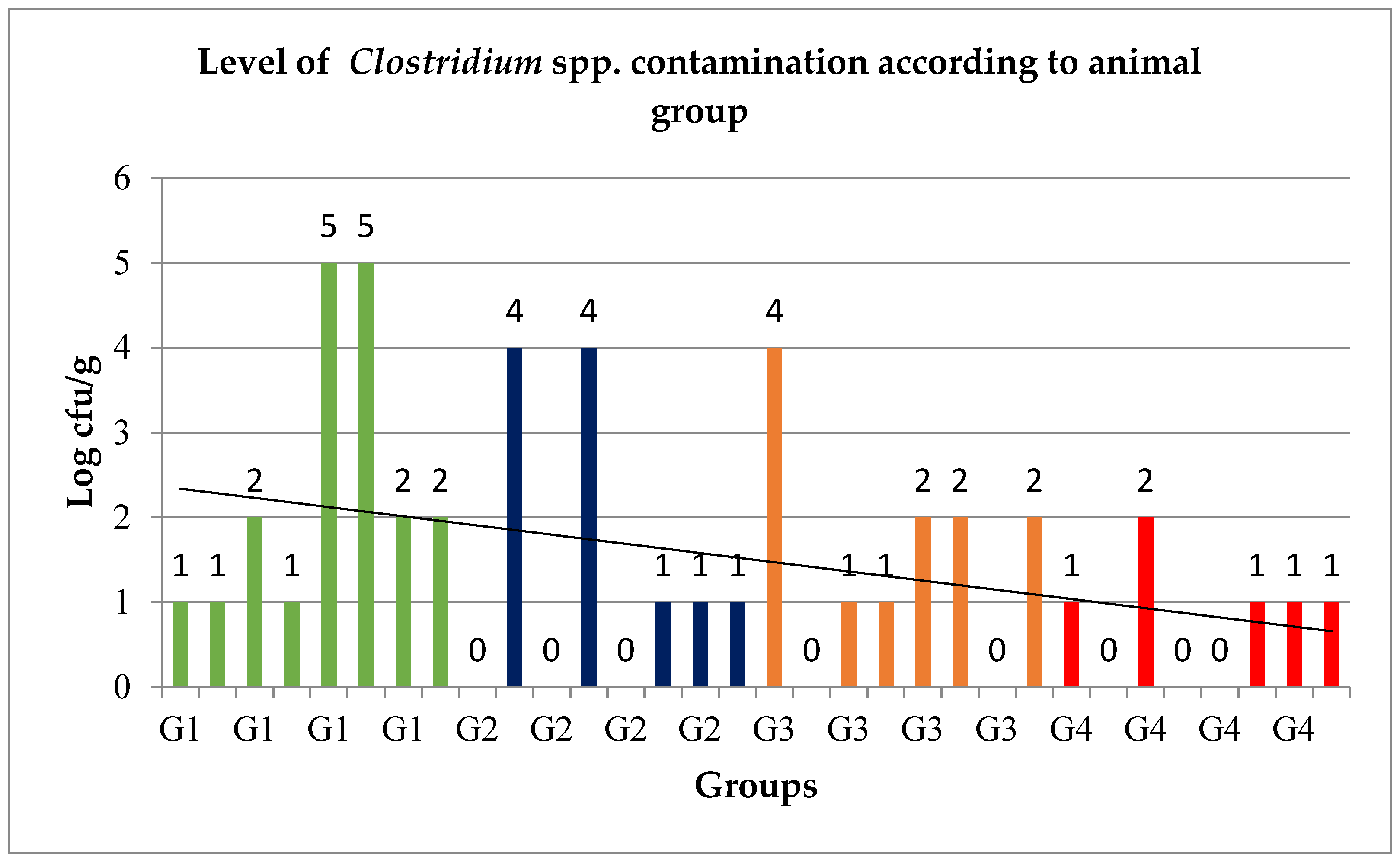
3.1. Level of Clostridium spp. Contamination and Statistical Analysis Results
3.2. Identification of Isolated Strains
3.3. Real-Time PCR Analysis Results
3.4. Multiplex PCR Results for Clostridium Perfringens Detection
3.5. Results of the 16S rDNA Gene Sequencing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, J.; Wang, F.; Yin, Y.; Ma, X. The Nutritional Applications of Garlic (Allium sativum) as Natural Feed Additives in Animals. PeerJ 2021, 9, e11934. [Google Scholar] [CrossRef] [PubMed]
- Regulation—1831/2003-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2003/1831/oj (accessed on 15 October 2024).
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and Biological Properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [PubMed]
- Ilić, D.; Nikolić, V.; Nikolić, L.; Stankovic, M.; Stanojević, L.; Cakic, M. Allicin and Related Compounds: Biosynthesis, Synthesis and Pharmacological Activity. Facta Univ. Ser. Phys. Chem. Technol. 2011, 9, 9–20. [Google Scholar] [CrossRef]
- Salehi, B.; Zucca, P.; Orhan, I.E.; Azzini, E.; Adetunji, C.O.; Mohammed, S.A.; Banerjee, S.K.; Sharopov, F.; Rigano, D.; Sharifi-Rad, J.; et al. Allicin and Health: A Comprehensive Review. Trends Food Sci. Technol. 2019, 86, 502–516. [Google Scholar] [CrossRef]
- Miron, T.; Bercovici, T.; Rabinkov, A.; Wilchek, M.; Mirelman, D. [3H]Allicin: Preparation and Applications. Anal. Biochem. 2004, 331, 364–369. [Google Scholar] [CrossRef]
- Nadeem, M.S.; Kazmi, I.; Ullah, I.; Muhammad, K.; Anwar, F. Allicin, an Antioxidant and Neuroprotective Agent, Ameliorates Cognitive Impairment. Antioxidants 2021, 11, 87. [Google Scholar] [CrossRef]
- Ogbuewu, I.P.; Okoro, V.M.; Mbajiorgu, E.F.; Mbajiorgu, C.A. Beneficial Effects of Garlic in Livestock and Poultry Nutrition: A Review. Agric. Res. 2018, 4, 411–426. [Google Scholar] [CrossRef]
- Puvača, N.; Ljubojević, D.; Kostadinović, L.; Lukač, D.; Lević, J.; Popović, S.; Đuragić, O. Spices and Herbs in Broilers Nutrition: Effects of Garlic (Allium sativum L.) on Broiler Chicken Production. Worlds Poult. Sci. J. 2015, 71, 533–538. [Google Scholar] [CrossRef]
- Kothari, D.; Lee, W.-D.; Niu, K.-M.; Kim, S.-K. The Genus Allium as Poultry Feed Additive: A Review. Animals 2019, 9, 1032. [Google Scholar] [CrossRef]
- Kirubakaran, A.; Moorthy, M.; Chitra, R.; Prabakar, G. Influence of Combinations of Fenugreek, Garlic, and Black Pepper Powder on Production Traits of the Broilers. Vet. World 2016, 9, 470. [Google Scholar] [CrossRef]
- Khan, R.U.; Nikousefat, Z.; Tufarelli, V.; Naz, S.; Javdani, M.; Laudadio, V. Garlic (Allium sativum) Supplementation in Poultry Diets: Effect on Production and Physiology. Worlds Poult. Sci. J. 2012, 68, 417–424. [Google Scholar] [CrossRef]
- El-Ghany, W.A.A. Potential Effects of Garlic (Allium sativum L.) on the Performance, Immunity, Gut Health, Anti-Oxidant Status, Blood Parameters, and Intestinal Microbiota of Poultry: An Updated Comprehensive Review. Anim. Open Access J. 2024, 14, 498. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Scaldaferri, F.; Petito, V.; Gasbarrini, A. Commensal Clostridia: Leading Players in the Maintenance of Gut Homeostasis. Gut Pathog. 2013, 5, 23. [Google Scholar] [CrossRef]
- Manson, J.M.; Rauch, M.; Gilmore, M.S. The Commensal Microbiology of the Gastrointestinal Tract. In GI Microbiota and Regulation of the Immune System; Huffnagle, G.B., Noverr, M.C., Eds.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2008; Volume 635, pp. 15–28. ISBN 978-0-387-79989-6. [Google Scholar]
- Guo, P.; Zhang, K.; Ma, X.; He, P. Clostridium Species as Probiotics: Potentials and Challenges. J. Anim. Sci. Biotechnol. 2020, 11, 24. [Google Scholar] [CrossRef]
- Ley, R.E.; Bäckhed, F.; Turnbaugh, P.; Lozupone, C.A.; Knight, R.D.; Gordon, J.I. Obesity Alters Gut Microbial Ecology. Proc. Natl. Acad. Sci. USA 2005, 102, 11070. [Google Scholar] [CrossRef]
- Kalia, V.C.; Mukherjee, T.; Bhushan, A.; Joshi, J.; Shankar, P.; Huma, N. Analysis of the Unexplored Features of Rrs (16S rDNA) of the Genus Clostridium. BMC Genom. 2011, 12, 18. [Google Scholar] [CrossRef]
- Council Regulation (EC) No 1099/2009 of 24 September 2009 on the Protection of Animals at the Time of killingText with EEA Relevance. Available online: https://eur-lex.europa.eu/eli/reg/2009/1099/oj/eng (accessed on 12 December 2024).
- PN-R-64791:1994; Animal Feeding Stuffs—Requirements and Microbiological Examinations. Polish Committee for Standardization: Warsaw, Poland, 2013.
- Raphael, B.H.; Andreadis, J.D. Real-Time PCR Detection of the Nontoxic Nonhemagglutinin Gene as a Rapid Screening Method for Bacterial Isolates Harboring the Botulinum Neurotoxin (A-G) Gene Complex. J. Microbiol. Methods 2007, 71, 343–346. [Google Scholar] [CrossRef]
- Rood, J.I.; Adams, V.; Lacey, J.; Lyras, D.; McClane, B.A.; Melville, S.B.; Moore, R.J.; Popoff, M.R.; Sarker, M.R.; Songer, J.G.; et al. Expansion of the Clostridium Perfringens Toxin-Based Typing Scheme. Anaerobe 2018, 53, 5–10. [Google Scholar] [CrossRef]
- Vaneechoutte, M.; Cartwright, C.P.; Williams, E.C.; Jäger, B.; Tichy, H.-V.; De Baere, T.; De Rouck, A.; Verschraegen, G. Evaluation of 16S rRNA Gene Restriction Analysis for the Identification of Cultured Organisms of Clinically Important Clostridium Species. Anaerobe 1996, 2, 249–256. [Google Scholar] [CrossRef]
- Grenda, T.; Kukier, E.; Kwiatek, K. Methods and Difficulties in Detection of Clostridium Botulinum and Its Toxins. Pol. J. Vet. Sci. 2014, 17, 195–205. [Google Scholar] [CrossRef]
- Navarro, M.A.; McClane, B.A.; Uzal, F.A. Mechanisms of Action and Cell Death Associated with Clostridium Perfringens Toxins. Toxins 2018, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Uzal, F.A. Diseases Produced by Clostridium Perfringens Type A in Mammalian Species. In Clostridial Diseases of Animals; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 107–116. ISBN 978-1-118-72829-1. [Google Scholar]
- Immerseel, F.V.; Buck, J.D.; Pasmans, F.; Huyghebaert, G.; Haesebrouck, F.; Ducatelle, R. Clostridium Perfringens in Poultry: An Emerging Threat for Animal and Public Health. Avian Pathol. 2004, 33, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Tian, R.; Imanian, B.; Williamson, C.H.D.; Johnson, S.L.; Daligault, H.E.; Schill, K.M. Integration of Complete Plasmids Containing Bont Genes into Chromosomes of Clostridium Parabotulinum, Clostridium Sporogenes, and Clostridium Argentinense. Toxins 2021, 13, 473. [Google Scholar] [CrossRef]
- Poulain, B.; Popoff, M.R. Why Are Botulinum Neurotoxin-Producing Bacteria So Diverse and Botulinum Neurotoxins So Toxic? Toxins 2019, 11, 34. [Google Scholar] [CrossRef]
- Lee, W.H.; Riemann, H. The Genetic Relatedness of Proteolytic Clostridium Botulinum Strains. J. Gen. Microbiol. 1970, 64, 85–90. [Google Scholar] [CrossRef]
- Gong, R.; Ye, X.; Wang, S.; Ren, Z. Isolation, Identification, and Biological Characteristics of Clostridium Sartagoforme from Rabbit. PLoS ONE 2021, 16, e0259715. [Google Scholar] [CrossRef]
- Lee, W.-K.; Fujisawa, T.; Kawamura, S.; Itoh, K.; Mitsuoka, T. Clostridium Intestinalis Sp. Nov., an Aerotolerant Species Isolated from the Feces of Cattle and Pigs. Int. J. Syst. Bacteriol. 1989, 39, 334–336. [Google Scholar] [CrossRef]
- Elsayed, S.; Zhang, K. Bacteremia Caused by Clostridium intestinale. J. Clin. Microbiol. 2005, 43, 2018–2020. [Google Scholar] [CrossRef]
- Cassir, N.; Benamar, S.; La Scola, B. Clostridium Butyricum: From Beneficial to a New Emerging Pathogen. Clin. Microbiol. Infect. 2016, 22, 37–45. [Google Scholar] [CrossRef]
- Williamson, C.H.D.; Vazquez, A.J.; Hill, K.; Smith, T.J.; Nottingham, R.; Stone, N.E.; Sobek, C.J.; Cocking, J.H.; Fernández, R.A.; Caballero, P.A.; et al. Differentiating Botulinum Neurotoxin-Producing Clostridia with a Simple, Multiplex PCR Assay. Appl. Environ. Microbiol. 2017, 83, e00806-17. [Google Scholar] [CrossRef]
- Ankri, S.; Mirelman, D. Antimicrobial Properties of Allicin from Garlic. Microbes Infect. 1999, 1, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Jonkers, D.; Van Den Broek, E.; Van Dooren, I.; Thijs, C.; Dorant, E.; Hageman, G.; Stobberingh, E. Antibacterial Effect of Garlic and Omeprazole on Helicobacter Pylori. J. Antimicrob. Chemother. 1999, 43, 837–839. [Google Scholar] [CrossRef] [PubMed]
| The Group from Which the Strain Was Isolated | Sequencing Results According to BLAST Analysis | % Similarity | Accession Number |
|---|---|---|---|
| G1–G4 | Clostridium sporogenes | 89–97% | CP082942.1 |
| G1–G4 | 92% | CP013242.1 | |
| G1–G4 | 90–96% | DQ278864.1 | |
| G1–G4 | 95% | CP084367.1 | |
| G1–G4 | 94–95% | MT356160.1 | |
| G1–G4 | 94% | CP011663.1 | |
| G1 | Clostridium sartagoforme | 87% | OP862452.1 |
| G1 | 93% | MN646980.1 | |
| G4 | 95% | MW450913.1 | |
| G3 | Clostridium butyricum | 94% | AY540106.1 |
| G2 | Clostridium intestinale | 79% | MK559547.1 |
| G2 | Clostridium perfringens | 94–96% | ON870866.1 |
| G3 | 95–96% | ON870867.1 | |
| G2 | Clostridium saccharolyticum | 94% | CP002109.1 |
| G4 | 95% | FJ957875.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makuch, A.; Ziomek, M.; Sapała, M.; Drabik, K.; Batkowska, J.; Domaradzki, P.; Patyra, E.; Grenda, T. The Impact of Allicin on the Growth of Clostridium spp. in the Digestive Track of Quails. Animals 2025, 15, 906. https://doi.org/10.3390/ani15070906
Makuch A, Ziomek M, Sapała M, Drabik K, Batkowska J, Domaradzki P, Patyra E, Grenda T. The Impact of Allicin on the Growth of Clostridium spp. in the Digestive Track of Quails. Animals. 2025; 15(7):906. https://doi.org/10.3390/ani15070906
Chicago/Turabian StyleMakuch, Aleksandra, Monika Ziomek, Magdalena Sapała, Kamil Drabik, Justyna Batkowska, Piotr Domaradzki, Ewelina Patyra, and Tomasz Grenda. 2025. "The Impact of Allicin on the Growth of Clostridium spp. in the Digestive Track of Quails" Animals 15, no. 7: 906. https://doi.org/10.3390/ani15070906
APA StyleMakuch, A., Ziomek, M., Sapała, M., Drabik, K., Batkowska, J., Domaradzki, P., Patyra, E., & Grenda, T. (2025). The Impact of Allicin on the Growth of Clostridium spp. in the Digestive Track of Quails. Animals, 15(7), 906. https://doi.org/10.3390/ani15070906







