Simple Summary
Salmonella contamination in poultry remains a significant public health concern, impacting both the poultry industry and consumers. Effective control measures must span the entire poultry production continuum, from farm to table, to mitigate the risk of contamination and outbreaks.
Abstract
This mini-review presents common strategies for controlling Salmonella in poultry, addressing combined pre-harvest and post-harvest interventions to create a multi-hurdle approach. The goal is to highlight integrated approaches that enhance overall food safety and sustainability within the poultry industry. Current pre-harvest and post-harvest strategies are discussed, including industry practices and regulatory frameworks. Emphasis is placed on the implementation of biosecurity measures, vaccination, feed management, and environmental control in pre-harvest settings, as well as processing plant interventions such as antimicrobials for carcass decontamination, sanitation, and quality control measures. Pre-harvest strategies that have shown promise include enhanced biosecurity protocols, selective vaccinations for pathogenic Salmonella strains, and advanced feeding regimens. Post-harvest interventions, such as antimicrobial application for decontamination, have improved sanitation practices, and pathogen reduction technologies are also critical in reducing Salmonella prevalence. An integrated approach that combines both pre-harvest and post-harvest measures is essential for an effective Salmonella control program. Implementing a continuum of control strategies for Salmonella in poultry production is vital for ensuring food safety and protecting public health. Collaborative efforts between researchers, industry stakeholders, and policymakers are necessary to address emerging issues and enhance overall effectiveness.
1. Introduction
Poultry has emerged as the most consumed meat product, and it is projected to remain the world’s largest imported livestock commodity by volume over the next decade [1,2]. In 2023, broiler chicken was the most consumed animal protein in the United States, with per capita consumption reaching approximately 99.5 pounds [3]. This consumption rate is expected to increase to around 105 pounds in 2025, reflecting the ongoing consumer preference for poultry products [3]. This surge in demand can be attributed to affordability, nutritional value, and versatility in culinary applications [1,4]. As a result, domestic production has significantly increased and continued to increase in the United States [1,5], which stands as the world’s largest producer and second largest exporter of broilers [5]. Poultry production involves multiple stages before products reach consumers. For broilers, it begins with raising chicks from hatch to performance, followed by processing, resulting in diverse poultry products for consumption or use as by-products [6]. In the United States, the poultry industry operates through a vertically integrated system encompassing production (hatcheries, feed mills, breeders, and broilers), processing (deboning, cutting, and packaging), and distribution [7]. This system enhances efficiency and quality control throughout the supply chain. Furthermore, U.S. poultry production comprises both commercial and small-scale operations, which differ in production and management approaches. The commercial sector, which includes broiler chickens, turkeys, and table egg layers, relies on controlled breeding programs to manage genetics and optimize productivity [8]. Here, broiler chickens and turkeys are transferred directly from hatcheries to broiler or turkey grower farms [9]. Small-scale poultry operations, including backyard poultry and specialty poultry like pasture, free-range, cage-free, and organic operations, sometimes involve multiple sources and may have contact with each other, not adhering to the all-in-all-out management systems typically seen in commercial operations [9,10,11]. This variability can introduce challenges in disease management and biosecurity [10]. In fact, poultry has been identified as a significant reservoir for foodborne pathogens, notably Salmonella and Campylobacter [7]. Consequently, food safety is a critical consideration across all stages of the poultry production continuum from pre-harvest (farm) to post-harvest (processing plants) and consumers. While many mitigation efforts have been developed, evaluated, and found to be effective, these enteric pathogens remain a significant food safety and public health challenge [12]. Therefore, continued efforts to enhance safety standards and educate consumers are essential to protect public health while meeting the growing demand for poultry products. This mini-review combines common strategies that have been reported and proven at pre-harvest and post-harvest production stages and discussed strategies to integrate these systems to create more comprehensive multi-hurdle and multi-technology approaches, as combined efforts are better than a single strategy.
2. Salmonella Complexity in Poultry Production
Salmonella enterica is one of the most significant pathogens in poultry production, posing a significant risk to food safety and public health [13]. This pathogen is diverse, having many serotypes that are categorized into two primary illness types. Typhoidal Salmonella causes illnesses like typhoid fever, a severe illness that can be life-threatening if untreated, and non-typhoidal Salmonella is commonly linked to foodborne infections that can spread through contaminated poultry products [14]. Nontyphoidal Salmonella infection can cause symptoms ranging from mild to severe gastrointestinal issues such as abdominal pain, diarrhea, fever, and nausea with potential long-term health problems like reactive arthritis [15,16]. According to the Centers for Disease Control and Prevention (CDC), Salmonella infections are usually self-limiting, lasting 4–7 days, but can last longer when the infection spreads to other parts of the body, leading to complications [16]. This diversity in Salmonella enterica is further expressed through host adaptation, environmental prevalence and persistence, and virulence. While some serotypes are broad host-adapted like the serotypes of Enteritidis and Typhimurium (non-typhoidal), others like Typhi (typhoidal) thrive in specific hosts [12,13,14]. Salmonella enterica includes over 2500 serotypes, and only a few, such as Enteritidis, Heidelberg, Infantis, I 4,[5],12:i:−, Kentucky, and Typhimurium, have been historically linked to poultry [17,18]. Interestingly, despite the prevalence of the Kentucky serotype in poultry, it is less frequently associated with salmonellosis, unlike others, e.g., Enteritidis, Infantis, I 4,[5],12:i:−, Newport, and Typhimurium [19,20,21,22]. Moreover, recent data indicate that the Infantis serotype has become an emergent serotype in post-harvest poultry production in the United States [20]. These serotypes are unique and diverse in colonization and virulence in poultry and humans [13,20] and differ in their response to common mitigation strategies.
Nontyphoidal Salmonella colonizes the intestinal tract of poultry, causing the contamination of meat during processing and handling. Poultry and its products are vulnerable to contamination at various points in the production chain, from farms to processing plants. On farms, Salmonella can be introduced through multiple sources, such as contaminated feed and the farm environment through water, equipment, and personnel [13,23,24]. Vertical transmission from breeder flocks to chicks is a critical route, by which infected hens can transmit Salmonella directly to their offspring via contaminated eggs [25]. Horizontal transmission is also common, occurring among birds through direct contact and shared spaces [13]. At the processing stage, cross-contamination can occur during slaughtering, defeathering, evisceration, and sectioning [13]. Improper handling of carcasses during scalding, poor hygiene practices in evisceration, poor cleaning and sanitation of surfaces and equipment, or inadequate antimicrobial treatment can further facilitate the spread of Salmonella, increasing the risk of contaminated products reaching consumers [20,26]. The effective control of Salmonella will require a comprehensive strategy throughout the production continuum, from farms to processing plants.
With diverse serotypes exhibiting varying levels of pathogenicity [20,27], managing Salmonella requires a comprehensive, risk-based approach across the entire food production chain. Key strategies for controlling Salmonella at the farm level include the use of synthetic and natural compounds, such as vaccines, to enhance the immune response in chickens [13,25]. Effective litter management, feed fortification with probiotics and/or prebiotics, and drinking water sanitation are common methods employed to reduce the pathogen load [28,29]. These measures aim to limit Salmonella colonization and spread among poultry populations. In poultry processing plants, multi-hurdle technology is applied to further reduce Salmonella levels. This includes the use of various chilling methods, chemical treatments, and mechanical interventions designed to minimize bacterial contamination [30,31,32,33]. For example, chilling conditions can be optimized to inhibit bacterial growth, while chemical washes or sprays may be used to kill bacteria on carcass surfaces [31]. Further, Salmonella control during processing relies on effective monitoring programs, adherence to good manufacturing practices (GMPs), and Hazard Analysis Critical Control Point (HACCP) protocols [34]. These programs ensure that contamination is detected and addressed promptly, maintaining the safety of poultry products for consumers.
Given the complexity of Salmonella, increased knowledge about specific serotypes through surveillance and monitoring will enable more targeted interventions [20]. Different serotypes may respond differently to control measures, so tailored approaches can enhance the effectiveness of interventions [35]. Ultimately, controlling Salmonella within the poultry industry is a collaborative effort involving producers, processors, and consumers. Each stakeholder plays a crucial role in ensuring that poultry products are safe for consumption. Producers must implement effective on-farm practices, processors need to apply rigorous interventions at the plant level, and consumers need to practice safe food handling to mitigate the risk of Salmonella infection (Figure 1). To address these challenges, the industry is increasingly adopting advanced technologies and practices, such as improved biosecurity measures and enhanced surveillance systems that evaluate serotype populations [12,18,20,36], as well as the use of novel feed additives and vaccine preparations like electron-beam-inactivated vaccines [37,38] to reduce pathogen loads. Additionally, ongoing research efforts aim to further improve poultry health and welfare while minimizing the environmental impact of production. More recently, whole genome sequencing (WGS), an application of genomics, has been used in the diagnosis, epidemiology, and surveillance of Salmonella [39,40].
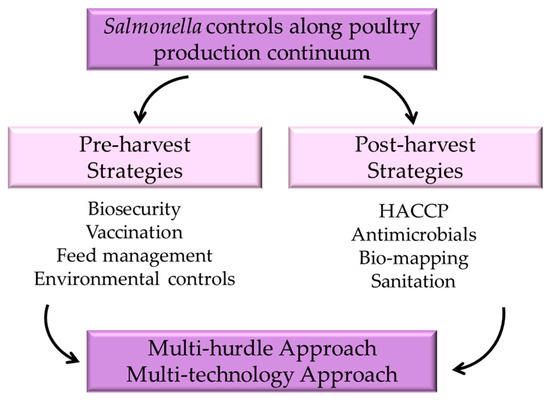
Figure 1.
Strategies to control Salmonella in poultry production.
3. Pre-Harvest Control Strategies
The USDA’s recently proposed framework, which could require the testing of incoming flocks for Salmonella before processing, has spurred renewed interest in understanding Salmonella dynamics within poultry production environments [41]. These interests include understanding the interactions between poultry and their environment and developing novel pre-harvest intervention strategies. As previously noted, poultry are particularly susceptible to Salmonella colonization from various sources during live production, including hatcheries, contaminated feed, breeder flocks, farm environments, litter, and during feed withdrawal [13,28,42]. Among these, the hatchery is one of the most significant contributors to Salmonella prevalence, with a reported 48.5% prevalence [28]. Consequently, obtaining Salmonella-free chicks is critical to reducing Salmonella incidence at this early stage. Intervention strategies targeting foodborne pathogens in pre-harvest broiler production focus on Salmonella and Campylobacter, specifically Salmonella serotypes frequently associated with human illness, such as Typhimurium and Enteritidis [7]. While Salmonella Kentucky is the most isolated in live production samples, it is less prevalent in processing plant samples, indicating that processing control measures are effectively mitigating this serotype [20,35,36].
Salmonella can contaminate raw feed ingredients at several stages of manufacturing. Factors influencing microbial multiplication at feed plants include moisture levels, feed composition, and thermal processing intensity [43]. The studies reviewed by [28] show that Salmonella prevalence in poultry feed can range from 0 to 100% and 0 to 40% according to studies within the United States. The removal of antibiotics from poultry production has created a demand for alternative feed amendments that can replicate some of the gut health and performance benefits traditionally provided by antibiotics [44,45]. Despite the need for alternatives, the effectiveness of some of these compounds as feed amendments has been mixed [46], especially since Salmonella is very diverse; hence, using a broad-spectrum antimicrobial agent that would be effective on multiple serotypes and strains is critical. In recent years, non-antibiotic alternatives in poultry production have gained attention [29]. These include feed-based interventions like probiotics, prebiotics, phytobiotics, and postbiotics [44,45,46]. The concept of competitive exclusion, in which beneficial bacteria outcompete pathogens for space and nutrients in the digestive tract, has been explored using probiotics [47,48,49,50]. Probiotics have shown potential in mitigating Salmonella infections [51], but many feed additives lack similar efficacy. More research is needed to identify effective feed additives with consistent efficacy. Beyond feed-based approaches, nonfeed-based alternatives, such as antimicrobials, vaccines, and in ovo strategies, are common interventions to combat the Salmonella burden in poultry [29,52]. Vaccination is a crucial health management strategy for boosting poultry flock immunity and is widely used in breeders and broilers to reduce Salmonella colonization and prevalence [29]. Vaccines are among the most effective and cost-efficient tools for preventing diseases in birds [53]. Typically, poultry vaccines use Salmonella serotypes Typhimurium and/or Enteritidis [54], which are broad-spectrum serotypes colonizing many food animals and humans and implicated in many foodborne outbreaks. Available vaccine types include live attenuated, inactivated, and subunit vaccines, offering various options for disease prevention [55,56]. Advances in Salmonella vaccines include the evaluation of electron-beam (eBeam) irradiation technology in vaccine preparation [57]. This has been used to prepare an inactivated Salmonella Enteritidis vaccine that was found to reduce cecal colonization [37]. Apart from single-serotype vaccines, this could potentially be used to evaluate multi-serotype vaccines for effective Salmonella control during pre-harvest poultry production.
In addition to enhancing flock performance through an effective feeding regimen and vaccination, effective poultry drinking water sanitation is vital for pre-harvest food safety, aiming to minimize foodborne pathogens and protect consumer health [58,59]. While the acceptable microbial load for poultry drinking water is 1000 CFUs per milliliter of aerobic bacterial count, the presence of E. coli and other pathogens is unacceptable (0 CFU/mL) [60]. Salmonella contamination in drinking water systems is typically minimal, with a prevalence ranging from 0 to 11% [28], and water treatment further reduces the risk. However, the optimal temperature (around 25 °C), low flow rates, and nutrient availability can promote microbial contamination and biofilm formation, complicating disinfection strategies [28,29,59]. Various chemical-based sanitizers are used to disinfect poultry drinking water, though their efficacy remains uncertain, highlighting the need to demonstrate the effectiveness of various water sanitation strategies. Biofilm removal in the drinking water system is also vital for the effective reduction in opportunistic and pathogenic microbial populations in the drinking water system [58,61]. Robust biosecurity measures are crucial in controlling Salmonella transmission and improving food safety [62]. Physical barriers, including fences, mesh wire, footbaths, and farm equipment disinfection, are key components of biosecurity programs [63,64]. Additionally, rodent and fly control, red mite management, and disinfection between flocks are recommended to reduce Salmonella incidence and disrupt disease cycles [65]. Proper litter management, such as composting, is essential in lowering Salmonella contamination, as fresh wood shavings are linked to higher contamination than older litter [66,67]. Biosecurity practices are among the most cost-efficient and effective preventive measures for managing disease risks to the economy, environment, and public health [68]. They not only reduce infectious disease risks but also hold regulatory importance. The U.S. Food Safety Modernization Act’s Preventive Control for Animal Food regulation is a valuable resource for ensuring poultry product safety [69]. In summary, an integrated approach encompassing control from the hatchery to the farm through feed amendments, vaccination, water sanitation, biosecurity measures, and regulatory compliance is essential for effectively controlling Salmonella in poultry production and ensuring pre-harvest food safety.
4. Post-Harvest Strategies
While pre-harvest strategies focus on preventing and managing contamination at the farm level, post-harvest interventions aim to minimize or eliminate contamination during the stages of processing, packaging, and distribution. Although the microbiological quality of poultry meat is influenced by the health of live birds, it is crucial to ensure there is no cross-contamination during processing operations. Government regulations are essential in establishing and enforcing standards for controlling Salmonella in the poultry industry [70]. The U.S. Department of Agriculture Food Safety and Inspection Service (USDA-FSIS) has set specific performance standards for Salmonella in poultry products [70]. These standards dictate a certain percentage of chicken samples that may test positive for Salmonella, ensuring food safety [30,70]. To comply with these standards, poultry processing plants are required to implement a Hazard Analysis Critical Control Point (HACCP) plan in addition to existing regulations with strict adherence to good manufacturing practices and thorough sanitation procedures throughout the processing stages [13,71]. These systems are designed to identify and manage potential hazards throughout the processing steps, and adherence to these guidelines can result in meaningful improvements in food safety [24]. While these guidelines provide a foundation, they have not always produced the desired food safety result. Poultry integrators have adopted additional strategies, including the bio-mapping of processing environments [72,73]. Microbial mapping, also called bio-mapping, has become an invaluable tool for monitoring and managing Salmonella contamination, allowing processors to identify contamination hotspots within the processing environment and promptly mitigate contamination risks [72,73,74,75]. This approach systematically samples and analyzes different areas within a processing plant to identify the locations most vulnerable to Salmonella contamination [76]. For example, ref. [74] evaluated the presence of Salmonella on chicken samples obtained after the bleeding, scalding, defeathering, carcass opening, evisceration, bird washing, prechilling, and chilling steps of poultry processing to demonstrate the utility of bio-mapping. This study revealed higher contamination levels, particularly after bleeding, scalding, defeathering, and evisceration [74]. Interestingly, there was also a decrease in contamination in areas to which specific treatments were applied. Likewise, ref. [75] evaluated Salmonella incidence across similar processing steps, with scalding and evisceration lines consistently being identified as high-risk areas. Furthermore, ref. [72] conducted a comprehensive bio-mapping study assessing not just the quantity of pathogens (Salmonella and Campylobacter) but also indicator organisms like aerobic bacteria and Enterobacteriaceae. This was performed under two different processing conditions, a normal chemical process with typical chemical interventions; and a reduced chemical process with minimal or no chemical interventions at different locations within a processing line, ranging from live receiving to post-chilling and parts (wings) processing [72]. The results suggest that the normal chemical process generally resulted in significantly lower Salmonella counts compared to the reduced chemical process at most locations. Generally, the study corroborates other studies [74,75,77] that highlight scalding, picking, and evisceration as key areas to reduce the bacterial load. These studies highlight the need for the effective disinfection of chicken carcasses at the primary processing (slaughter to chilling) stage to reduce the microbial load during secondary processing, including during deboning, portioning, and packaging [77].
Maintaining hygienic standards in poultry processing facilities is essential in reducing the risk of Salmonella contamination. Effective cleaning and sanitation, which include the routine application of approved cleaning agents and sanitizers, play a key role in this process [77]. This process is designed to minimize the presence of Salmonella on surfaces and equipment that are in direct contact with products [77]. Aside from equipment, poultry workers should maintain adequate hygienic standards. Workers are often in close contact during processing, making them potential vectors for cross-contamination if proper hygienic practices are not followed [78]. Commercial processing plants encourage frequent handwashing practices with the use of personal protective equipment (PPE) to prevent cross-contamination from employees. Commonly used sanitizers, such as chlorine-based compounds, peracetic acid (PAA), and quaternary ammonium compounds, have been recognized for their efficacy in processing plants [33,79,80]. To achieve comprehensive cleaning and sanitation, it is essential that processing equipment is hygienically designed to allow for easy access during cleaning and sanitation [77,81]. However, studies [77,82,83] have shown that Salmonella is resilient and continuously evolving past control strategies, persisting on processing surfaces after antimicrobial interventions. The authors [77] reported a high prevalence of Salmonella on several pieces of processing equipment, including the head puller, scalder, picker, and cropper, after sanitation. The prevalence varied between the plants evaluated in the study, and this could be due to variability in the sanitizers used by the plants and effective cleaning procedures. The application of sanitizers during processing stages such as scalding, eviscerating, internal/external bird washing, and chilling shows a paramount intervention strategy to ensure product safety; however, as previously noted, the use of sanitizers varies across processing facilities. Historically, chlorine has been widely used in poultry processing due to its cost effectiveness and the fact that it only requires a low concentration for pathogen reduction [33]. However, some studies have reported concerns about its actual effectiveness in certain applications within processing environments [32,84,85]. For instance, chlorine has been found to be less effective when applied as a spray [33,84] compared to other applications, like immersion. These authors suggest that spraying chlorine on carcass surfaces does not always reduce bacteria as expected, leading to bacterial persistence on carcasses and surfaces. Moreover, the use of higher concentrations of chlorine on poultry products has been associated with undesirable outcomes, such as the creation and release of trihalomethanes, a by-product of chlorine with organic compounds in water [86]. Peracetic acid (PAA), however, is the most popular disinfectant used in poultry processing [33]. Research has shown that even at low concentrations and permissible levels, PAA effectively reduces Salmonella post-chilling [31,84,87,88,89,90]. Ref. [31] compared PAA with chlorine and showed that PAA is more effective in reducing pathogen levels. Likewise, ref. [90] showed its efficacy was significantly better than cetylpyridinium chloride (CPC), achieving approximately 1.5 and 1.3 log10 reductions at 0.07 and 0.1% concentrations, respectively, while CPC achieved a 0.8 log reduction at 0.35 and 0.6%. Although the use of PAA in immersion methods typically shows more consistent outcomes, a review by [80] noted that spray applications tend to yield variable results. There is a growing interest in exploring the potential of combining PAA with other antimicrobials to enhance efficacy and prevent antimicrobial tolerance that could arise from consistent use. Quaternary ammonium compounds like CPC have been shown to be effective against Salmonella on poultry carcasses; however, the contact time set by the USDA could limit its usage, along with the need to perform a portable water rinse after its application [33,91,92]. In addition to these antimicrobials, other pathogen reduction technologies have been evaluated to control Salmonella in poultry products and during processing. Some of these approaches are electrochemically activated water (ECAW), ozone-based technologies, and bacteriophages [93,94,95]. However, some of these approaches have not been thoroughly evaluated, limiting our understanding of their efficacy. For instance, ECAW, which produces hypochlorous acid from the electrolysis of salt and water [93], was reported to reduce Salmonella counts in a simulated chiller environment at 200 ppm; however, this concentration is significantly higher than the 50 ppm recommended for contact with poultry carcasses in the chiller [91]. Likewise, ref. [94] evaluated ozonated water on chicken wings at three concentrations, 2.5, 5, and 10 ppm. The authors reported reduction levels lower than 1.0 log10 CFU/mL, which may not be practical for industry applications.
Despite the effectiveness of some of these interventions, Salmonella can persist on the surface of processing equipment and develop biofilms that are difficult to remove [82,96,97]. Factors like the improper usage of antimicrobials, exposure to sub-lethal concentrations, and temperature abuse could encourage biofilm formation and Salmonella persistence in processing environments [77,98,99]. Biofilms on processing equipment can protect Salmonella from sanitizers, allowing the survival and potential cross-contamination of subsequent batches of poultry products [77,83,100]. In addition, the quantification of Salmonella that persist on surfaces after processing is crucial for assessing cross-contamination risks, the effectiveness of sanitation practices, control measures, and public health risks. While current sanitary processing and processing measures have significantly reduced Salmonella contamination, there remains an ongoing need for continual improvements to address the challenges of emerging virulent serotypes, biofilm formation, antimicrobial tolerance and/or resistance, and other factors contributing to Salmonella survival and persistence.
5. Multi-Hurdle Approach to Controlling Salmonella
Combating Salmonella requires a multi-hurdle approach that aligns with a One Health perspective, emphasizing the interconnectedness of human, animal, and environmental health [101]. The complexity and adaptability of Salmonella necessitates a multifaceted approach to effectively lower contamination levels in poultry [102]. A summary of current strategies discussed in the review implemented individually or as a combined approach are highlighted in Table 1. However, no single intervention, whether it be pre-harvest measures or post-harvest strategies, has shown sufficient efficacy to significantly reduce Salmonella prevalence within the poultry production continuum [13,103,104]. This limitation highlights the challenges poultry integrators face in managing this pervasive pathogen that can persist under and adapt to diverse environmental conditions [77,96,105,106].
The multi-hurdle approach for controlling Salmonella in poultry is characterized by incorporating several interventions that work synergistically to minimize contamination risks [13,107,108,109,110,111]. This approach will integrate multiple control measures to create a composite effect that is more effective than individualized measures [112]. By deploying several strategies simultaneously, producers can target different stages of the poultry production chain, from pre-harvest to processing, thereby addressing potential contamination points (control points) more thoroughly. This comprehensive strategy will focus on identifying and addressing microbial hazards at various critical control points throughout the poultry production process and establishing mitigation plans. This plan will emphasize the importance of integrating controls from the earliest stages of poultry production, specifically during the pre-harvest phase, when management practices including biosecurity and Salmonella-free flocks at the breeder stage are critical [104,107]. This holistic methodology ensures that even if one control measure fails or is less effective, others will still contribute to overall pathogen reduction.
For instance, a successful pre-harvest multi-hurdle approach will employ combined interventions such as vaccination, a feeding strategy to induce competitive exclusion, water treatments for biofilm control and to improve gut health, and enhanced hygiene practices to prevent Salmonella and other enteric pathogens from proliferating in the birds [13,29,38,59]. A robust multi-hurdle vaccination program could include the established vaccination program in combination with the application of autogenous vaccines to maximize the Salmonella reduction. An autogenous vaccine is a custom vaccine prepared from Salmonella serotypes detected in a flock through surveillance and monitoring [113]. This vaccine is then used on subsequent flocks on the same farm. Post-harvest, the combination of strategies highlighted in Table 1 will ensure a continued reduction and prevent pathogen introduction into the supply chain. This method of using a combined approach will contribute to a more resilient food safety system by applying multiple corrective actions to tackle Salmonella contamination across the entire production chain [114]. While some studies have reported a significant reduction when two or more applications [109,115] are used, others have not seen similar effect [116], meaning there is a need for continued efforts to study the effectiveness of combining applications post-harvest. These efforts should also establish a methodology for applying certain measures, as combined synergistic applications differ from sequential synergistic applications [108,109,110,115].
It is noteworthy that the use of plant-derived, food-grade phytochemical nano emulsions represents a promising intervention in the multi-hurdle multi-technology approach to control Salmonella and other pathogens in poultry production [117,118,119]. These phytochemicals are developed from various plant sources and prepared in nano-emulsions to increase their dispersion in aqueous medium [120,121]. They are designed to be safe for consumption while effectively targeting pathogens. Authors have shown that employing such interventions can significantly reduce the colonization and presence of harmful bacteria in poultry, thereby enhancing overall food safety [120,121,122]. These efforts can be effectively combined with existing efforts like biosecurity measures restricting access to poultry farms, ensuring proper sanitation practices, managing external vectors that may carry pathogens, and other antimicrobial applications like bacteriophages [64,123,124].
Moreover, by optimizing factors like ventilation, litter management, and waste disposal, growers can create environments that are less conducive to the growth of harmful bacteria, significantly reducing contamination risks [125]. Reducing the microbial load on farms can significantly enhance the effectiveness of the processing strategies previously discussed. The United States Department of Agriculture (USDA) and the Food and Drug Administration (FDA) are key regulatory agencies that endorse the multi-hurdle approach in their guidelines for controlling Salmonella in poultry [104,126]. Their support reflects a recognition of the limitations of individual interventions and the need for a comprehensive strategy to manage food safety risks associated with poultry production. The regulatory emphasis on the multi-hurdle approach also encourages collaboration among stakeholders in the poultry industry, including producers, processors, and suppliers. Such coordination is vital for implementing effective interventions and aligning practices across the production continuum to ensure that each link contributes to the goal of reducing Salmonella contamination. This shared vision facilitates the development of best practices and innovative solutions, further enhancing food safety. The investigation of novel interventions like bacteriophages reflects a commitment to continually improving food safety protocols in poultry production [127,128]. Bacteriophages have continued to gain attention due to their ability to selectively infect and kill Salmonella, presenting a promising method for reducing its prevalence in poultry products without negatively impacting the broader microbiota essential for poultry health [95,129]. Their application could provide a complementary strategy within the multi-hurdle approach, potentially enhancing its overall effectiveness against Salmonella. By integrating cutting-edge technologies and biological solutions within the multi-hurdle approach, the industry aims to adapt to evolving challenges posed by pathogens like Salmonella, ensuring that poultry remains a safe choice for consumers.

Table 1.
Management strategies and their mode of application in different studies.
Table 1.
Management strategies and their mode of application in different studies.
| Strategies | Type of Application | Application | References |
|---|---|---|---|
| Feed management | Prebiotics | In feed | [47,48,50,51] |
| Probiotics, phytobiotics, postbiotics, feed additives | In feed | [28,29] | |
| In ovo strategies | Bioactive substances | In Ovo | [52,130] |
| Vaccines | Live attenuated, inactivated, subunit, killed | In Ovo, oral, intramuscular | [29,37,38,54,55,57] |
| Bacteriophages | Lytic phage lysates | Intra-cloacal | [131] |
| Encapsulated phage | In drinking water | [132,133] | |
| Lytic phage | Chicken breast fillet and skin | [115] | |
| Drinking water management | Sanitizers | In drinking water | [58,59,60,61] |
| Biosecurity | Physical barriers | General on-farm practices | Physical barriers |
| Rodent and fly control Red mite management | Sanitation protocols | [65,134] | |
| Litter management | Fresh wood shavings | Composting | [66,67] |
| HACCP | Good manufacturing practices | Sanitation procedures | [24,30,34] |
| Bio-mapping | Identification of contamination hotspots | Sampling at processing | [72,73,74,75,77] |
| Antimicrobials | Peracetic acid (PAA) | In water processing | [31,32,84,87,88,89,90] |
| Sanitation | Chlorine-based compounds | Sprays | [79,80] |
| Quaternary ammonium compounds | Sprays | [33,80,82,84] | |
| Multi-technology | Pre-harvest | [48,54,62,65,124] | |
| Post-harvest | [108,109,111,115,116] |
6. Conclusions and Future Directions
Adopting a comprehensive and integrative strategy for controlling Salmonella along the poultry production continuum allows for a robust framework that can be adopted for other pathogens and poultry species like turkey and duck production. These strategies will not only focus on eliminating existing pathogens but on preventing future contamination, thereby fostering a sustainable environment for poultry production. However, a potential limitation to the multi-hurdle multi-technology approach is the cost implication of adopting multiple strategies rather than a few single approaches. Therefore, research looking into risk assessments and cost benefits of this control measure is critical to the successful adoption of this multi-strategy approach. As the poultry industry evolves, the continual development and assessment of new interventions will be necessary to advance the control of foodborne pathogens and other pathogenic microbial populations effectively. The continuous evaluation and refinement of these interventions are essential to adapt to emerging challenges, such as evolving pathogen profiles and changing production practices. Studies focusing on innovative interventions and technologies intended to complement the established approaches will be crucial in mitigating microbial evolution. For example, research looking into the use of specific bacteriophages targeting specific Salmonella serotypes or a combination of bacteriophages targeting a wide range of serotypes is promising to eliminate serotypes of concern in poultry and reduce public health risks. This line of inquiry will represent efforts to utilize biological methods alongside traditional interventions, potentially offering additional pathways for pathogen control. Future advancements in technology and methods, including machine learning and microbiome studies, may further optimize the multi-hurdle approach, leading to improved control measures for Salmonella in poultry production.
Author Contributions
All authors (E.B., T.T.O., T.K. and T.O.) contributed to the conceptualization, writing, and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the start-up funds awarded to T.O. from the University of Arkansas Division of Agriculture.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge the members of the Obe lab, whose invaluable suggestions improved the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- United States Department of Agriculture USDA. Poultry & Eggs. Available online: https://www.ers.usda.gov/topics/animal-products/poultry-eggs (accessed on 20 July 2024).
- Shahbandeh, M. Global Chicken Meat Production 2012–2024. Available online: https://www.statista.com/statistics/237637/production-of-poultry-meat-worldwide-since-1990/ (accessed on 20 July 2024).
- National Chicken Council NCC. Per Capita Consumption of Poultry and Livestock, 1965 to Forecast 2022, in Pounds. Available online: https://www.nationalchickencouncil.org/about-the-industry/statistics/per-capita-consumption-of-poultry-and-livestock-1965-to-estimated-2012-in-pounds/ (accessed on 20 July 2024).
- Poultry World. The Future of Chicken: Poultry Beyond 2050. Available online: https://www.poultryworld.net/the-industrymarkets/market-trends-analysis-the-industrymarkets-2/the-future-of-chicken-poultry-beyond-2050/ (accessed on 20 July 2024).
- United States Department of Agriculture USDA. Poultry. Available online: https://www.climatehubs.usda.gov/topics/poultry (accessed on 20 July 2024).
- Ricke, S.C. Poultry food safety and foodborne illness. In Encyclopedia of Meat Sciences, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 47–55. [Google Scholar] [CrossRef]
- Alali, W.Q.; Hofacre, C.L. Preharvest food safety in broiler chicken production. Microbiol. Spectr. 2016, 4, 1128. [Google Scholar] [CrossRef] [PubMed]
- Neeteson, A.M.; Avendaño, S.; Koerhuis, A.; Duggan, B.; Souza, E.; Mason, J.; Ralph, J.; Rohlf, P.; Burnside, T.; Kranis, A.; et al. Evolutions in commercial meat poultry breeding. Animals 2023, 13, 3150. [Google Scholar] [CrossRef] [PubMed]
- Pepin, K.M.; Spackman, E.; Brown, J.D.; Pabilonia, K.L.; Garber, L.P.; Weaver, J.T.; Kennedy, D.A.; Patyk, K.A.; Huyvaert, K.P.; Miller, R.S.; et al. Using quantitative disease dynamics as a tool for guiding response to avian influenza in poultry in the United States of America. Prev. Vet. Med. 2014, 113, 376–397. [Google Scholar] [CrossRef] [PubMed]
- Ricke, S.C.; Rothrock, M.J. Gastrointestinal microbiomes of broilers and layer hens in alternative production systems. Poult. Sci. 2020, 99, 660–669. [Google Scholar] [CrossRef] [PubMed]
- El Jeni, R.; Dittoe, D.K.; Olson, E.G.; Lourenco, J.; Seidel, D.S.; Ricke, S.C.; Callaway, T.R. An overview of health challenges in alternative poultry production systems. Poult. Sci. 2021, 100, 101173. [Google Scholar] [CrossRef] [PubMed]
- Obe, T.; Siceloff, A.T.; Crowe, M.G.; Scott, H.M.; Shariat, N.W. Combined Quantification and Deep Serotyping for Salmonella Risk Profiling in Broiler Flocks. Appl. Environ. Microbiol. 2023, 89, e02035-22. [Google Scholar] [CrossRef]
- Obe, T.; Boltz, T.; Kogut, M.; Ricke, S.C.; Brooks, L.A.; Macklin, K.; Peterson, A. Controlling Salmonella: Strategies for feed, the farm, and the processing plant. Poult. Sci. 2023, 102, 103086. [Google Scholar] [CrossRef]
- Gal-Mor, O.; Boyle, E.C.; Grassl, G.A. Same species, different diseases: How and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front. Microbiol. 2014, 5, 391. [Google Scholar] [CrossRef]
- Mujahid, S.; Hansen, M.; Miranda, R.; Newsom-Stewart, K.; Rogers, J.E. Prevalence and antibiotic resistance of Salmonella and Campylobacter isolates from raw chicken breasts in retail markets in the United States and comparison to data from the plant level. Life 2023, 13, 642. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention CDC. Salmonella Infection (Salmonellosis). Available online: https://www.cdc.gov/salmonella/signs-symptoms/index.html (accessed on 24 July 2024).
- Wagenaar, J.A.; Hendriksen, R.S.; Carrique-Mas, J. Practical considerations of surveillance of Salmonella serovars other than Enteritidis and Typhimurium. Rev. Sci. Tech. Off. Int. Epiz. 2013, 32, 509–519. [Google Scholar] [CrossRef]
- Foley, S.L.; Nayak, R.; Hanning, I.B.; Johnson, T.J.; Han, J.; Ricke, S.C. Population Dynamics of Salmonella enterica Serotypes in Commercial Egg and Poultry Production. Appl. Environ. Microbiol. 2011, 77, 4273–4279. [Google Scholar] [CrossRef]
- Shah, D.H.; Paul, N.C.; Sischo, W.C.; Crespo, R.; Guard, J. Population dynamics and antimicrobial resistance of the most prevalent poultry-associated Salmonella serotypes. Poult. Sci. 2017, 96, 687–702. [Google Scholar] [CrossRef]
- Siceloff, A.T.; Waltman, D.; Shariat, N.W. Regional Salmonella differences in United States broiler production from 2016 to 2020 and the contribution of multi-serovar populations to Salmonella surveillance. Appl. Environ. Microbiol. 2022, 88, e00204-22. [Google Scholar] [CrossRef]
- Tack, D.M.; Ray, L.; Griffin, P.M.; Cieslak, P.R.; Dunn, J.; Rissman, T.; Jervis, R.; Lathrop, S.; Muse, A.; Duwell, M.; et al. Preliminary Incidence and Trends of Infections with Pathogens Transmitted Commonly Through Food—Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2016–2019. Morb. Mortal. Wkly. Rep. 2020, 69, 509–514. [Google Scholar] [CrossRef]
- Tack, D.M.; Marder, E.P.; Griffin, P.M.; Cieslak, P.R.; Dunn, J.; Hurd, S.; Scallan, E.; Lathrop, S.; Muse, A.; Ryan, P.; et al. Preliminary Incidence and Trends of Infections with Pathogens Transmitted Commonly Through Food—Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2015–2018. Morb. Mortal. Wkly. Rep. 2019, 68, 369–373. [Google Scholar] [CrossRef]
- Park, S.H.; Aydin, M.; Khatiwara, A.; Dolan, M.C.; Gilmore, D.F.; Bouldin, J.L.; Ahn, S.; Ricke, S.C. Current and emerging technologies for rapid detection and characterization of Salmonella in poultry and poultry products. Food Microbiol. 2014, 38, 250–262. [Google Scholar] [CrossRef]
- O’Bryan, C.A.; Ricke, S.C.; Marcy, J.A. Public health impact of Salmonella spp. on raw poultry: Current concepts and future prospects in the United States. Food Control 2022, 132, 108539. [Google Scholar] [CrossRef]
- Dórea, F.C.; Cole, D.J.; Hofacre, C.; Zamperini, K.; Mathis, D.; Doyle, M.P.; Lee, M.D.; Maurer, J.J. Effect of Salmonella vaccination of breeder chickens on contamination of broiler chicken carcasses in integrated poultry operations. Appl. Environ. Microbiol. 2010, 76, 7820–7825. [Google Scholar] [CrossRef]
- Bailey, J.S.; Stern, N.J.; Fedorka-Cray, P.; Craven, S.E.; Cox, N.A.; Cosby, D.E.; Ladely, S.; Musgrove, M.T. Sources and movement of Salmonella through integrated poultry operations: A multistate epidemiological investigation. J. Food Prot. 2001, 64, 1690–1697. [Google Scholar] [CrossRef]
- Grimont, P.A.D.; Weill, F.X. Antigenic Formulae of the Salmonella Serovars, 9th ed.; WHO Collaborating Center for Reference and Research on Salmonella; Institut Pasteur: Paris, France; Available online: https://www.pasteur.fr/sites/default/files/veng_0.pdf (accessed on 24 July 2024).
- Wang, J.; Vaddu, S.; Bhumanapalli, S.; Mishra, A.; Applegate, T.; Singh, M.; Thippareddi, H. A systematic review and meta-analysis of the sources of Salmonella in poultry production (pre-harvest) and their relative contributions to the microbial risk of poultry meat. Poul. Sci. 2023, 102, 102566. [Google Scholar] [CrossRef]
- Raut, R.; Maharjan, P.; Fouladkhah, A.C. Practical Preventive Considerations for Reducing the Public Health Burden of Poultry-Related Salmonellosis. Int. J. Environ. Res. Public Health 2023, 20, 6654. [Google Scholar] [CrossRef]
- Wideman, N.; Bailey, M.; Bilgili, S.F.; Thippareddi, H.; Wang, L.; Bratcher, C.; Sanchez-Plata, M.; Singh, M. Evaluating Best Practices for Campylobacter and Salmonella Reduction in Poultry Processing Plants. Poult. Sci. 2016, 95, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Bauermeister, L.J.; Bowers, J.W.J.; Townsend, J.C.; McKee, S.R. The Microbial and Quality Properties of Poultry Carcasses Treated with Peracetic Acid as an Antimicrobial Treatment. Poult. Sci. 2008, 87, 2390–2398. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Morey, A.; Bilgili, S.F.; McKee, S.R.; Garner, L.J. Effectiveness of Several Antimicrobials and the Effect of Contact Time in Reducing Salmonella and Campylobacter on Poultry Drumsticks. J. Appl. Poult. Res. 2019, 28, 1143–1149. [Google Scholar] [CrossRef]
- Bourassa, D.V. Antimicrobial Use in Poultry Processing. Available online: https://www.food-safety.com/articles/5581-antimicrobial-use-in-poultry-processing (accessed on 12 August 2024).
- Abdul-Rahiman, U.A.; Nordin, N.; Abdul-Mutalib, N.A.; Sanny, M. Holistic approaches to reducing Salmonella contamination in the poultry industry. Pertanika J. Trop. Agric. Sci. 2021, 44, 1–23. [Google Scholar] [CrossRef]
- Rasamsetti, S.; Shariat, N.W. Biomapping Salmonella serovar complexity in broiler carcasses and parts during processing. Food Microbiol. 2023, 110, 104149. [Google Scholar] [CrossRef]
- Rothrock, M.J.; Guard, J.Y.; Oladeinde, A. Salmonella diversity along the farm-to-fork continuum of pastured poultry flocks in the Southeastern United States. Front. Anim. Sci. 2021, 2, 761930. [Google Scholar] [CrossRef]
- Jesudhasan, P.R.; McReynolds, J.L.; Byrd, A.J.; He, H.; Genovese, K.J.; Droleskey, R.; Swaggerty, C.L.; Kogut, M.H.; Duke, S.; Nisbet, D.J.; et al. Electron-Beam–Inactivated Vaccine Against Salmonella Enteritidis Colonization in Molting Hens. Avian Dis. 2015, 59, 165–170. [Google Scholar] [CrossRef]
- Praveen, C.; Bhatia, S.S.; Alaniz, R.C.; Droleskey, R.E.; Cohen, N.D.; Jesudhasan, P.R.; Pillai, S.D. Assessment of microbiological correlates and immunostimulatory potential of electron beam inactivated metabolically active yet non culturable (MAyNC) Salmonella Typhimurium. PLoS ONE 2021, 16, e0243417. [Google Scholar] [CrossRef] [PubMed]
- Shaw, S.L.; Esteban, J.E.; Kissler, B.W.; Freiman, J.L.; Tillman, G.E. Use of Whole-Genome Sequencing at the Food Safety and Inspection Service to Detect and Investigate Foodborne Illness Outbreaks. Food Prot. Trends 2020, 40, 268–269. Available online: https://www.nxtbook.com/nxtbooks/trilix/fpt_20200708/ (accessed on 25 August 2024).
- Didelot, X.; Fraser, C.; Gardy, J.; Colijn, C. Genomic infectious disease epidemiology in partially sampled and ongoing outbreaks. Mol. Biol. Evol. 2017, 34, msw275. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture USDA-FSIS. Proposed Regulatory Framework to Reduce Salmonella Illnesses Attributable to Poultry. Available online: https://www.fsis.usda.gov/inspection/inspection-programs/inspection-poultry-products/reducing-salmonella-poultry/proposed (accessed on 12 August 2024).
- Rothrock, M.J.; Al Hakeem, W.G.; Oladeinde, A.; Looft, T.; Li, X.; Guard, J.Y. Salmonella Biomapping of a Commercial Broiler Hatchery. J. Food Prot. 2024, 87, 100347. [Google Scholar] [CrossRef] [PubMed]
- Jones, F.T. A review of practical Salmonella control measures in animal feed. J. Appl. Poult. Res. 2011, 20, 102–113. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Salem, H.M.; El-Tahan, A.M.; Soliman, M.M.; Youssef, G.B.A.; Taha, A.E.; Soliman, S.M.; Ahmed, A.E.; El-kott, A.F.; et al. Alternatives to antibiotics for organic poultry production: Types, modes of action and impacts on bird’s health and production. Poult. Sci. 2022, 101, 101696. [Google Scholar] [CrossRef]
- Wickramasuriya, S.S.; Ault, J.; Ritchie, S.; Gay, C.G.; Lillehoj, H.S. Alternatives to Antibiotic Growth Promoters for Poultry: A Bibliometric Analysis of the Research Journals. Poult. Sci. 2024, 103, 103987. [Google Scholar] [CrossRef] [PubMed]
- Ricke, S.C. Strategies to Improve Poultry Food Safety, a Landscape Review. Annu. Rev. Anim. Biosci. 2021, 9, 379–400. [Google Scholar] [CrossRef]
- Micciche, A.C.; Foley, S.L.; Pavlidis, H.O.; McIntyre, D.R.; Ricke, S.C. A Review of Prebiotics Against Salmonella in Poultry: Current and Future Potential for Microbiome Research Applications. Front. Vet. Sci. 2018, 5, 191. [Google Scholar] [CrossRef]
- Kimminau, E.A.; Karnezos, T.P.; Berghaus, R.D.; Jones, M.K.; Baxter, J.A.; Hofacre, C.L. Combination of Probiotic and Prebiotic Impacts Salmonella Enteritidis Infection in Layer Hens. J. Appl. Poult. Res. 2021, 30, 100200. [Google Scholar] [CrossRef]
- Juricova, H.; Matiasovicova, J.; Faldynova, M.; Sebkova, A.; Kubasova, T.; Prikrylova, H.; Karasova, D.; Crhanova, M.; Havlickova, H.; Rychlik, I. Probiotic Lactobacilli Do Not Protect Chickens against Salmonella Enteritidis Infection by Competitive Exclusion in the Intestinal Tract but in Feed, Outside the Chicken Host. Microorganisms 2022, 10, 219. [Google Scholar] [CrossRef]
- Fulnechek, D.L. Effective Salmonella Control Requires Involvement of Entire Production Chain. Poult. Health Today. Available online: https://www.thepoultrysite.com/articles/effective-salmonella-control-requires-involvement-of-entire-production-chain-1 (accessed on 12 August 2024).
- Higgins, S.E.; Higgins, J.P.; Wolfenden, A.D.; Henderson, S.N.; Torres-Rodriguez, A.; Tellez, G.; Hargis, B. Evaluation of a Lactobacillus-Based Probiotic Culture for the Reduction of Salmonella Enteritidis in Neonatal Broiler Chicks. Poult. Sci. 2008, 87, 27–31. [Google Scholar] [CrossRef]
- Ruvalcaba-Gómez, J.M.; Villagrán, Z.; Valdez-Alarcón, J.J.; Martínez-Núñez, M.; Gomez-Godínez, L.J.; Ruesga-Gutiérrez, E.; Anaya-Esparza, L.M.; Arteaga-Garibay, R.I.; Villarruel-López, A. Non-Antibiotic Strategies to Control Salmonella Infection in Poultry. Animals 2022, 12, 102. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Villanueva, K.Y.; Akerele, G.O.; Al Hakeem, W.G.; Renu, S.; Shanmugasundaram, R.; Selvaraj, R.K. A Novel Approach against Salmonella: A Review of Polymeric Nanoparticle Vaccines for Broilers and Layers. Vaccines 2021, 9, 1041. [Google Scholar] [CrossRef] [PubMed]
- Renu, S.; Han, Y.; Dhakal, S.; Lakshmanappa, Y.S.; Ghimire, S.; Feliciano-Ruiz, N.; Senapati, S.; Narasimhan, B.; Selvaraj, R.; Renukaradhya, G.J. Chitosan-Adjuvanted Salmonella Subunit Nanoparticle Vaccine for Poultry Delivered through Drinking Water and Feed. Carbohydr. Polym. 2020, 243, 116434. [Google Scholar] [CrossRef]
- Rabie, N.S.; Amin Girh, Z.M.S. Bacterial Vaccines in Poultry. Bull. Natl. Res. Centre 2020, 44, 15. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; McWhorter, A.R.; Andrews, D.M.; Underwood, G.J.; Chousalkar, K.K. Challenges in Vaccinating Layer Hens against Salmonella Typhimurium. Vaccines 2020, 8, 696. [Google Scholar] [CrossRef]
- Kogut, M.H.; McReynolds, J.L.; He, H.; Genovese, K.J.; Jesudhasan, P.R.; Davidson, M.A.; Cepeda, M.A.; Pillai, S.D. Electron-beam Irradiation Inactivation of Salmonella: Effects on Innate Immunity and Induction of Protection Against Salmonella enterica serovar Typhimurium Challenge of Chickens. Procedia Vaccinol. 2012, 6, 47–63. [Google Scholar] [CrossRef]
- Maharjan, P.; Clark, T.; Kuenzel, C.; Foy, M.K.; Watkins, S. On farm monitoring of the impact of water system sanitation on microbial levels in broiler house water supplies. J. Appl. Poult. Res. 2016, 25, 266–271. [Google Scholar] [CrossRef]
- Ogundipe, T.T.; Betia, S.; Obe, T. Applied Research Note: Microbial composition of the biofilm of poultry drinking water system. J. Appl. Poult. Res. 2024, 33, 100403. [Google Scholar] [CrossRef]
- Watkins, S. Water: Identifying and Correcting Challenges. The Poultry Site. Available online: https://www.thepoultrysite.com/articles/water-identifying-and-correcting-challenges (accessed on 12 August 2024).
- Maharjan, P.; Huff, G.; Zhang, W.; Watkins, S. Effects of chlorine and hydrogen peroxide sanitation in low bacterial content water on biofilm formation model of poultry brooding house waterlines. Poultr. Sci. 2017, 96, 2145–2150. [Google Scholar] [CrossRef]
- Fraser, R.W.; Williams, N.T.; Powell, L.F.; Cook, A.J.C. Reducing Campylobacter and Salmonella Infection: Two Studies of the Economic Cost and Attitude to Adoption of On-Farm Biosecurity Measures. Zoonoses Public Health 2010, 57, e109–e115. [Google Scholar] [CrossRef]
- Aiyedun, J.O.; Oludairo, O.O.; Olorunsola, I.D.; Daodu, O.B.; Furo, N.A. Effectiveness of Biosecurity Measures in Some Selected Farms in Kwara State, Nigeria. J. Res. For. Wildl. Environ. 2018, 10, 17–23. Available online: https://www.ajol.info/index.php/jrfwe/article/view/174785 (accessed on 25 August 2024).
- Shaji, S.; Selvaraj, R.K.; Shanmugasundaram, R. Salmonella Infection in Poultry: A Review on the Pathogen and Control Strategies. Microorganisms 2023, 11, 2814. [Google Scholar] [CrossRef]
- Gosling, R.J.; Martelli, F.; Wintrip, A.; Sayers, A.R.; Wheeler, K.; Davies, R.H. Assessment of Producers’ Response to Salmonella Biosecurity Issues and Uptake of Advice on Laying Hen Farms in England and Wales. Br. Poult. Sci. 2014, 55, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Volkova, V.V.; Wills, R.W.; Hubbard, S.A.; Magee, D.L.; Byrd, J.A.; Bailey, R.H. Risk Factors Associated with Detection of Salmonella in Broiler Litter at the Time of New Flock Placement. Zoonoses Public Health 2011, 58, 158–168. [Google Scholar] [CrossRef]
- Eid, S.; Hassan, H.; Atfeehy, N.; Selim, K.; Oksh, A. Composting: A Biosecurity Measure to Maximize the Benefit of Broilers’ Litter. J. Adv. Vet. Anim. Res. 2023, 10, 458. [Google Scholar] [CrossRef] [PubMed]
- Conan, A.; Goutard, F.L.; Sorn, S.; Vong, S. Biosecurity Measures for Backyard Poultry in Developing Countries: A Systematic Review. BMC Vet. Res. 2012, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Fouladkhah, A. The Need for Evidence-Based Outreach in the Current Food Safety Regulatory Landscape. J. Ext. 2017, 55, 20. [Google Scholar] [CrossRef]
- United States Department of Agriculture USDA-FSIS. Performance Standards Salmonella Verification Program for Raw Poultry Products. Available online: https://www.fsis.usda.gov/policy/fsis-directives/10250.2 (accessed on 15 August 2024).
- United states Department of Agriculture USDA-FSIS. Pathogen Reduction; Hazard Analysis and Critical Control Point (HACCP) Systems. Available online: https://www.federalregister.gov/documents/1996/07/25/96-17837/pathogen-reduction-hazard-analysis-and-critical-control-point-haccp-systems (accessed on 25 August 2024).
- De Villena, J.F.; Vargas, D.A.; Bueno López, R.; Chávez-Velado, D.R.; Casas, D.E.; Jiménez, R.L.; Sanchez-Plata, M.X. Bio-Mapping Indicators and Pathogen Loads in a Commercial Broiler Processing Facility Operating with High and Low Antimicrobial Intervention Levels. Foods 2022, 11, 775. [Google Scholar] [CrossRef]
- Chavez-Velado, D.R.; Vargas, D.A.; Sanchez-Plata, M.X. Bio-Mapping Salmonella and Campylobacter Loads in Three Commercial Broiler Processing Facilities in the United States to Identify Strategic Intervention Points. Foods 2024, 13, 180. [Google Scholar] [CrossRef]
- Rivera-Pérez, W.; Barquero-Calvo, E.; Zamora-Sanabria, R. Salmonella Contamination Risk Points in Broiler Carcasses During Slaughter Line Processing. J. Food Prot. 2014, 77, 2031–2034. [Google Scholar] [CrossRef]
- Boubendir, S.; Arsenault, J.; Quessy, S.; Thibodeau, A.; Fravalo, P.; Thériault, W.P.; Fournaise, S.; Gaucher, M.L. Salmonella Contamination of Broiler Chicken Carcasses at Critical Steps of the Slaughter Process and in the Environment of Two Slaughter Plants: Prevalence, Genetic Profiles, and Association with the Final Carcass Status. J. Food Prot. 2021, 84, 321–332. [Google Scholar] [CrossRef]
- University of Georgia. Customized Biomapping and Data Collection Improve Food Safety in Poultry Processing. The Poultry Site. Available online: https://www.thepoultrysite.com/articles/biomapping-and-data-collection-improve-food-safety-in-poultry-processing (accessed on 25 August 2024).
- Obe, T.; Nannapaneni, R.; Schilling, W.; Zhang, L.; McDaniel, C.; Kiess, A. Prevalence of Salmonella enterica on Poultry Processing Equipment after Completion of Sanitization Procedures. Poult. Sci. 2021, 99, 4539–4548. [Google Scholar] [CrossRef] [PubMed]
- Ehuwa, O.; Jaiswal, A.K.; Jaiswal, S. Salmonella, Food Safety and Food Handling Practices. Foods 2021, 10, 907. [Google Scholar] [CrossRef]
- Mead, G.C.; Adams, B.W.; Parry, R.T. The Effectiveness of In-Plant Chlorination in Poultry Processing. Br. Poult. Sci. 1975, 16, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Thames, H.T.; Sukumaran, A.T. A Review of Salmonella and Campylobacter in Broiler meat: Emerging challenges and food safety measures. Foods 2020, 9, 776. [Google Scholar] [CrossRef]
- Northcutt, J.K.; Jones, D.R. A Survey of Water Uses and Common Industry Practices in Commercial Broiler Processing Facilities. J. Appl. Poult. Res. 2004, 13, 48–54. [Google Scholar] [CrossRef]
- Obe, T.; Nannapaneni, R.; Schilling, W.; Zhang, L.; Kiess, A. Antimicrobial tolerance, biofilm formation, and molecular characterization of Salmonella isolates from poultry processing equipment. J. Appl. Poult. Res. 2021, 30, 100195. [Google Scholar] [CrossRef]
- Obe, T.; Richards, A.K.; Shariat, N.W. Differences in Biofilm Formation of Salmonella Serovars on two Surfaces under two Temperature Conditions. J. Appl. Microbiol. 2022, 132, 2410–2420. [Google Scholar] [CrossRef]
- Nagel, G.M.; Bauermeister, L.J.; Bratcher, C.L.; Singh, M.; McKee, S.R. Salmonella and Campylobacter reduction and quality characteristics of poultry carcasses treated with various antimicrobials in a post-chill immersion tank. Int. J. Food Microbiol. 2013, 165, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Northcutt, J.K.; Smith, D.P.; Musgrove, M.T.; Ingram, K.D.; Hinton, A., Jr. Processing Products, and Food Safety Microbiological Impact of Spray Washing Broiler Carcasses Using Different Chlorine Concentrations and Water Temperatures. Poult. Sci. 2005, 84, 1648–1652. [Google Scholar] [CrossRef]
- Bartenfeld, L.N.; Fletcher, D.L.; Northcutt, J.K.; Bourassa, D.V.; Cox, N.A.; Buhr, R.J. The Effect of High-level Chlorine Carcass Drench on the Recovery of Salmonella and Enumeration of Bacteria from Broiler Carcasses. Poultr. Sci. 2014, 93, 2893–2899. [Google Scholar] [CrossRef]
- Cano, C.; Meneses, Y.; Chaves, B.D. Application of Peroxyacetic Acid for Decontamination of Raw Poultry Products and Comparison to Other Commonly Used Chemical Antimicrobial Interventions: A review. J. Food Prot. 2021, 84, 1772–1783. [Google Scholar] [CrossRef] [PubMed]
- Kataria, J.; Morey, A. Antimicrobial Interventions in Poultry Processing to Improve Shelf Life and Safety of Poultry Meat: A Review with Special Attention to Salmonella spp. J. Food Qual. Hazards Control 2020, 7, 52–59. [Google Scholar] [CrossRef]
- Vaddu, S.; Wang, J.; Sidhu, G.; Leone, C.; Singh, M.; Thippareddi, H. Relative resistance of Salmonella Serotypes (Typhimurium, Infantis, and Reading) to Peroxyacetic Acid on Chicken Wings. Poultr. Sci. 2024, 103, 103935. [Google Scholar] [CrossRef]
- Chen, X.; Bauermeister, L.J.; Hill, G.N.; Singh, M.; Bilgili, S.F.; McKee, S.R. Efficacy of Various Antimicrobials on Reduction of Salmonella and Campylobacter and Quality Attributes of Ground Chicken Obtained from Poultry Parts treated in a Post Chill Decontamination Tank. J. Food Prot. 2014, 77, 1882–1888. [Google Scholar] [CrossRef]
- United States Department of Agriculture USDA-FSIS. Safe and Suitable Ingredients Used in the Production of Meat, Poultry, and Egg Products (Last Reviewed 7 August 2024). Available online: https://www.fsis.usda.gov/policy/fsis-directives/7120.1 (accessed on 30 August 2024).
- Waldroup, A.L.; Beers, K.L.; Cook, P.E.; Dell, E.A.; Odglen, R.; Baker, R.A.; Coleman, C.W.; Smith, B.A.; Maingi, B.W. The Effects of Cetylpyridinium Chloride (Cecure CPC Antimicrobial) on ® 1 Campylobacter Spp. on Raw Poultry: A Review. Int. J. Poult. Sci. 2010, 9, 305–308. [Google Scholar] [CrossRef]
- Wilsmann, D.E.; Carvalho, D.; Chitolina, G.Z.; Borges, K.; Furian, T.Q.; Martins, A.C.; Webber, B.; Nascimento, V.P. Electrochemically Activated Water Presents Bactericidal Effect Against Salmonella Heidelberg Isolated from Poultry Origin. Foodborne Pathog. Dis. 2020, 17, 228–233. [Google Scholar] [CrossRef]
- Cano, C.; Sullivan, G.A.; Chaves, B.D. Evaluation of Ozonated Water as a Potential Intervention to Reduce Salmonella and Indicator Organisms on Raw Chicken Wing Sections. IAFP. 2023, 43, 472–478. [Google Scholar] [CrossRef]
- Abd-El Wahab, A.; Basiouni, S.; El-Seedi, H.R.; Ahmed, M.F.E.; Bielke, L.R.; Hargis, B.; Tellez-Isaias, G.; Eisenreich, W.; Lehnherr, H.; Kittler, S.; et al. An overview of the use of Bacteriophages in the Poultry Industry: Successes, Challenges, and Possibilities for Overcoming Breakdowns. Front. Microbiol. 2023, 14, 1136638. [Google Scholar] [CrossRef]
- Obe, T.; Kiess, A.S.; Nannapaneni, R. Antimicrobial Tolerance in Salmonella: Contributions to Survival and Persistence in Processing Environments. Animals 2024, 14, 578. [Google Scholar] [CrossRef]
- Merino, L.; Procura, F.; Trejo, F.M.; Bueno, D.J.; Golowczyc, M.A. Biofilm formation by Salmonella sp. in the Poultry Industry: Detection, control and eradication strategies. Food Res. Int. 2019, 119, 530–540. [Google Scholar] [CrossRef]
- Davidson, P.M.; Harrison, M.A. Resistance and Adaptation to Food Antimicrobials, Sanitizers, and Other Process Controls. IFT 2002, 56, 11. Available online: https://www.researchgate.net/publication/237373178 (accessed on 12 September 2024).
- Paz-Méndez, A.M.; Lamas, A.; Vázquez, B.; Miranda, J.M.; Cepeda, A.; Franco, C.M. Effect of Food Residues in Biofilm Formation on Stainless Steel and Polystyrene Surfaces by Salmonella enterica Strains Isolated from Poultry Houses. Foods 2017, 6, 106. [Google Scholar] [CrossRef] [PubMed]
- Obe, T.; Nannapaneni, R.; Sharma, C.S.; Kiess, A. Homologous Stress Adaptation, Antibiotic Resistance, and Biofilm Forming Ability of Salmonella enterica serovar Heidelberg ATCC8326 on different Food-Contact Surfaces Following Exposure to Sublethal Chlorine Concentrations. Poultr. Sci. 2018, 97, 951–961. [Google Scholar] [CrossRef]
- United States Department of Agriculture USDA. One Health. Available online: https://www.usda.gov/topics/animals/one-health (accessed on 12 September 2024).
- Galán-Relaño, Á.; Valero Díaz, A.; Huerta Lorenzo, B.; Gómez-Gascón, L.; Mena Rodríguez, M.A.; Carrasco Jiménez, E.; Pérez Rodríguez, F.; Astorga Márquez, R.J. Salmonella and Salmonellosis: An Update on Public Health Implications and Control Strategies. Animals 2023, 13, 3666. [Google Scholar] [CrossRef] [PubMed]
- Whitworth, J. No Single Solution to Control Salmonella in Poultry Meat, say Experts. Food Safety News. Available online: https://www.foodsafetynews.com/2022/10/no-single-solution-to-control-salmonella-in-poultry-meat-say-experts (accessed on 12 September 2024).
- The National Advisory Committee on Microbiological Criteria in Foods. Response to Questions Posed by the Food Safety and Inspection Service: Enhancing Salmonella Control in Poultry Products. J. Food Prot. 2023, 87, 100168. [Google Scholar] [CrossRef]
- Condell, O.; Iversen, C.; Cooney, S.; Power, K.A.; Walsh, C.; Burgess, C.; Fanning, S. Efficacy of Biocides Used in the Modern Food Industry To Control Salmonella enterica, and Links between Biocide Tolerance and Resistance to Clinically Relevant Antimicrobial Compounds. Appl. Environ. Microbiol. 2012, 78, 3087–3097. [Google Scholar] [CrossRef]
- Molina-González, D.; Alonso-Calleja, C.; Alonso-Hernando, A.; Capita, R. Effect of Sub-lethal Concentrations of Biocides on the Susceptibility to Antibiotics of Multi-Drug-Resistant Salmonella enterica strains. Food Control 2014, 40, 329–334. [Google Scholar] [CrossRef]
- Food Safety Magazine Editorial Team. Multi-Hurdle Approach Crucial to Controlling Salmonella in Poultry, Reports FAO/WHO. Available online: https://www.food-safety.com/articles/9157-multi-hurdle-approach-crucial-to-controlling-salmonella-in-poultry-reports-fao-who (accessed on 12 September 2024).
- Milillo, S.R.; Ricke, S.C. Synergistic Reduction of Salmonella in a Model Raw Chicken Media using a Combined Thermal and Acidified Organic Acid Salt Intervention Treatment. J. Food Sci. 2010, 75, M121–M125. [Google Scholar] [CrossRef]
- Yadav, B.; Roopesh, M.S. Synergistically enhanced Salmonella Typhimurium reduction by sequential treatment of organic acids and atmospheric cold plasma and the mechanism study. Food Microbiol. 2022, 104, 103976. [Google Scholar] [CrossRef]
- Thornton, G. How to Reduce Salmonella on Poultry Parts. WATTPoultry. Available online: https://www.wattagnet.com/broilers-turkeys/processing-slaughter/article/15514337/how-to-reduce-salmonellaon-poultry-part (accessed on 12 September 2024).
- Pelyuntha, W.; Vongkamjan, K. Control of Salmonella in Chicken Meat by a Phage Cocktail in Combination with Propionic Acid and Modified Atmosphere Packaging. Foods 2023, 12, 4181. [Google Scholar] [CrossRef] [PubMed]
- Mogren, L.; Windstam, S.; Boqvist, S.; Vågsholm, I.; Söderqvist, K.; Rosberg, A.K.; Lindén, J.; Mulaosmanovic, E.; Karlsson, M.; Uhlig, E.; et al. The Hurdle Approach–A Holistic Concept for Controlling Food Safety Risks Associated with Pathogenic Bacterial Contamination of Leafy Green Vegetables: A Review. Front. Microbiol. 2018, 9, 1965. [Google Scholar] [CrossRef]
- Ceva Poultry. Autogenous Vaccines. Available online: https://poultry.ceva.com/poultry-vaccines/autogenous-vaccines/ (accessed on 12 December 2024).
- Montoro-Dasi, L.; Lorenzo-Rebenaque, L.; Marco-Fuertes, A.; Vega, S.; Marin, C. Holistic Strategies to Control Salmonella Infantis: An Emerging Challenge in the European Broiler Sector. Microorganisms 2023, 11, 1765. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, A.T.; Nannapaneni, R.; Kiess, A.; Sharma, C.S. Reduction of Salmonella on Chicken meat and Chicken Skin by Combined or Sequential Application of Lytic Bacteriophage with Chemical Antimicrobials. Int. J. Food Microbiol. 2015, 207, 8–15. [Google Scholar] [CrossRef]
- Manjankattil, S.; Nair, D.V.T.; Peichel, C.; Noll, S.; Johnson, T.J.; Cox, R.B.; Donoghue, A.M.; Kollanoor Johny, A. Effect of caprylic acid alone or in combination with peracetic acid against multidrug-resistant Salmonella Heidelberg on chicken drumsticks in a soft scalding temperature-time setup. Poultr Sci. 2021, 100, 101421. [Google Scholar] [CrossRef]
- United States Department of Agriculture USDA. Multi-Hurdle Approaches for Controlling Foodborne Pathogens in Poultry. Available online: https://www.nal.usda.gov/research-tools/food-safety-research-projects/multi-hurdle-approaches-controlling-foodborne (accessed on 12 December 2024).
- Upadhyaya, I.; Yin, H.-B.; Surendran Nair, M.; Chen, C.-H.; Lang, R.; Darre, M.J.; Venkitanarayanan, K. Inactivation of Salmonella enteritidis on shell eggs by coating with phytochemicals. Poultr. Sci. 2016, 95, 2106–2111. [Google Scholar] [CrossRef]
- Micciche, A.; Rothrock, M.J.; Yang, Y.; Ricke, S.C. Essential Oils as an Intervention Strategy to Reduce Campylobacter in Poultry Production: A Review. Front. Microbiol. 2019, 10, 1058. [Google Scholar] [CrossRef]
- Almuzaini, A.M. Phytochemicals: Potential alternative strategy to fight Salmonella enterica serovar Typhimurium. Front Vet Sci. 2023, 10, 1188752. [Google Scholar] [CrossRef]
- Allen, J.; Balasubramanian, B.; Rankin, K.; Shah, T.; Donoghue, A.M.; Upadhyaya, I.; Sartini, B.; Luo, Y.; Upadhyay, A. Trans-cinnamaldehyde nanoemulsion wash Inactivates Salmonella Enteritidis on shelled eggs without affecting egg color. Poult. Sci. 2023, 102, 102523. [Google Scholar] [CrossRef]
- Nair, D.V.T.; Manjankattil, S.; Peichel, C.; Martin, W.; Donoghue, A.M.; Venkitanarayanan, K.; Kollanoor Johny, A. Effect of plant-derived antimicrobials, eugenol, carvacrol, and β-resorcylic acid against Salmonella on organic chicken wings and carcasses. Poult. Sci. 2023, 102, 102886. [Google Scholar] [CrossRef] [PubMed]
- Trampel, D.W.; Holder, T.G.; Gast, R.K. Integrated farm management to prevent Salmonella Enteritidis contamination of eggs. J. Appl. Poult. Res. 2014, 23, 353–365. [Google Scholar] [CrossRef]
- Moon, S.H.; Waite-Cusic, J.; Huang, E. Control of Salmonella in Chicken Meat using a Combination of a Commercial Bacteriophage and Plant-based Essential oils. Food Control 2020, 110, 106984. [Google Scholar] [CrossRef]
- Lamichhane, B.; Mawad, A.M.M.; Saleh, M.; Kelley, W.G.; Harrington, P.J.; Lovestad, C.W.; Amezcua, J.; Sarhan, M.M.; El Zowalaty, M.E.; Ramadan, H.; et al. Salmonellosis: An Overview of Epidemiology, Pathogenesis, and Innovative Approaches to Mitigate the Antimicrobial Resistant Infections. Antibiotics 2024, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture USDA-FSIS. Guideline for Controlling Salmonella in Raw Poultry. Available online: https://www.fsis.usda.gov/policy/federal-register-rulemaking/federal-register-rules/salmonella-framework-raw-poultry-products (accessed on 12 December 2024).
- Thanki, A.M.; Hooton, S.; Whenham, N.; Salter, M.G.; Bedford, M.R.; O’Neill, H.V.M.; Clokie, M.R.J. A Bacteriophage Cocktail Delivered in Feed Significantly Reduced Salmonella Colonization in Challenged Broiler Chickens. Emerg. Microbes Infect. 2023, 12, 2217947. [Google Scholar] [CrossRef]
- Doughman, E. Bacteriophages Could Improve Salmonella Control in Poultry. WATTPoultry. Available online: https://www.wattagnet.com/broilers-turkeys/food-safety/news/15710635/bacteriophages-could-improve-salmonella-control-in-poultry (accessed on 10 January 2025).
- Żbikowska, K.; Michalczuk, M.; Dolka, B. The use of bacteriophages in the poultry industry. Animals 2020, 10, 872. [Google Scholar] [CrossRef]
- Shehata, A.M.; Paswan, V.K.; Attia, Y.A.; Abdel-Moneim, A.M.E.; Abougabal, M.S.; Sharaf, M.; Elmazoudy, R.; Alghafari, W.T.; Osman, M.A.; Farag, M.R.; et al. Managing Gut Microbiota through In Ovo Nutrition Influences Early-Life Programming in Broiler Chickens. J. Anim. 2021, 11, 3491. [Google Scholar] [CrossRef]
- Wong, C.L.; Sieo, C.C.; Tan, W.S.; Abdullah, N.; Hair-Bejo, M.; Abu, J.; Ho, Y.W. Evaluation of a lytic bacteriophage, Φ st1, for biocontrol of Salmonella enterica serovar Typhimurium in chickens. Int. J. Food Microbiol. 2014, 172, 92–101. [Google Scholar] [CrossRef]
- Lorenzo-Rebenaque, L.; Malik, D.J.; Catalá-Gregori, P.; Marin, C.; Sevilla-Navarro, S. In vitro and in vivo gastrointestinal survival of non-encapsulated and microencapsulated salmonella bacteriophages: Implications for bacteriophage therapy in poultry. Pharmaceuticals 2021, 14, 434. [Google Scholar] [CrossRef]
- Lorenzo-Rebenaque, L.; Casto-Rebollo, C.; Diretto, G.; Frusciante, S.; Rodríguez, J.C.; Ventero, M.-P.; Molina-Pardines, C.; Vega, S.; Marin, C.; Marco-Jiménez, F. Examining the effects of salmonella phage on the caecal microbiota and metabolome features in salmonella-free broilers. Front. Genet. 2022, 13, 713. [Google Scholar] [CrossRef] [PubMed]
- Eltholth, M.M.; Mohamed, R.A.; Elgohary, F.A.; Abo Elfadl, E.A. Assessment of Biosecurity Practices in broiler Chicken Farms in Gharbia Governorate, Egypt. Alex. J. Vet. Sci. 2016, 49, 68–77. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).