Epidural Inflammatory Pseudotumor in the Cervical Spine: A Case Report of a Bernese Mountain Dog
Simple Summary
Abstract
1. Introduction
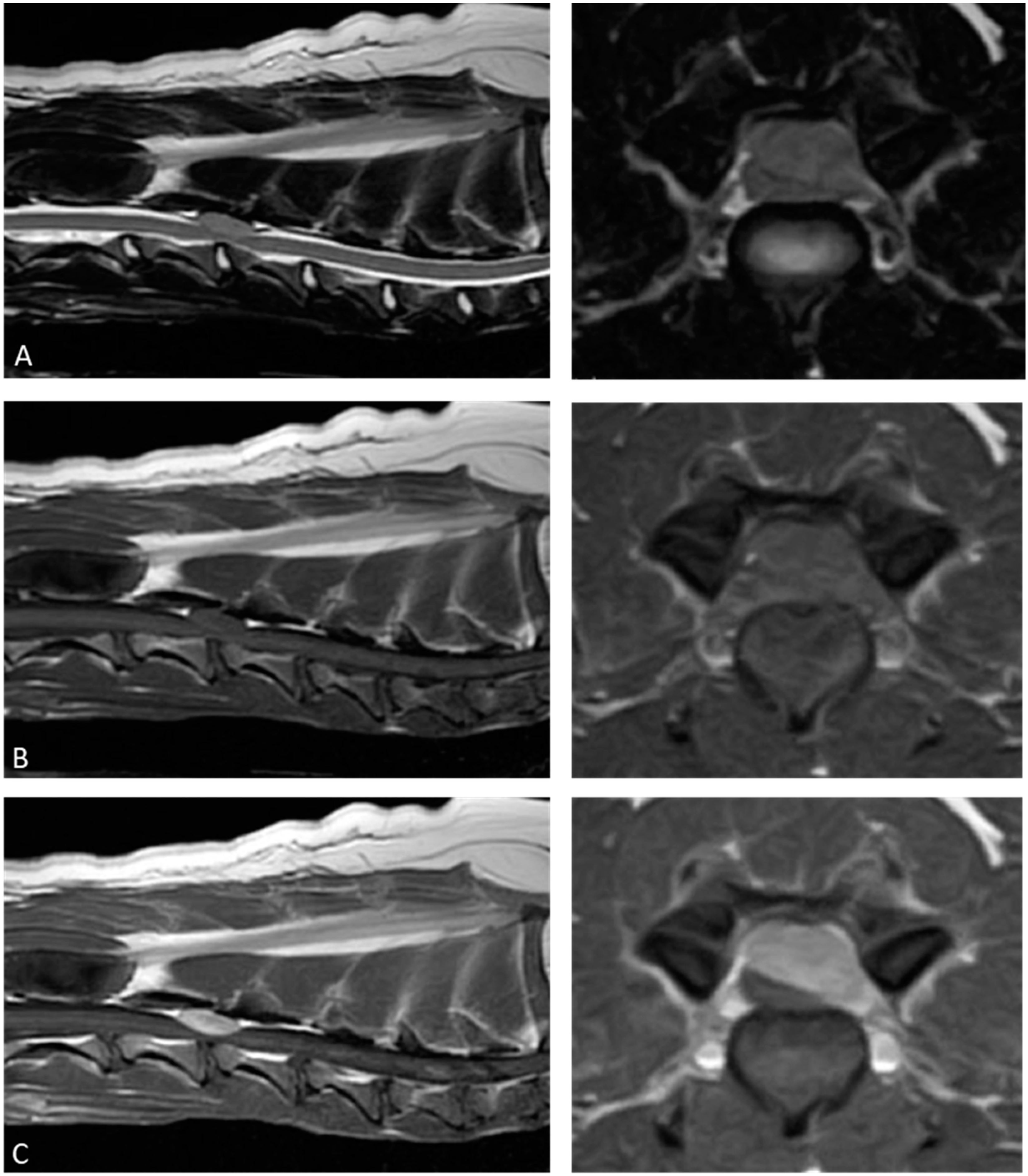
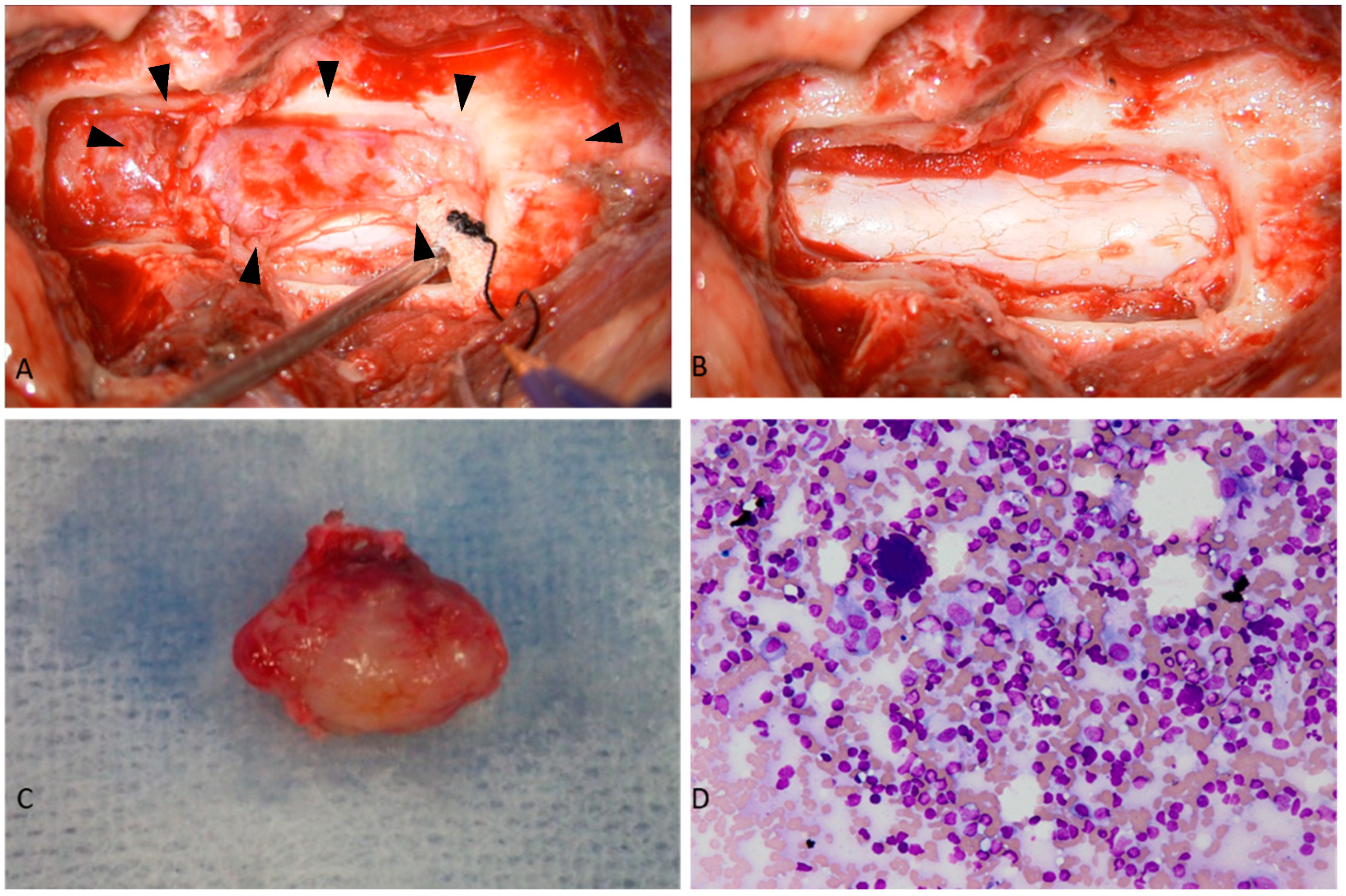
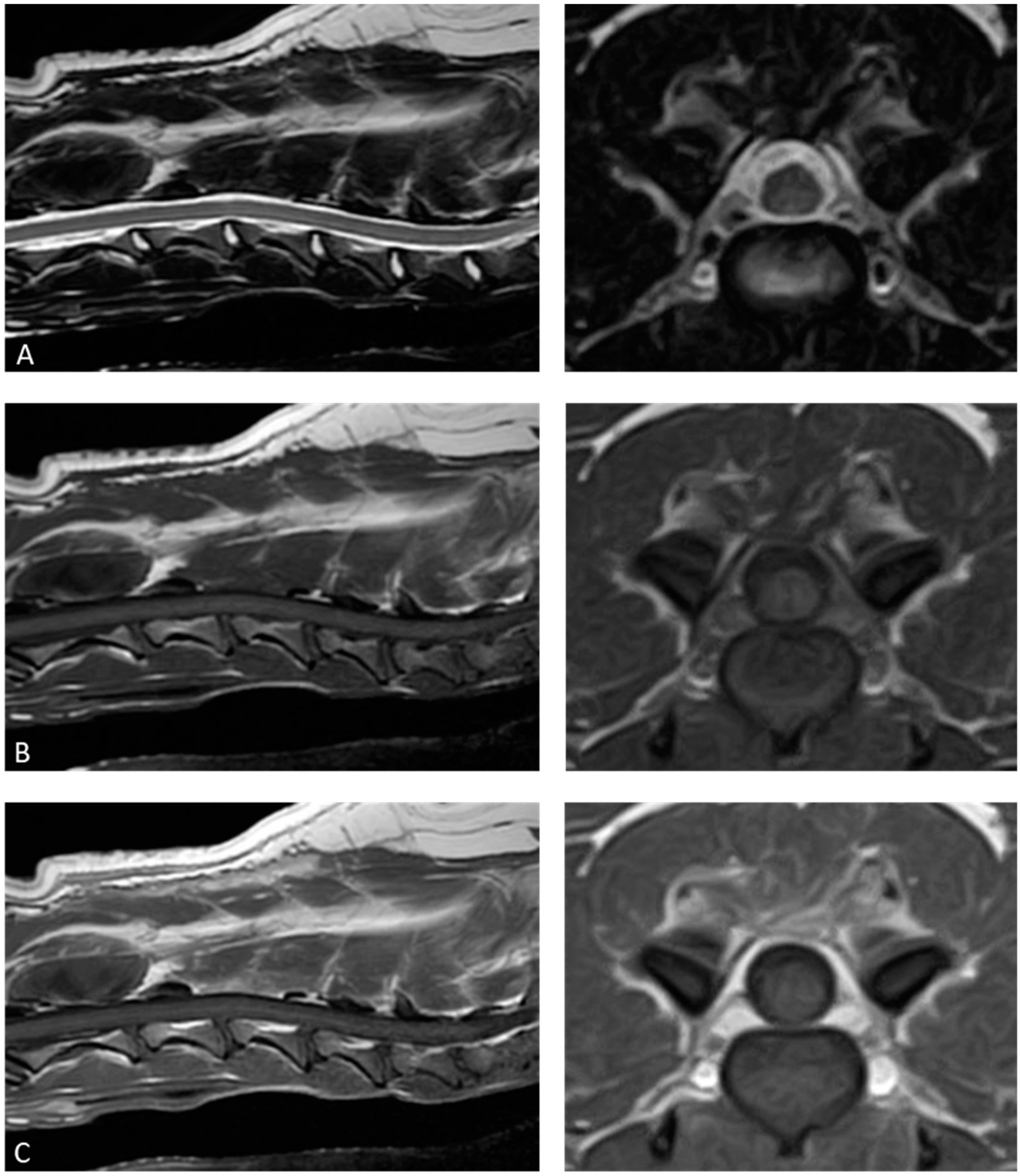
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MRI | Magnetic resonance imaging |
| IPT | Inflammatory pseudotumor |
References
- Narla, L.D.; Newman, B.; Spottswood, S.S.; Narla, S.; Kolli, R. Inflammatory pseudotumor. RadioGraphics 2003, 23, 719–729. [Google Scholar] [CrossRef]
- Seol, H.J.; Kim, S.S.; Kim, J.E.; Lee, S.H.; Won, J.Y. Inflammatory pseudotumor in the epidural space of the thoracic spine: A case report and literature review of MR imaging findings. AJNR Am. J. Neuroradiol. 2005, 26, 2667–2670. [Google Scholar]
- Boutarbouch, M.; Arkha, Y.; Rifi, L.; Derraz, S.; El Ouahabi, A.; El Khamlichi, A. Intradural cervical inflammatory pseudotumor mimicking epidural hematoma in a pregnant woman: Case report and review of the literature. Surg. Neurol. 2008, 69, 302–305. [Google Scholar] [CrossRef]
- Yoshimura, K.; Ssasaki, M.; Kojima, M.; Tsuruzono, K.; Matsumoto, K.; Wakayama, A.; Yoshimine, T. Spontaneous regression of inflammatory pseudotumor in the cauda equina: A case report. NMC Case Rep. J. 2016, 4, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.E.; Moncure, C.W. Pseudotumor of the orbit in a prosimian primate (lesser bush baby—Galago-senegalensis). J. Med. Primatol. 1973, 2, 369–377. [Google Scholar] [CrossRef]
- Miller, S.A.; Van der Woerdt, A.; Bartick, T.E. Retrobulbar pseudotumor of the orbit in a cat. J. Am. Vet. Med. Assoc. 2000, 216, 356–358. [Google Scholar] [CrossRef]
- Billson, F.M.; Miller-Michau, T.; Mould, J.R.B.; Davidson, M.G. Idiopathic sclerosing orbital pseudotumor in seven cats. Vet. Ophthalmol. 2006, 9, 45–51. [Google Scholar] [CrossRef] [PubMed]
- van der Woerdt, A. Orbital inflammatory disease and pseudotumor in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2008, 38, 389–401. [Google Scholar] [CrossRef]
- Knight, C.; Fan, E.; Riis, R.; McDonough, S. Inflammatory myofibroblastic tumors in two dogs. Vet. Pathol. 2009, 46, 273–276. [Google Scholar] [CrossRef]
- Miller, M.A.; Fales, W.H.; McCracken, W.S.; O’Bryan, M.A.; Jarnagin, J.J.; Payeur, J.B. Inflammatory pseudotumor in a cat with cutaneous mycobacteriosis. Vet. Pathol. 1999, 36, 161–163. [Google Scholar] [CrossRef]
- Gärtner, F.; Santos, M.; Gillette, D.; Schmitt, F. Inflammatory pseudotumour of the spleen in a dog. Vet. Rec. 2002, 150, 697–698. [Google Scholar] [CrossRef]
- Wako, Y.; Okazaki, Y.; Tomonari, Y.; Doi, T.; Kanno, T.; Katsuta, O.; Tsuchitani, M. A pseudotumorous nodular lesion of the subcutis in a beagle dog comparable to calcifying fibrous pseudotumor in human. J. Toxicol. Pathol. 2005, 18, 199–202. [Google Scholar] [CrossRef][Green Version]
- Böhme, B.; Ngendahayo, P.; Hamaide, A.; Heimann, M. Inflammatory pseudotumours of the urinary bladder in dogs resembling human myofibroblastic tumours: A report of eight cases and comparative pathology. Vet. J. 2010, 183, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Loderstedt, S.; Walmsley, G.L.; Summers, B.A.; Cappello, R.; Volk, H.A. Neurological, imaging and pathological features of a meningeal inflammatory pseudotumour in a Maltese terrier. J. Small Anim. Pract. 2010, 51, 387–392. [Google Scholar] [CrossRef]
- Reyes, H.M.; Fiorentino, E.; Matiasek, K.; Menchetti, M. Intradural-extramedullary inflammatory pseudotumour of the cervical spinal cord of a dog. Vet. Radiol. Ultrasound 2024, 65, 513–517. [Google Scholar] [CrossRef]
- Kuniya, T.; Shimoyama, Y.; Sano, M.; Watanabe, N. Inflammatory pseudotumour arising in the epidural space of a dog. Vet. Rec. Case Rep. 2014, 2, e000095. [Google Scholar] [CrossRef]
- Castro Carnes, L.; Gutierrez-Quintana, R.; Marchesi, F.; Morris, J.; Kaczmarska, A. Inflammatory pseudotumour presenting as a cervical extradural mass in a 1-year-old Bernese Mountain dog. Vet. Rec. Case Rep. 2024, 13, e1052. [Google Scholar] [CrossRef]
- Yamashita, M.; Osaki, T.; Murahata, Y.; Sunden, Y.; Morita, R.; Imagawa, T.; Okamoto, Y. Imaging and pathological findings of intramedullary inflammatory pseudotumour in a miniature dachshund: A case report. BMC Vet. Res. 2019, 15, 459. [Google Scholar] [CrossRef]
- Chávez-Peón Berle, E.; Hallman, C.; Kleinhenz, K.; Plattner, B.L. Multifocal spinal inflammatory myofibroblastic tumors in a juvenile paraparetic dog. Vet. Radiol. Ultrasound 2023, 64, E14–E18. [Google Scholar] [CrossRef]
- Despeyroux-Ewers, M.; Catalaâ, I.; Collin, L.; Cognard, C.; Loubes-Lacroix, F.; Manelfe, C. Inflammatory myofibroblastic tumour of the spinal cord: Case report and review of the literature. Neuroradiology 2003, 45, 812–817. [Google Scholar] [CrossRef]
- Liccardo, G.; Lunardi, P.; Menniti, A.; Floris, R.; Pastore, F.S.; Fraioli, B. Calcifying pseudotumor of the spine: Description of a case and review of the literature. Eur. Spine J. 2003, 12, 548–551. [Google Scholar] [CrossRef]
- Checrallah, A.; Riachi, M.; Slaba, S. Inflammatory pseudotumors of the lung with spontaneous regression. Leban. Med. J. 2005, 53, 229–233. [Google Scholar]
- Zhao, J.J.; Ling, J.Q.; Fang, Y.; Gao, X.D.; Shu, P.; Shen, K.T.; Qin, J.; Sun, Y.H.; Qin, X.Y. Intra-abdominal inflammatory myofibroblastic tumor: Spontaneous regression. World J. Gastroenterol. 2014, 20, 13625–13631. [Google Scholar] [CrossRef] [PubMed]
- Gilliard, C.; De Coene, B.; Lahdou, J.B.; Boutsen, Y.; Noël, H.; Godfraind, C. Cervical epidural pseudotumor and multifocal fibrosclerosis: Case report and review of the literature. J. Neurosurg. 2000, 93, 152–156. [Google Scholar] [CrossRef]
- Aizawa, T.; Sato, T.; Tanaka, Y.; Kishimoto, K.; Watanabe, M.; Kokubun, S. Intramedullary plasma cell granuloma in the cervicothoracic spine. Case report. J. Neurosurg. 2002, 97, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.K.; Chang, K.H.; Suh, Y.L.; Jung, H.W.; Park, S.H. Inflammatory myofibroblastic tumor of the central nervous system: Clinicopathologic analysis of 10 cases. J. Neuropathol. Exp. Neurol. 2005, 64, 254–259. [Google Scholar] [CrossRef][Green Version]
- Yoon, S.H.; Kim, K.J.; Chung, S.K.; Kim, H.J.; Choe, G.; Chung, S.B.; Jin, Y.J. Inflammatory myofibroblastic tumor in the intradural extramedullary space of the lumbar spine with spondylolisthesis: Case report and review of the literature. Eur. Spine J. 2010, 19 (Suppl. S2), S153–S157. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Murakami, H.; Demura, S.; Yoshioka, K.; Okamoto, Y.; Hayashi, H.; Tsuchiya, H. Epidural inflammatory pseudotumor in the thoracic spine in a patient with polymyalgia rheumatica. Spine J. 2012, 12, e1–e4. [Google Scholar] [CrossRef][Green Version]
- Eimoto, T.; Yanaka, M.; Kurosawa, M.; Ikeya, F. Plasma cell granuloma (inflammatory pseudotumor) of the spinal-cord meninges: Report of a case. Cancer 1978, 41, 1929–1936. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, Y.; Kitoh, R.; Satoh, M.; Yoshigae, Y.; Nibe, K.; Uchida, K.; Matsunaga, S. Epidural Inflammatory Pseudotumor in the Cervical Spine: A Case Report of a Bernese Mountain Dog. Animals 2025, 15, 1049. https://doi.org/10.3390/ani15071049
Inoue Y, Kitoh R, Satoh M, Yoshigae Y, Nibe K, Uchida K, Matsunaga S. Epidural Inflammatory Pseudotumor in the Cervical Spine: A Case Report of a Bernese Mountain Dog. Animals. 2025; 15(7):1049. https://doi.org/10.3390/ani15071049
Chicago/Turabian StyleInoue, Yoshiyuki, Rie Kitoh, Moe Satoh, Yuki Yoshigae, Kazumi Nibe, Kazuyuki Uchida, and Satoru Matsunaga. 2025. "Epidural Inflammatory Pseudotumor in the Cervical Spine: A Case Report of a Bernese Mountain Dog" Animals 15, no. 7: 1049. https://doi.org/10.3390/ani15071049
APA StyleInoue, Y., Kitoh, R., Satoh, M., Yoshigae, Y., Nibe, K., Uchida, K., & Matsunaga, S. (2025). Epidural Inflammatory Pseudotumor in the Cervical Spine: A Case Report of a Bernese Mountain Dog. Animals, 15(7), 1049. https://doi.org/10.3390/ani15071049



