Swine Gut Lactic Acid Bacteria and Their Exopolysaccharides Differentially Modulate Toll-like Receptor Signaling Depending on the Agave Fructans Used as a Carbon Source
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Animals
2.3. Fecal Sample Collection
2.4. Isolation of Lactic Acid Bacteria (LAB) Strains
2.5. Graminan-Type Fructans
2.6. Determination of Growth Curves of LAB
2.7. Selection Criteria for LAB Strains
2.8. Extraction and Quantification of EPSs from the LAB Culture Supernatant
2.9. EPS Production by LAB Using Agave GTFs as Carbon Sources
2.10. Assays with Reporter Cell Lines
2.11. Statistical Analyses
3. Results
3.1. LAB Strains Isolated from Swine Feces and Their EPS Production
3.2. The LAB Strain Growth Was GTF Dependent
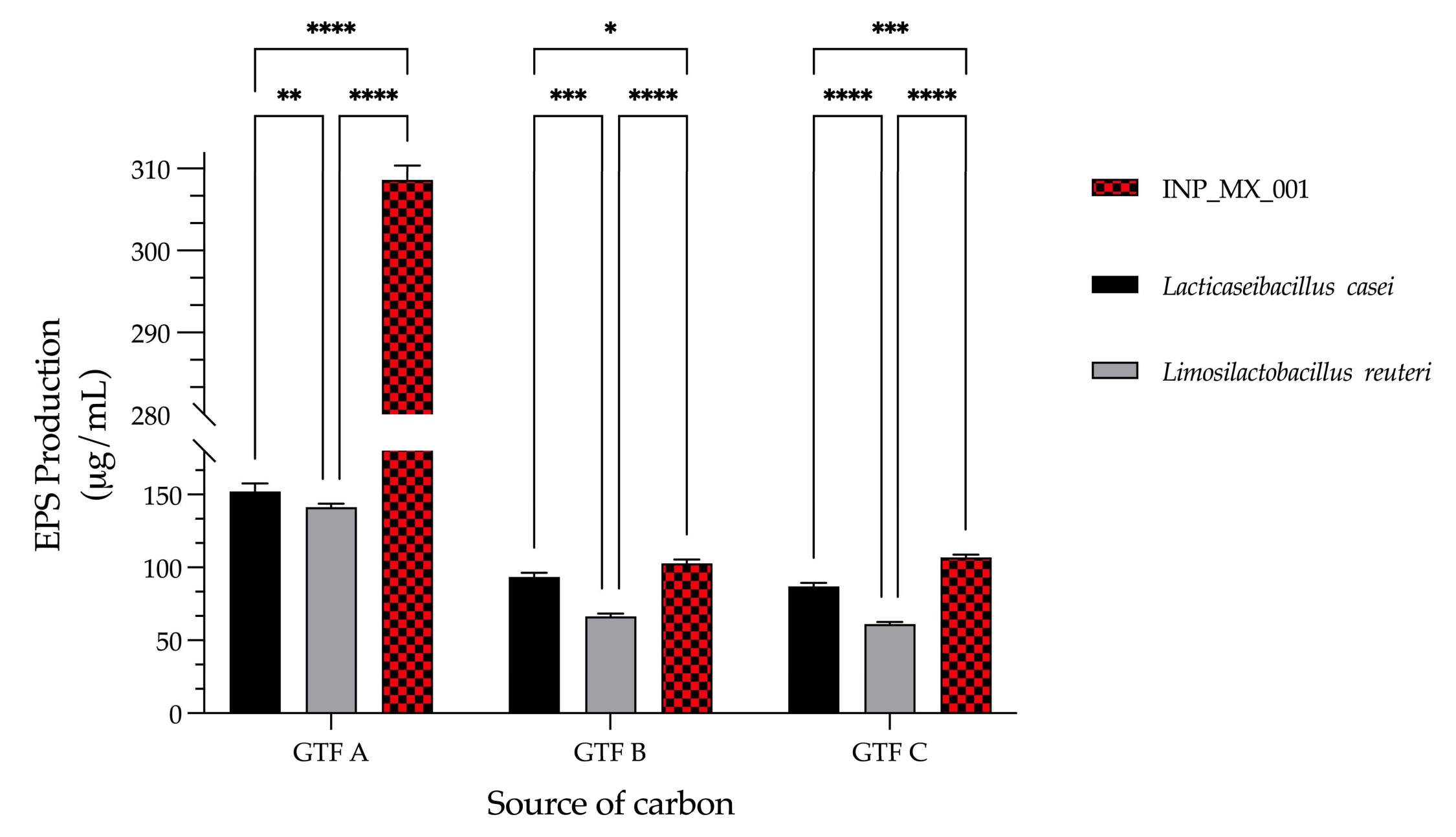
3.3. The LAB Strain Isolated from Swine Was the Best EPS Producer with GTFs as a Carbon Source Compared with Other Well-Characterized Lactobacilli
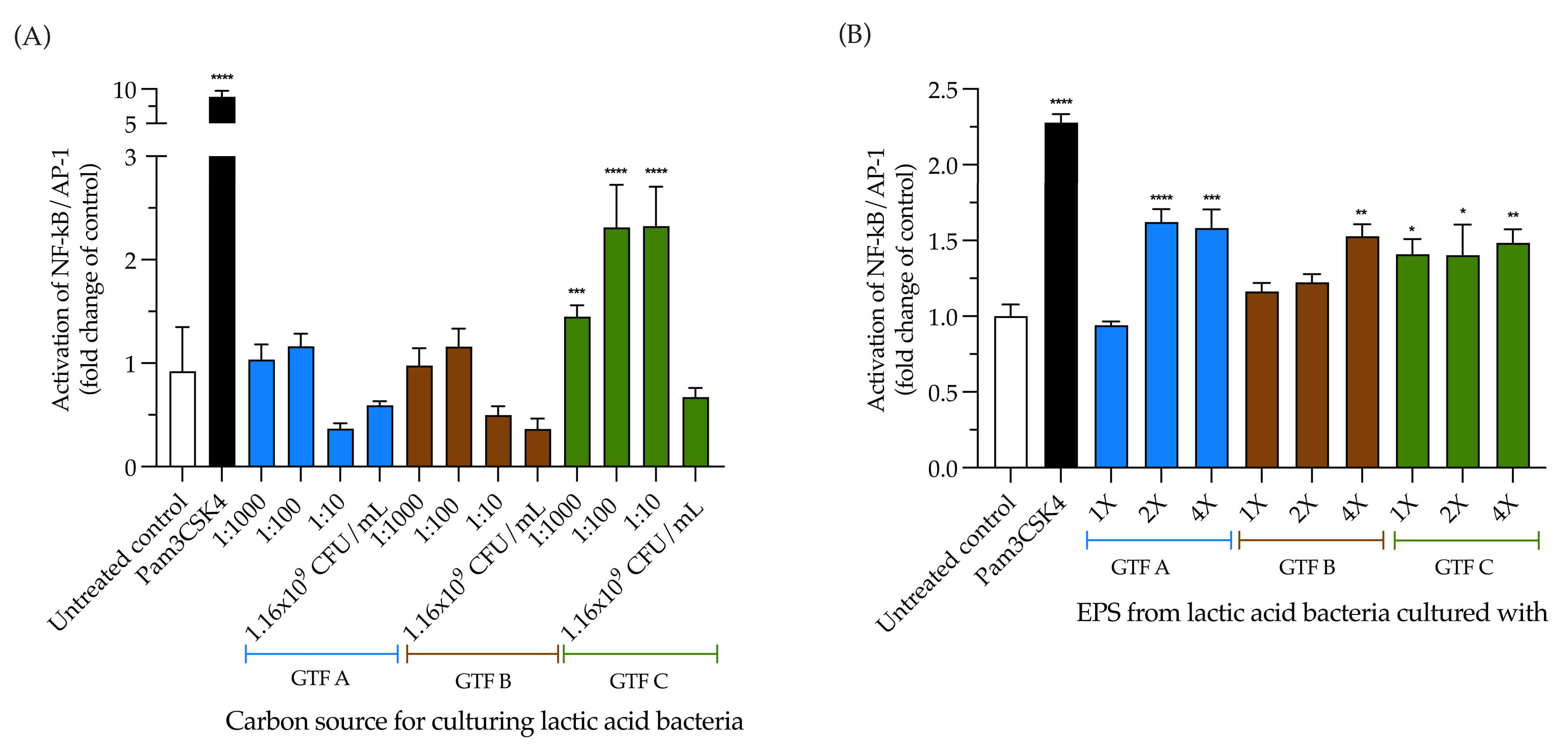
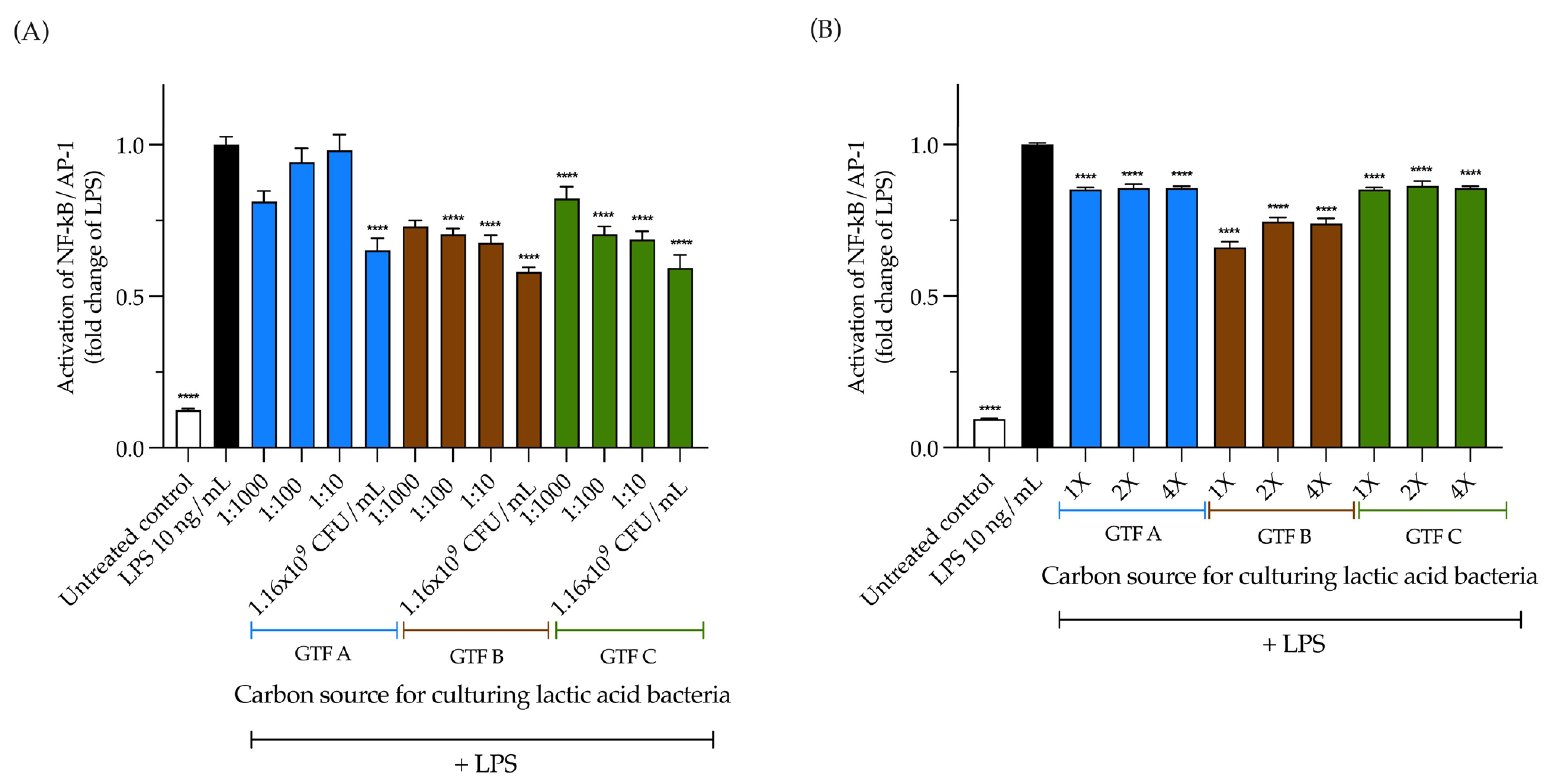
3.4. LAB Activate the NF-κB Pathway Independently of the Carbon Source, but Its Activation Is EPS Dependent
3.5. The Activation of TLR2 by LAB Is Carbon Source Dependent; Conversely, EPSs Produced by LAB Independently Activate TLR2
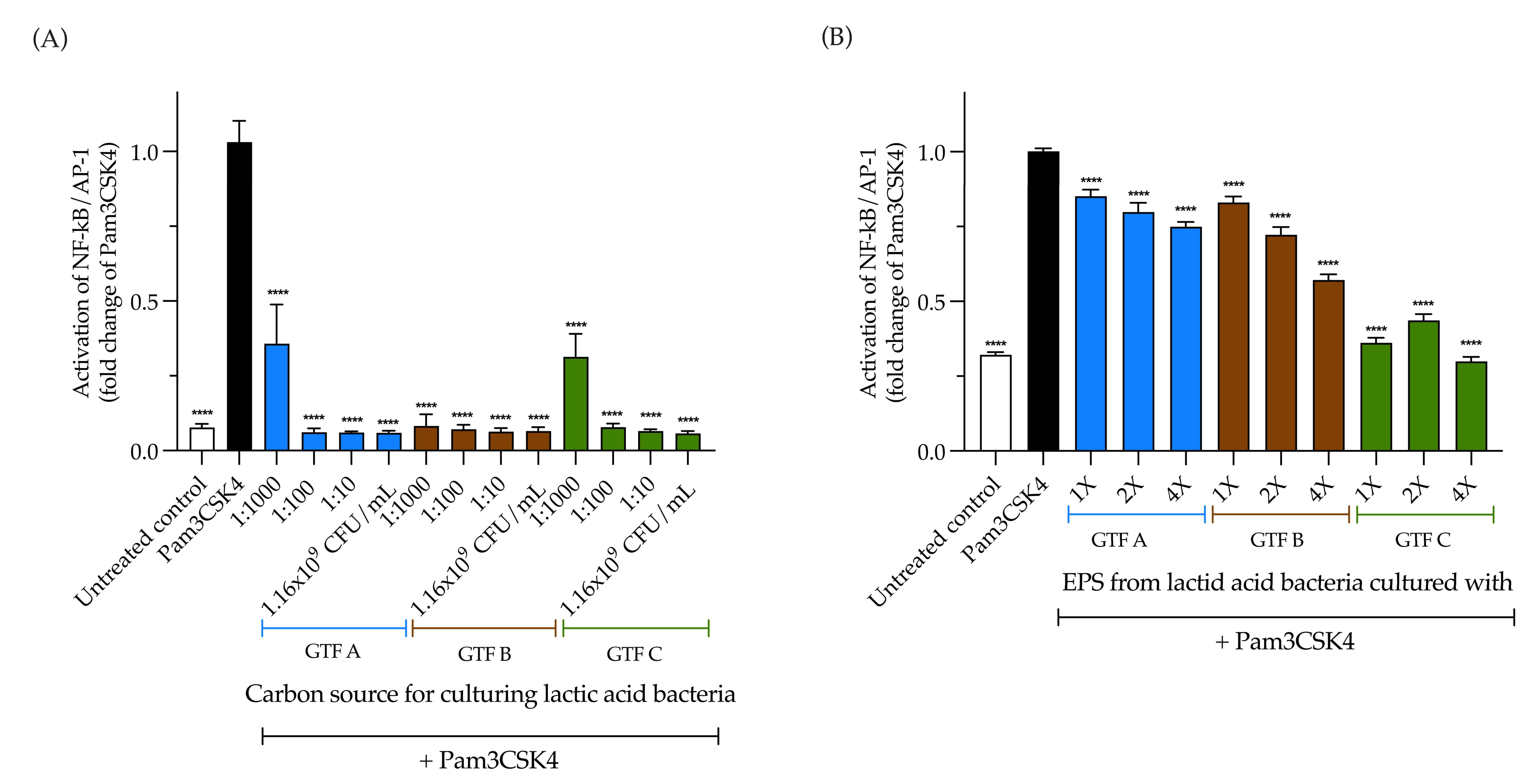
3.6. LAB Strongly Inhibit TLR2 Activation, While EPSs Produced by LAB Inhibit It in a Carbon Source-Dependent Manner
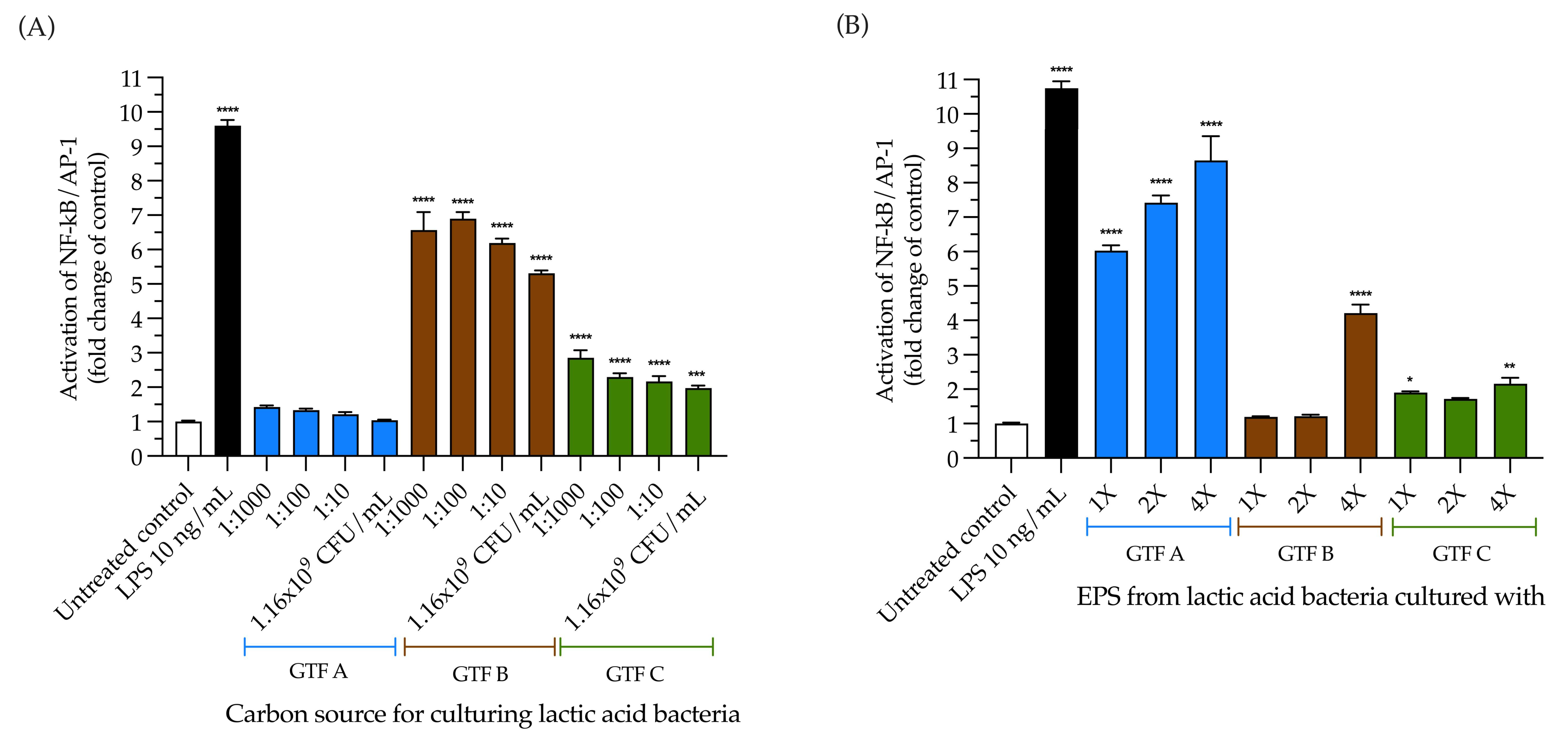
3.7. The Selected LAB Strain Grown with All the GTFs Except GTF A Activated TLR4, and the EPSs from GTF A Activated It in a Dose-Dependent Fashion
3.8. The Inhibition of TLR4 Activation by the LAB Strain Is Carbon Source and Dose Dependent, While Its EPSs Inhibit It Independently of These Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the pig as a human biomedical model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef] [PubMed]
- Bertho, N.; Meurens, F. The pig as a medical model for acquired respiratory diseases and dysfunctions: An immunological perspective. Mol. Immunol. 2021, 135, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Estellé, J.; Kiilerich, P.; Ramayo-Caldas, Y.; Xia, Z.; Feng, Q.; Liang, S.; Pedersen, A.; Kjeldsen, N.J.; Liu, C.; et al. A reference gene catalogue of the pig gut microbiome. Nat. Microbiol. 2016, 1, 16161. [Google Scholar] [CrossRef] [PubMed]
- Drewnowski, A. Perspective: The place of pork meat in sustainable healthy diets. Adv. Nutr. 2024, 15, 100213. [Google Scholar]
- Gresse, R.; Chaucheyras-Durand, F.; Fleury, M.A.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Gut Microbiota Dysbiosis in Postweaning Piglets: Understanding the Keys to Health. Trends Microbiol. 2017, 25, 851–873. [Google Scholar] [CrossRef]
- Fernández-Lainez, C.; López-Velázquez, G.; de Vos, P. Health benefits of Inulin and Agavin-type Fructans in Food: Impact on Microbiota, Immune and Gut Barrier Function. In The Book of Fructans; Elsevier: Amsterdam, The Netherlands, 2023; pp. 211–234. [Google Scholar]
- Del Fabbro, S.; Calder, P.C.; Childs, C.E. Microbiota-independent immunological effects of non-digestible oligosaccharides in the context of inflammatory bowel diseases. Proc. Nutr. Soc. 2020, 79, 468–478. [Google Scholar] [CrossRef]
- Akatsu, H. Exploring the Effect of Probiotics, Prebiotics, and Postbiotics in Strengthening Immune Activity in the Elderly. Vaccines 2021, 9, 136. [Google Scholar] [CrossRef]
- Kang, Y.; Cai, Y. Commentary: Boosting Vaccine-Elicited Respiratory Mucosal and Systemic COVID-19 Immunity in Mice With the Oral Lactobacillus plantarum. Front. Nutr. 2022, 9, 846379. [Google Scholar] [CrossRef]
- Tomczyk, G.; Niczyporuk, J.S.; Kozdruń, W.; Sawicka-Durkalec, A.; Bocian, Ł.; Barabasz, M.; Michalski, M. Probiotic supplementation as an alternative to antibiotics in broiler chickens. J. Vet. Res. 2024, 68, 147–154. [Google Scholar] [CrossRef]
- Taghinezhad, S.S.; Mohseni, A.H.; Bermúdez-Humarán, L.G.; Casolaro, V.; Cortes-Perez, N.G.; Keyvani, H.; Simal-Gandara, J. Probiotic-Based Vaccines May Provide Effective Protection against COVID-19 Acute Respiratory Disease. Vaccines 2021, 9, 466. [Google Scholar] [CrossRef]
- Gutiérrez-Castrellón, P.; Gandara-Martí, T.; Abreu, Y.A.A.T.; Nieto-Rufino, C.D.; López-Orduña, E.; Jiménez-Escobar, I.; Jiménez-Gutiérrez, C.; López-Velazquez, G.; Espadaler-Mazo, J. Probiotic improves symptomatic and viral clearance in Covid19 outpatients: A randomized, quadruple-blinded, placebo-controlled trial. Gut Microbes 2022, 14, 2018899. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.; Chang, S.Y.; Bogere, P.; Won, K.; Choi, J.Y.; Choi, Y.J.; Lee, H.K.; Hur, J.; Park, B.Y.; Kim, Y.; et al. Beneficial roles of probiotics on the modulation of gut microbiota and immune response in pigs. PLoS ONE 2019, 14, e0220843. [Google Scholar] [CrossRef]
- Helmy, Y.A.; Kathayat, D.; Ghanem, M.; Jung, K.; Closs, G., Jr.; Deblais, L.; Srivastava, V.; El-Gazzar, M.; Rajashekara, G. Identification and characterization of novel small molecule inhibitors to control Mycoplasma gallisepticum infection in chickens. Vet. Microbiol. 2020, 247, 108799. [Google Scholar] [CrossRef]
- Akutko, K.; Stawarski, A. Probiotics, Prebiotics and Synbiotics in Inflammatory Bowel Diseases. J. Clin. Med. 2021, 10, 2466. [Google Scholar] [CrossRef]
- Habteweld, H.A.; Asfaw, T. Novel Dietary Approach with Probiotics, Prebiotics, and Synbiotics to Mitigate Antimicrobial Resistance and Subsequent Out Marketplace of Antimicrobial Agents: A Review. Infect. Drug Resist. 2023, 16, 3191–3211. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, Y.; Wang, H.; Zhang, H.; Chen, W.; Lu, W. Lactic acid bacteria-derived exopolysaccharide: Formation, immunomodulatory ability, health effects, and structure-function relationship. Microbiol. Res. 2023, 274, 127432. [Google Scholar] [CrossRef]
- Polak-Berecka, M.; Waśko, A.; Kubik-Komar, A. Optimization of culture conditions for exopolysaccharide production by a probiotic strain of Lactobacillus rhamnosus E/N. Pol. J. Microbiol. 2014, 63, 253–257. [Google Scholar]
- Min, W.H.; Fang, X.B.; Wu, T.; Fang, L.; Liu, C.L.; Wang, J. Characterization and antioxidant activity of an acidic exopolysaccharide from Lactobacillus plantarum JLAU103. J. Biosci. Bioeng. 2019, 127, 758–766. [Google Scholar] [CrossRef]
- Samanta, A.K.; Jayapal, N.; Senani, S.; Kolte, A.P.; Sridhar, M. Prebiotic inulin: Useful dietary adjuncts to manipulate the livestock gut microflora. Braz. J. Microbiol. 2013, 44, 1–14. [Google Scholar] [CrossRef]
- Pang, D.J.; Huang, C.; Chen, M.L.; Chen, Y.L.; Fu, Y.P.; Paulsen, B.S.; Rise, F.; Zhang, B.Z.; Chen, Z.L.; Jia, R.Y.; et al. Characterization of Inulin-Type Fructan from Platycodon grandiflorus and Study on Its Prebiotic and Immunomodulating Activity. Molecules 2019, 24, 1199. [Google Scholar] [CrossRef]
- Csernus, B.; Czeglédi, L. Physiological, antimicrobial, intestine morphological, and immunological effects of fructooligosaccharides in pigs. Arch. Anim. Breed. 2020, 63, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Kawasaki, N.; Tamiya, T.; Anzai, S.; Toyohara, K.; Nishiyama, A.; Kitazono, E. Comparison of the prebiotic properties of native chicory and synthetic inulins using swine fecal cultures. Biosci. Biotechnol. Biochem. 2020, 84, 1486–1496. [Google Scholar] [CrossRef]
- Praznik, W.; Löppert, R.; Rubio, J.M.C.; Zangger, K.; Huber, A. Structure of fructo-oligosaccharides from leaves and stem of Agave tequilana Weber, var. azul. Carbohydr. Res. 2013, 381, 64–73. [Google Scholar] [CrossRef]
- Van den Ende, W. Multifunctional fructans and raffinose family oligosaccharides. Front. Plant Sci. 2013, 4, 247. [Google Scholar] [CrossRef]
- Chen, W.; Tan, D.; Yang, Z.; Tang, J.; Bai, W.; Tian, L. Fermentation patterns of prebiotics fructooligosaccharides-SCFA esters inoculated with fecal microbiota from ulcerative colitis patients. Food Chem. Toxicol. 2023, 180, 114009. [Google Scholar]
- Gonçalves, C.S.N.; Simões, L.S.; Gonçalves, D.A.M.; Berni, P.; Teixeira, J. Fructooligosaccharides production and the health benefits of prebiotics. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Niu, Z.; Zou, M.; Bei, T.; Zhang, N.; Li, D.; Wang, M.; Li, C.; Tian, H. Effect of fructooligosaccharides on the colonization of Lactobacillus rhamnosus AS 1.2466 T in the gut of mice. Food Sci. Hum. Wellness 2023, 12, 607–613. [Google Scholar]
- Fernández-Lainez, C.; Aan de Stegge, M.; Silva-Lagos, L.A.; López-Velázquez, G.; de Vos, P. β(2→1)-β(2→6) and β(2→1) fructans protect from impairment of intestinal tight junction’s gene expression and attenuate human dendritic cell responses in a fructan-dependent fashion. Carbohydr. Polym. 2023, 320, 121259. [Google Scholar] [CrossRef]
- Fernández-Lainez, C.; Akkerman, R.; Oerlemans, M.; Logtenberg, M.; Schols, H.; Silva-Lagos, L.; López-Velázquez, G.; de Vos, P. β (2→6)-Type fructans attenuate proinflammatory responses in a structure dependent fashion via Toll-like receptors. Carbohydr. Polym. 2022, 277, 118893. [Google Scholar] [CrossRef]
- Fernández-Lainez, C.; Logtenberg, M.J.; Tang, X.; Schols, H.A.; López-Velázquez, G.; de Vos, P. β(2→1) chicory and β(2→1)-β(2→6) agave fructans protect the human intestinal barrier function in vitro in a stressor-dependent fashion. Food Funct. 2022, 13, 6737–6748. [Google Scholar] [CrossRef]
- Márquez-López, R.E.; Santiago-García, P.A.; López, M.G. Agave Fructans in Oaxaca’s Emblematic Specimens: Agave angustifolia Haw. and Agave potatorum Zucc. Plants 2022, 11, 1834. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, S.; Li, G.; Chen, M.; Liu, H.; Ji, H. Two doses of Lactobacillus induced different microbiota profiles and serum immune indices in pigs. J. Funct. Foods 2023, 102, 105405. [Google Scholar] [CrossRef]
- Kenny, M.; Smidt, H.; Mengheri, E.; Miller, B. Probiotics–do they have a role in the pig industry? Animal 2011, 5, 462–470. [Google Scholar] [CrossRef]
- Méndez-Palacios, N.; Méndez-Mendoza, M.; Vázquez-Flores, F.; Castro-Colombres, J.G.; Ramírez-Bribiesca, J.E. Productive and economic parameters of pigs supplemented from weaning to finishing with prebiotic and probiotic feed additives. Anim. Sci. J. 2018, 89, 994–1001. [Google Scholar] [CrossRef]
- Verspreet, J.; Hansen, A.H.; Dornez, E.; Delcour, J.A.; Van den Ende, W.; Harrison, S.J.; Courtin, C.M. LC-MS analysis reveals the presence of graminan-and neo-type fructans in wheat grains. J. Cereal Sci. 2015, 61, 133–138. [Google Scholar] [CrossRef]
- Dowarah, R.; Verma, A.K.; Agarwal, N.; Singh, P.; Singh, B.R. Selection and characterization of probiotic lactic acid bacteria and its impact on growth, nutrient digestibility, health and antioxidant status in weaned piglets. PLoS ONE 2018, 13, e0192978. [Google Scholar] [CrossRef]
- Aouadhi, C.; Turki, M.; Maaroufi, A. Screening and characterization of lactic acid bacteria with antibacterial effect against heat-resistant spore outgrowth of Bacillus sporothermodurans. Int. Dairy J. 2024, 158, 106047. [Google Scholar] [CrossRef]
- Mueller, M.; Reiner, J.; Fleischhacker, L.; Viernstein, H.; Loeppert, R.; Praznik, W. Growth of selected probiotic strains with fructans from different sources relating to degree of polymerization and structure. J. Funct. Foods 2016, 24, 264–275. [Google Scholar] [CrossRef]
- Ferrari, M.; Hameleers, L.; Stuart, M.C.A.; Oerlemans, M.M.P.; de Vos, P.; Jurak, E.; Walvoort, M.T.C. Efficient isolation of membrane-associated exopolysaccharides of four commercial bifidobacterial strains. Carbohydr. Polym. 2022, 278, 118913. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.t.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- O’Donnell, M.M.; Forde, B.M.; Neville, B.; Ross, P.R.; O’Toole, P.W. Carbohydrate catabolic flexibility in the mammalian intestinal commensal Lactobacillus ruminis revealed by fermentation studies aligned to genome annotations. In Microbial Cell Factories; BioMed Central: London, UK, 2011; pp. 1–11. [Google Scholar]
- Lépine, A.F.; Konstanti, P.; Borewicz, K.; Resink, J.-W.; de Wit, N.J.; Vos, P.d.; Smidt, H.; Mes, J.J. Combined dietary supplementation of long chain inulin and Lactobacillus acidophilus W37 supports oral vaccination efficacy against Salmonella Typhimurium in piglets. Sci. Rep. 2019, 9, 18017. [Google Scholar] [CrossRef]
- Chen, S.; Suo, K.; Kang, Q.; Zhu, J.; Shi, Y.; Yi, J.; Lu, J. Active induction: A promising strategy for enhancing the bioactivity of lactic acid bacteria. Crit. Rev. Food Sci. Nutr. 2025, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Zhang, Q.; de Haan, B.J.; Zhang, H.; Faas, M.M.; de Vos, P. Identification of TLR2/TLR6 signalling lactic acid bacteria for supporting immune regulation. Sci. Rep. 2016, 6, 34561. [Google Scholar] [CrossRef] [PubMed]
- Akkerman, R.; Oerlemans, M.M.; Ferrari, M.; Fernández-Lainez, C.; Walvoort, M.T.; de Vos, P. Exopolysaccharides from Bifidobacterium longum subsp. infantis and Bifidobacterium adolescentis modulate Toll-like receptor signaling. Carbohydr. Polym. 2025, 349, 123017. [Google Scholar] [CrossRef]
- Kandler, O. Amino acid sequence of the murein and taxonomy of the genera Lactobacillus, Bifidobacterium, Leuconostoc and Pediococcus. Int. J. Syst. Evol. Microbiol. 1970, 20, 491–507. [Google Scholar] [CrossRef]
- Murofushi, Y.; Villena, J.; Morie, K.; Kanmani, P.; Tohno, M.; Shimazu, T.; Aso, H.; Suda, Y.; Hashiguchi, K.; Saito, T. The toll-like receptor family protein RP105/MD1 complex is involved in the immunoregulatory effect of exopolysaccharides from Lactobacillus plantarum N14. Mol. Immunol. 2015, 64, 63–75. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Wang, S.; Liu, H.; Zhang, D.; Wang, Y.; Ji, H. Swine-derived probiotic Lactobacillus plantarum modulates porcine intestinal endogenous host defense peptide synthesis through TLR2/MAPK/AP-1 signaling pathway. Front. Immunol. 2019, 10, 2691. [Google Scholar]
- Lin, M.-H.; Yang, Y.-L.; Chen, Y.-P.; Hua, K.-F.; Lu, C.-P.; Sheu, F.; Lin, G.-H.; Tsay, S.-S.; Liang, S.-M.; Wu, S.-H. A novel exopolysaccharide from the biofilm of Thermus aquaticus YT-1 induces the immune response through Toll-like receptor 2. J. Biol. Chem. 2011, 286, 17736–17745. [Google Scholar] [CrossRef]
- Laplanche, V.; Armiento, S.; Speciale, I.; Šuligoj, T.; Crost, E.H.; Lamprinaki, D.; Vaux, L.; Gotts, K.; De Castro, C.; Juge, N. The human gut symbiont Ruminococcus gnavus displays strain-specific exopolysaccharides modulating the host immune response. Carbohydr. Polym. 2025, 347, 122754. [Google Scholar] [CrossRef]
- Wang, X.; Ye, C.; Xun, T.; Mo, L.; Tong, Y.; Ni, W.; Huang, S.; Liu, B.; Zhan, X.; Yang, X. Bacteroides Fragilis Polysaccharide A Ameliorates Abnormal Voriconazole Metabolism Accompanied With the Inhibition of TLR4/NF-κB Pathway. Front. Pharmacol. 2021, 12, 663325. [Google Scholar] [CrossRef]
- Yue, F.; Han, H.; Xu, J.; Yao, X.; Qin, Y.; Zhang, L.; Sun, X.; Huang, J.; Zhang, F.; Lü, X. Effects of exopolysaccharides form Lactobacillus plantarum KX041 on high fat diet-induced gut microbiota and inflammatory obesity. Int. J. Biol. Macromol. 2024, 289, 138803. [Google Scholar] [CrossRef]
- Chen, L.; Wang, D.; Liu, W.; Zhou, S.; Gu, Q.; Zhou, T. Immunomodulation of exopolysaccharide produced by Lacticaseibacillus rhamnosus ZFM216 in cyclophosphamide-induced immunosuppressed mice by modulating gut microbiota. Int. J. Biol. Macromol. 2024, 283, 137619. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, H.; Stanton, C.; Ross, R.P.; Zhao, J.; Chen, W.; Yang, B. Alleviative effects of exopolysaccharides from Limosilactobacillus mucosae CCFM1273 against ulcerative colitis via modulation of gut microbiota and inhibition of Fas/Fasl and TLR4/NF-κB pathways. Int. J. Biol. Macromol. 2024, 260, 129346. [Google Scholar] [CrossRef]
- Kalinina, O.; Talley, S.; Zamora-Pineda, J.; Paik, W.; Campbell, E.M.; Knight, K.L. Amelioration of Graft-versus-Host Disease by Exopolysaccharide from a Commensal Bacterium. J. Immunol. 2021, 206, 2101–2108. [Google Scholar] [CrossRef]
- Paik, W.; Alonzo, F., 3rd; Knight, K.L. Suppression of Staphylococcus aureus Superantigen-Independent Interferon Gamma Response by a Probiotic Polysaccharide. Infect. Immun. 2020, 88, 10–1128. [Google Scholar] [CrossRef]
- Galván-Moroyoqui, J.M.; Del Carmen Domínguez-Robles, M.; Meza, I. Pathogenic bacteria prime the induction of Toll-like receptor signalling in human colonic cells by the Gal/GalNAc lectin Carbohydrate Recognition Domain of Entamoeba histolytica. Int. J. Parasitol. 2011, 41, 1101–1112. [Google Scholar] [CrossRef]
- Re, F.; Strominger, J.L. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J. Biol. Chem. 2001, 276, 37692–37699. [Google Scholar]
- Murtaugh, M.P.; Johnson, C.R.; Xiao, Z.; Scamurra, R.W.; Zhou, Y. Species specialization in cytokine biology: Is interleukin-4 central to the TH1–TH2 paradigm in swine? Dev. Comp. Immunol. 2009, 33, 344–352. [Google Scholar] [CrossRef]
- Silva-Lagos, L.; Ijaz, A.; Buwalda, P.; Kassai, S.; Klostermann, C.E.; Leemhuis, H.; Veldhuizen, E.J.A.; Schols, H.A.; López-Velázquez, G.; de Vos, P. Immunostimulatory effects of isomalto/malto-polysaccharides via TLR2 and TLR4 in preventing doxycycline-induced cytokine loss. Carbohydr. Polym. 2025, 350, 122980. [Google Scholar] [CrossRef]



| Cell Line | Cell Density | Agonist (Concentration) | Selection Antibiotic |
|---|---|---|---|
| THP1-XBlue™-MD2-CD14 | 5 × 105 cells/mL | LPS-EK Ultrapure (10 ng/mL) | Zeocin (200 µg/mL) G418 (250 µg/mL) |
| HEK-Blue™ hTLR2 | 2.8 × 105 cells/mL | Pam3CSK4 (100 ng/mL) hTLR2-TLR1 heterodimer | HEK-Blue selection™ |
| HEK-Blue™ hTLR4 | 1.4 × 105 cells/mL | LPS-EK Ultrapure (10 ng/mL) | HEK-Blue selection™ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanhueza-Carrera, E.A.; Fernández-Lainez, C.; Castro-De la Mora, C.; Ortega-Álvarez, D.; Mendoza-Camacho, C.; Cortéz-Sánchez, J.M.; Pérez-Guillé, B.; de Vos, P.; López-Velázquez, G. Swine Gut Lactic Acid Bacteria and Their Exopolysaccharides Differentially Modulate Toll-like Receptor Signaling Depending on the Agave Fructans Used as a Carbon Source. Animals 2025, 15, 1047. https://doi.org/10.3390/ani15071047
Sanhueza-Carrera EA, Fernández-Lainez C, Castro-De la Mora C, Ortega-Álvarez D, Mendoza-Camacho C, Cortéz-Sánchez JM, Pérez-Guillé B, de Vos P, López-Velázquez G. Swine Gut Lactic Acid Bacteria and Their Exopolysaccharides Differentially Modulate Toll-like Receptor Signaling Depending on the Agave Fructans Used as a Carbon Source. Animals. 2025; 15(7):1047. https://doi.org/10.3390/ani15071047
Chicago/Turabian StyleSanhueza-Carrera, Enrique A., Cynthia Fernández-Lainez, César Castro-De la Mora, Daniel Ortega-Álvarez, Claudia Mendoza-Camacho, Jesús Manuel Cortéz-Sánchez, Beatriz Pérez-Guillé, Paul de Vos, and Gabriel López-Velázquez. 2025. "Swine Gut Lactic Acid Bacteria and Their Exopolysaccharides Differentially Modulate Toll-like Receptor Signaling Depending on the Agave Fructans Used as a Carbon Source" Animals 15, no. 7: 1047. https://doi.org/10.3390/ani15071047
APA StyleSanhueza-Carrera, E. A., Fernández-Lainez, C., Castro-De la Mora, C., Ortega-Álvarez, D., Mendoza-Camacho, C., Cortéz-Sánchez, J. M., Pérez-Guillé, B., de Vos, P., & López-Velázquez, G. (2025). Swine Gut Lactic Acid Bacteria and Their Exopolysaccharides Differentially Modulate Toll-like Receptor Signaling Depending on the Agave Fructans Used as a Carbon Source. Animals, 15(7), 1047. https://doi.org/10.3390/ani15071047









