Zearalenone Depresses Lactation Capacity Through the ROS-Mediated PI3K/AKT Pathway
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Mouse Maintenance and Zearalenone Treatment
2.3. Sample Collection and Histomorphological Observation
2.4. Immunohistochemistry
2.5. Tissue Immunofluorescence Staining
2.6. TUNEL Assay
2.7. Cell Culture
2.8. Cell Viability Assay
2.9. Lactate Dehydrogenase Assay
2.10. Cell Proliferation Assay
2.11. Detection of Oxidative Stress Levels
2.12. Detection of ROS Level
2.13. Detection of Apoptosis
2.14. Western Blotting
2.15. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis
2.16. Statistical Analyses
3. Results
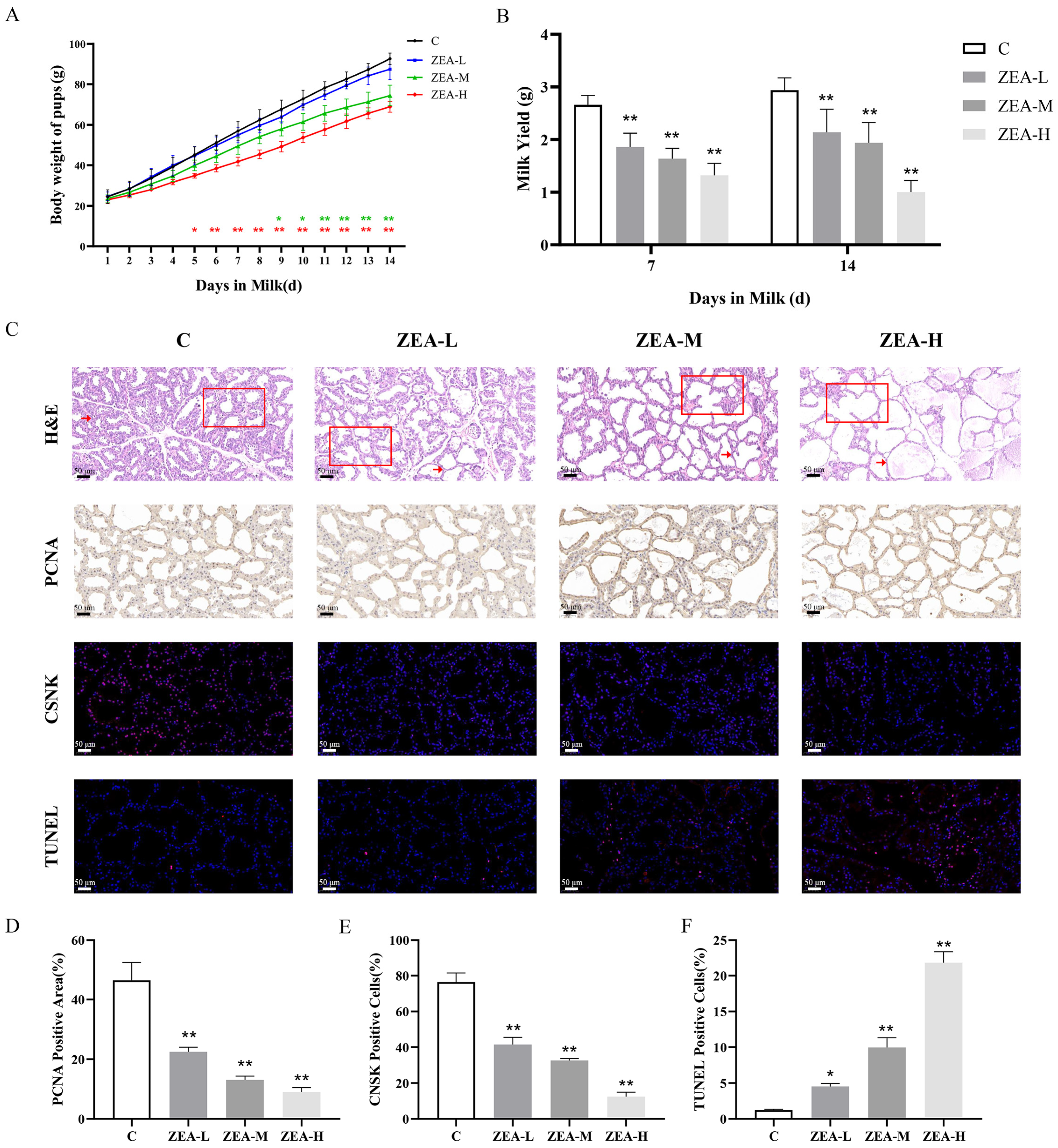
3.1. ZEN Induces Mammary Gland Damage in Mice
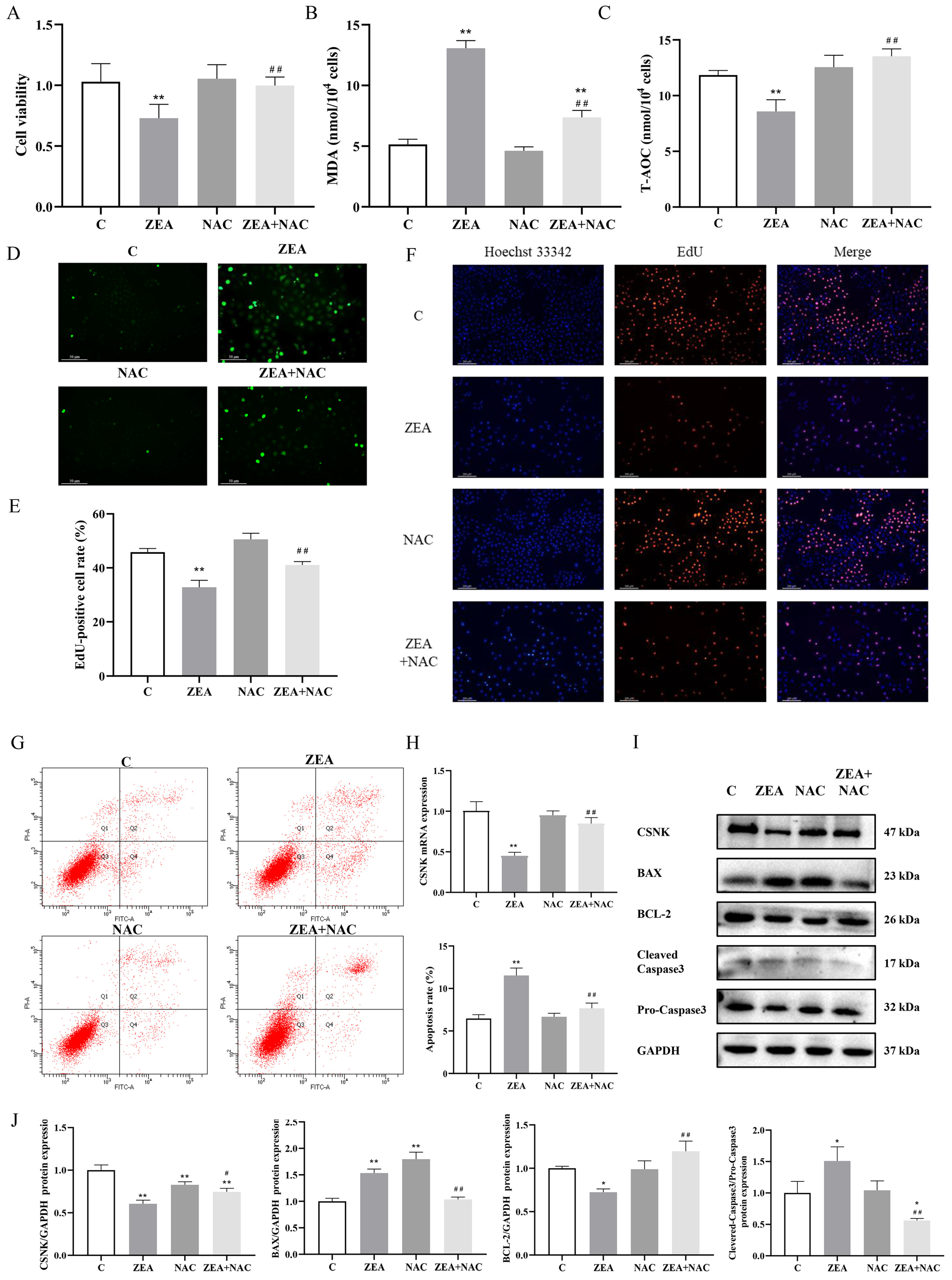
3.2. ZEA Inhibits MAC-T Cell Proliferation and Induces Apoptosis
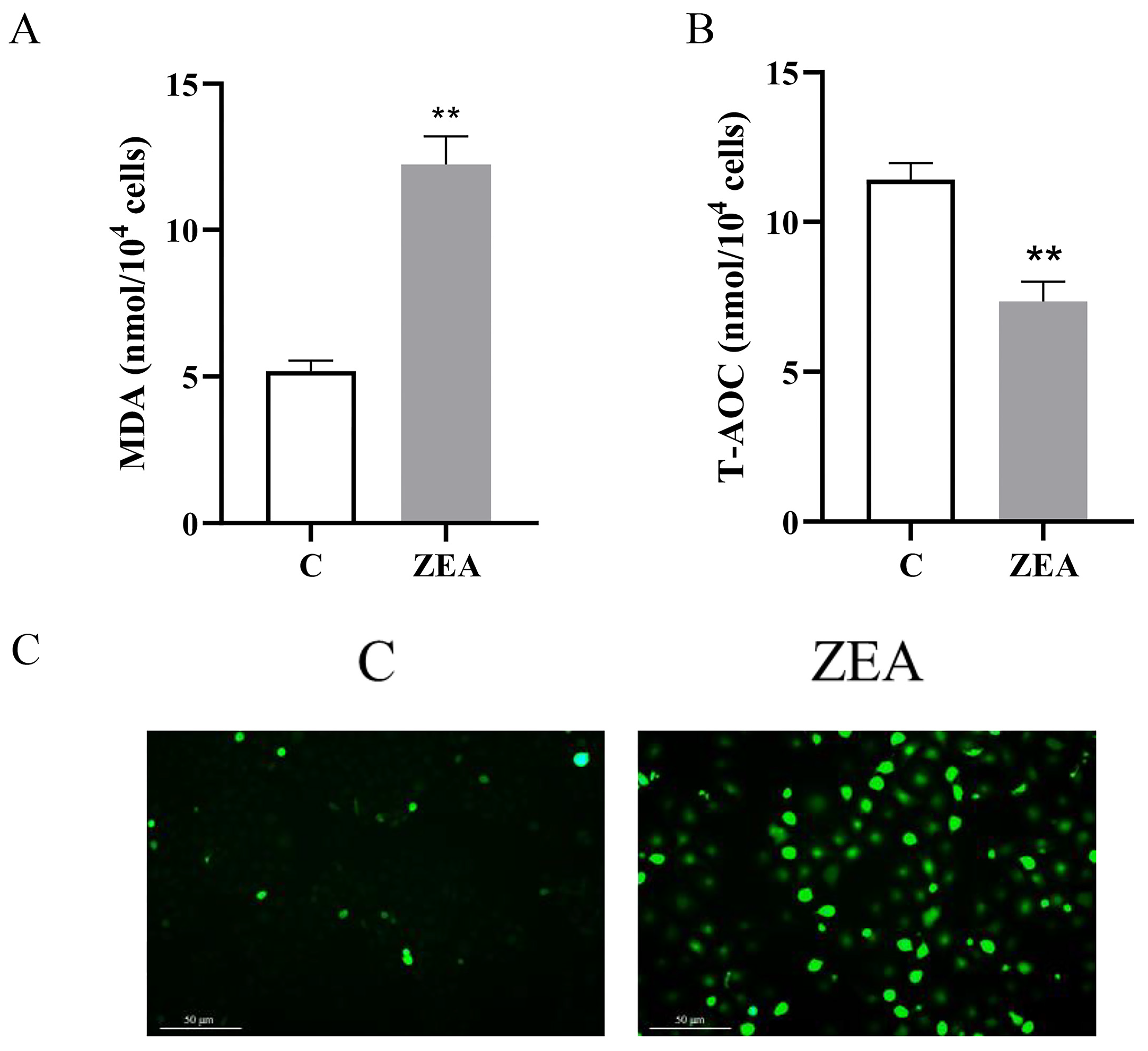
3.3. ZEA Induces Oxidative Stress in MAC-T
3.4. ROS Accumulation Is Linked to ZEA-Induced Proliferation and Apoptosis of MAC-T
3.5. ROS-Mediated PI3K/AKT Signaling Pathway Is Involved in ZEA Regulation of MAC-T
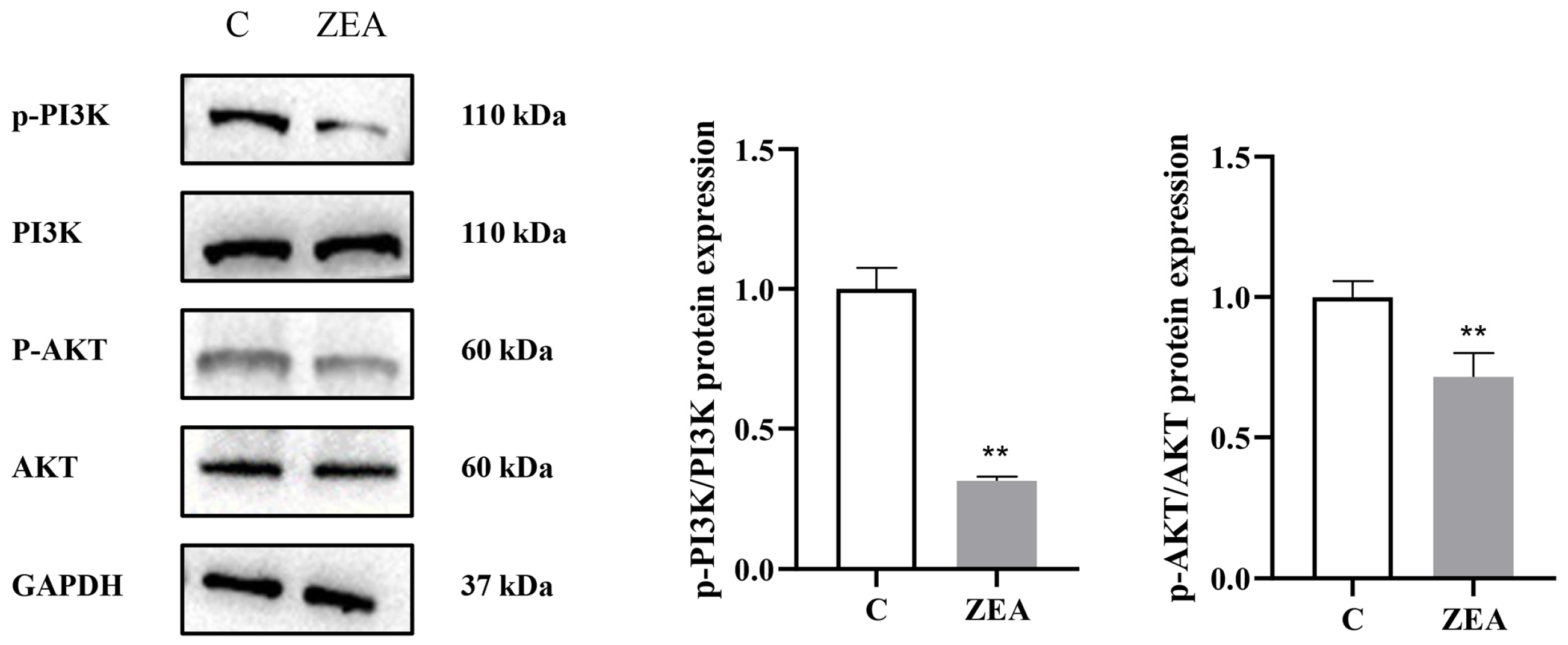
3.5.1. ZEA Inhibits PI3K/AKT Signaling Pathway in MAC-T
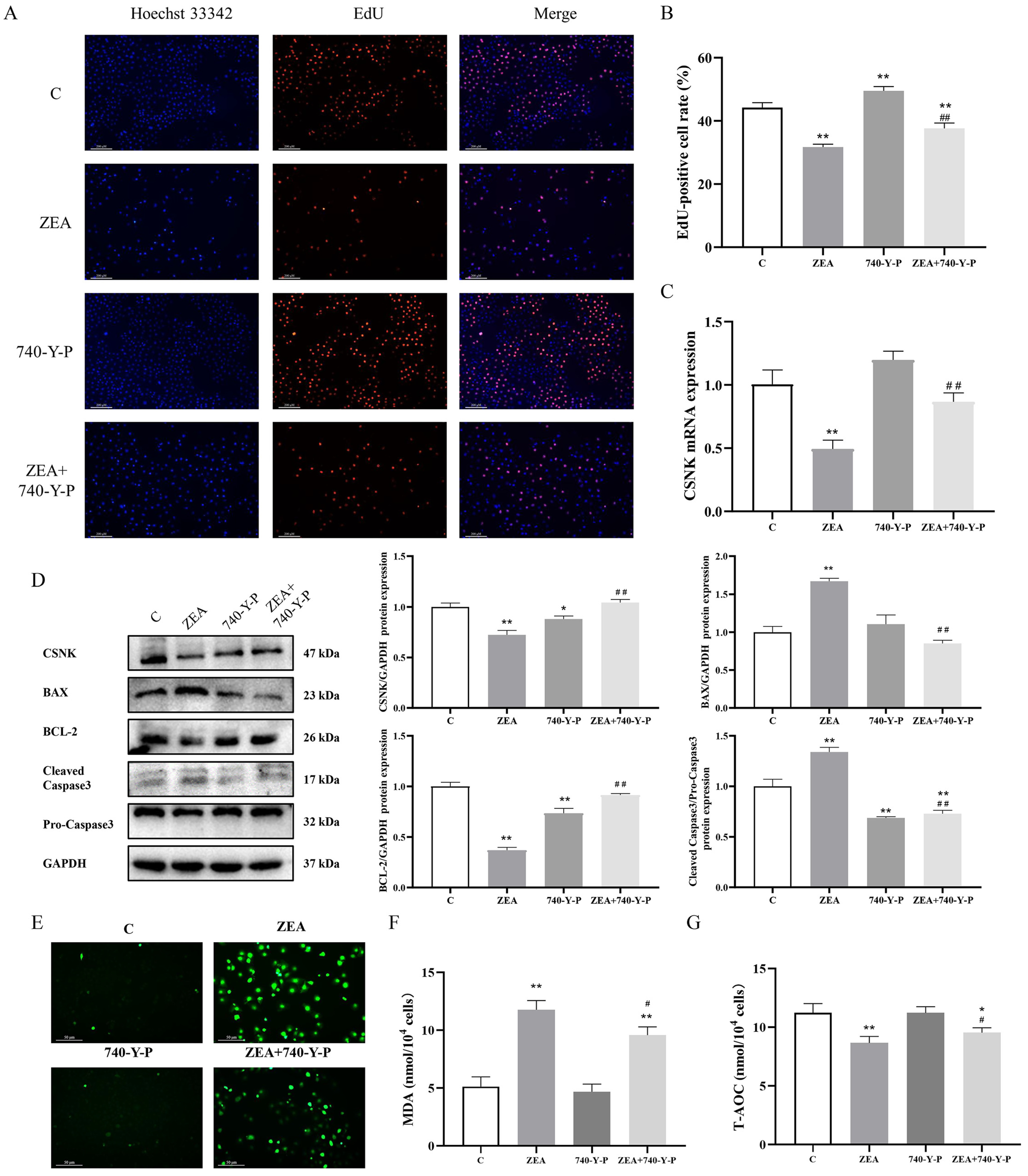
3.5.2. Involvement of PI3K/AKT Signaling Pathway in the Regulation of MAC-T by ZEA
3.5.3. The Inhibitory Effect of ZEA on PI3K/AKT Signaling Pathway Is ROS-Dependent
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Latham, R.L.; Boyle, J.T.; Barbano, A.; Loveman, W.G.; Brown, N.A. Diverse mycotoxin threats to safe food and feed cereals. Essays Biochem. 2023, 67, 797–809. [Google Scholar] [CrossRef]
- Marins-Goncalves, L.; Martins Ferreira, M.; Rocha Guidi, L.; De Souza, D. Is chemical analysis suitable for detecting mycotoxins in agricultural commodities and foodstuffs? Talanta 2023, 265, 124782. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.; Jackson, L.S.; Bullerman, L.B. Effects of processing on zearalenone. Adv. Exp. Med. Biol. 2002, 504, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2020, 60, 2710–2729. [Google Scholar] [CrossRef]
- Seeling, K.; Danicke, S.; Ueberschar, K.H.; Lebzien, P.; Flachowsky, G. On the effects of Fusarium toxin-contaminated wheat and the feed intake level on the metabolism and carry over of zearalenone in dairy cows. Food Addit. Contam. 2005, 22, 847–855. [Google Scholar] [CrossRef]
- Omar, S.S. Prevalence, level and health risk assessment of mycotoxins in the fried poultry eggs from Jordan. Environ. Res. 2021, 200, 111701. [Google Scholar] [CrossRef]
- Liu, J.; Applegate, T. Zearalenone (ZEN) in Livestock and Poultry: Dose, Toxicokinetics, Toxicity and Estrogenicity. Toxins 2020, 12, 377. [Google Scholar] [CrossRef]
- Rogowska, A.; Pomastowski, P.; Sagandykova, G.; Buszewski, B. Zearalenone and its metabolites: Effect on human health, metabolism and neutralisation methods. Toxicon 2019, 162, 46–56. [Google Scholar] [CrossRef]
- Mauro, T.; Hao, L.; Pop, L.C.; Buckley, B.; Schneider, S.H.; Bandera, E.V.; Shapses, S.A. Circulating zearalenone and its metabolites differ in women due to body mass index and food intake. Food Chem. Toxicol. 2018, 116 Pt B, 227–232. [Google Scholar] [CrossRef]
- Rubert, J.; Leon, N.; Saez, C.; Martins, C.P.; Godula, M.; Yusa, V.; Manes, J.; Soriano, J.M.; Soler, C. Evaluation of mycotoxins and their metabolites in human breast milk using liquid chromatography coupled to high resolution mass spectrometry. Anal. Chim. Acta 2014, 820, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Alshannaq, A.; Yu, J.H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public. Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed]
- Fleck, S.C.; Churchwell, M.I.; Doerge, D.R.; Teeguarden, J.G. Urine and serum biomonitoring of exposure to environmental estrogens II: Soy isoflavones and zearalenone in pregnant women. Food Chem. Toxicol. 2016, 95, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Falkauskas, R.; Bakutis, B.; Jovaisiene, J.; Vaiciulience, G.; Gerulis, G.; Kerziene, S.; Jaceviciene, I.; Jacevicius, E.; Baliukoniene, V. Zearalenone and Its Metabolites in Blood Serum, Urine, and Milk of Dairy Cows. Animals 2022, 12, 1651. [Google Scholar] [CrossRef]
- Goyarts, T.; Danicke, S.; Brussow, K.P.; Valenta, H.; Ueberschar, K.H.; Tiemann, U. On the transfer of the Fusarium toxins deoxynivalenol (DON) and zearalenone (ZON) from sows to their fetuses during days 35–70 of gestation. Toxicol. Lett. 2007, 171, 38–49. [Google Scholar] [CrossRef]
- Mahato, D.K.; Devi, S.; Pandhi, S.; Sharma, B.; Maurya, K.K.; Mishra, S.; Dhawan, K.; Selvakumar, R.; Kamle, M.; Mishra, A.K.; et al. Occurrence, Impact on Agriculture, Human Health, and Management Strategies of Zearalenone in Food and Feed: A Review. Toxins 2021, 13, 92. [Google Scholar] [CrossRef]
- Wang, S.; Fu, W.; Zhao, X.; Chang, X.; Liu, H.; Zhou, L.; Li, J.; Cheng, R.; Wu, X.; Li, X.; et al. Zearalenone disturbs the reproductive-immune axis in pigs: The role of gut microbial metabolites. Microbiome 2022, 10, 234. [Google Scholar] [CrossRef]
- Malekinejad, H.; Schoevers, E.J.; Daemen, I.J.; Zijlstra, C.; Colenbrander, B.; Fink-Gremmels, J.; Roelen, B.A. Exposure of oocytes to the Fusarium toxins zearalenone and deoxynivalenol causes aneuploidy and abnormal embryo development in pigs. Biol. Reprod. 2007, 77, 840–847. [Google Scholar] [CrossRef]
- Lo, E.K.K.; Wang, X.; Lee, P.K.; Wong, H.C.; Lee, J.C.; Gomez-Gallego, C.; Zhao, D.; El-Nezami, H.; Li, J. Mechanistic insights into zearalenone-accelerated colorectal cancer in mice using integrative multi-omics approaches. Comput. Struct. Biotechnol. J. 2023, 21, 1785–1796. [Google Scholar] [CrossRef]
- Ma, Z.; Li, Q.; Xu, H.; Li, Y.; Wang, S.; Xiong, Y.; Lan, D.; Li, j.; Xiong, X.; Fu, W. Zearalenone triggers programmed cell death and impairs milk fat synthesis via the AKT-mTOR-PPARgamma-ACSL4 pathway in bovine mammary epithelial cells. J. Anim. Sci. 2024, 3, 102. [Google Scholar] [CrossRef]
- Ersahin, T.; Tuncbag, N.; Cetin-Atalay, R. The PI3K/AKT/mTOR interactive pathway. Mol. Biosyst. 2015, 11, 1946–1954. [Google Scholar] [CrossRef] [PubMed]
- Ling, M.; Quan, L.; Lai, X.; Lang, L.; Li, F.; Yang, X.; Fu, Y.; Feng, S.; Yi, X.; Zhu, C.; et al. VEGFB Promotes Myoblasts Proliferation and Differentiation through VEGFR1-PI3K/Akt Signaling Pathway. Int. J. Mol. Sci. 2021, 22, 13352. [Google Scholar] [CrossRef]
- Xu, Z.J.; Liu, M.; Niu, Q.J.; Huang, Y.X.; Zhao, L.; Lei, X.G.; Sun, L.H. Both selenium deficiency and excess impair male reproductive system via inducing oxidative stress-activated PI3K/AKT-mediated apoptosis and cell proliferation signaling in testis of mice. Free Radic. Biol. Med. 2023, 197, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, W.; Chang, X.; Chen, C.; Guo, Z.; Yu, G.; Shao, W.; Wu, S.; Zhang, Q.; Zheng, F.; et al. ROS/mtROS promotes TNTs formation via the PI3K/AKT/mTOR pathway to protect against mitochondrial damages in glial cells induced by engineered nanomaterials. Part. Fibre Toxicol. 2024, 21, 1. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Wang, Z.; Lin, X.; Yan, Z.; Cao, Q.; Zhao, M.; Shi, K. MEN1/Menin regulates milk protein synthesis through mTOR signaling in mammary epithelial cells. Sci. Rep. 2017, 7, 5479. [Google Scholar] [CrossRef]
- Parveen, S.; Zhu, P.; Shafique, L.; Lan, H.; Xu, D.; Ashraf, S.; Ashraf, S.; Sherazi, M.; Liu, Q. Molecular Characterization and Phylogenetic Analysis of Casein Gene Family in Camelus ferus. Genes 2023, 14, 256. [Google Scholar] [CrossRef] [PubMed]
- Staiger, E.A.; Thonney, M.L.; Buchanan, J.W.; Rogers, E.R.; Oltenacu, P.A.; Mateescu, R.G. Effect of prolactin, beta-lactoglobulin, and kappa-casein genotype on milk yield in East Friesian sheep. J. Dairy. Sci. 2010, 93, 1736–1742. [Google Scholar] [CrossRef]
- Pizarro, M.G.; Landi, V.; Navas, F.J.; Leon, J.M.; Martinez, A.; Fernandez, J.; Delgado, J.V. Nonparametric analysis of casein complex genes’ epistasis and their effects on phenotypic expression of milk yield and composition in Murciano-Granadina goats. J. Dairy. Sci. 2020, 103, 8274–8291. [Google Scholar] [CrossRef]
- Liu, X.; Xi, H.; Han, S.; Zhang, H.; Hu, J. Zearalenone induces oxidative stress and autophagy in goat Sertoli cells. Ecotoxicol. Environ. Saf. 2023, 252, 114571. [Google Scholar] [CrossRef]
- Abuelo, A.; Hernandez, J.; Benedito, J.L.; Castillo, C. The importance of the oxidative status of dairy cattle in the periparturient period: Revisiting antioxidant supplementation. J. Anim. Physiol. Anim. Nutr. 2015, 99, 1003–1016. [Google Scholar] [CrossRef]
- Bai, J.; Zhou, Y.; Luo, X.; Hai, J.; Si, X.; Li, J.; Fu, H.; Dai, Z.; Yang, Y.; Wu, Z. Roles of stress response-related signaling and its contribution to the toxicity of zearalenone in mammals. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3326–3345. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.Q.; Zhao, A.H.; Wang, J.J.; Tian, Y.; Yan, Z.H.; Dri, M.; Shen, W.; De Felici, M.; Li, L. Oxidative stress as a plausible mechanism for zearalenone to induce genome toxicity. Gene 2022, 829, 146511. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Gao, S.; Ouyang, J.; Ma, L.; Bu, D. Impacts of Heat Stress-Induced Oxidative Stress on the Milk Protein Biosynthesis of Dairy Cows. Animals 2021, 11, 726. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Chen, Y.; Yang, M.; Li, J.; Wu, Y.; Fan, H.; Kong, X.; Ning, C.; Wang, S.; Xiao, W.; et al. Betulinic acid alleviates zearalenone-induced uterine injury in mice. Environ. Pollut. 2023, 316 Pt 1, 120435. [Google Scholar] [CrossRef]
- Gao, X.; Sun, L.; Zhang, N.; Li, C.; Zhang, J.; Xiao, Z.; Qi, D. Gestational Zearalenone Exposure Causes Reproductive and Developmental Toxicity in Pregnant Rats and Female Offspring. Toxins 2017, 9, 21. [Google Scholar] [CrossRef]
- Lin, J.; Zuo, C.; Liang, T.; Huang, Y.; Kang, P.; Xiao, K.; Liu, Y. Lycopene alleviates multiple-mycotoxin-induced toxicity by inhibiting mitochondrial damage and ferroptosis in the mouse jejunum. Food Funct. 2022, 13, 11532–11542. [Google Scholar] [CrossRef]
- Gao, X.; Xiao, Z.; Li, C.; Zhang, J.; Zhu, L.; Sun, L.; Zhang, N.; Khalil, M.M.; Rajput, S.A.; Qi, D. Prenatal exposure to zearalenone disrupts reproductive potential and development via hormone-related genes in male rats. Food Chem. Toxicol. 2018, 116 Pt B, 11–19. [Google Scholar] [CrossRef]
- Wu, K.; Jia, S.; Xue, D.; Rajput, S.A.; Liu, M.; Qi, D.; Wang, S. Dual effects of zearalenone on aflatoxin B1-induced liver and mammary gland toxicity in pregnant and lactating rats. Ecotoxicol. Environ. Saf. 2022, 245, 114115. [Google Scholar] [CrossRef]
- Yan, X.R.; Shi, T.; Xiao, J.Y.; Liu, Y.F.; Zheng, H.L. In Vitro transdifferentiated signatures of goat preadipocytes into mammary epithelial cells revealed by DNA methylation and transcriptome profiling. J. Biol. Chem. 2022, 298, 102604. [Google Scholar] [CrossRef]
- Hao, W.; Guan, S.; Li, A.; Wang, J.; An, G.; Hofstetter, U.; Schatzmayr, G. Mycotoxin Occurrence in Feeds and Raw Materials in China: A Five-Year Investigation. Toxins 2023, 15, 63. [Google Scholar] [CrossRef]
- Biscoto, G.L.; Salvato, L.A.; Alvarenga, E.R.; Dias, R.R.S.; Pinheiro, G.R.G.; Rodrigues, M.P.; Pinto, P.N.; Freitas, R.P.; Keller, K.M. Mycotoxins in Cattle Feed and Feed Ingredients in Brazil: A Five-Year Survey. Toxins 2022, 14, 552. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.J.; Derous, D.; Gerrard, A.; Wen, J.; Liu, X.; Tan, S.; Hambly, C.; Speakman, J.R. Limits to sustained energy intake. XXX. Constraint or restraint? Manipulations of food supply show peak food intake in lactation is constrained. J. Exp. Biol. 2020, 223 Pt 8, jeb208314. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.; Zhu, Z.; Zhu, S.; Han, L. A high dose of conjugated linoleic acid increases fatty liver and insulin resistance in lactating mice. PLoS ONE 2019, 14, e0214903. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Liu, Y.; Liao, X.; Wu, D.; Chen, Y.; Liang, Z.; Yuan, Z.; Li, R.; Yi, J.; et al. Tannic acid repair of zearalenone-induced damage by regulating the death receptor and mitochondrial apoptosis signaling pathway in mice. Environ. Pollut. 2021, 287, 117557. [Google Scholar] [CrossRef]
- Li, Y.; Cao, Y.; Wang, J.; Fu, S.; Cheng, J.; Ma, L.; Zhang, Q.; Guo, W.; Kan, X.; Liu, J. Kp-10 promotes bovine mammary epithelial cell proliferation by activating GPR54 and its downstream signaling pathways. J. Cell Physiol. 2020, 235, 4481–4493. [Google Scholar] [CrossRef]
- Lee, H.; An, G.; Lim, W.; Song, G. Pendimethalin exposure induces bovine mammary epithelial cell death through excessive ROS production and alterations in the PI3K and MAPK signaling pathways. Pestic. Biochem. Physiol. 2022, 188, 105254. [Google Scholar] [CrossRef]
- Shekar, P.C.; Goel, S.; Rani, S.D.; Sarathi, D.P.; Alex, J.L.; Singh, S.; Kumar, S. kappa-casein-deficient mice fail to lactate. Proc. Natl. Acad. Sci. USA 2006, 103, 8000–8005. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Zhang, J.; Hu, C.; Jiang, J.; Li, Y.; Peng, Z. ROS-induced lipid peroxidation modulates cell death outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis. Arch. Toxicol. 2023, 97, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Lin, Y.; Liu, L.; Bian, Y.; Zhang, L.; Gao, X.; Li, Q. 14-3-3gamma Regulates Lipopolysaccharide-Induced Inflammatory Responses and Lactation in Dairy Cow Mammary Epithelial Cells by Inhibiting NF-kappaB and MAPKs and Up-Regulating mTOR Signaling. Int. J. Mol. Sci. 2015, 16, 16622–16641. [Google Scholar] [CrossRef]
- Gannuscio, R.; Ponte, M.; Di Grigoli, A.; Maniaci, G.; Di Trana, A.; Bacchi, M.; Alabiso, M.; Bonanno, A.; Todaro, M. Feeding Dairy Ewes with Fresh or Dehydrated Sulla (Sulla coronarium L.) Forage. 1. Effects on Feed Utilization, Milk Production, and Oxidative Status. Animals 2022, 12, 2317. [Google Scholar] [CrossRef]
- Wang, H.; Hao, W.; Yang, L.; Li, T.; Zhao, C.; Yan, P.; Wei, S. Procyanidin B2 Alleviates Heat-Induced Oxidative Stress through the Nrf2 Pathway in Bovine Mammary Epithelial Cells. Int. J. Mol. Sci. 2022, 23, 7769. [Google Scholar] [CrossRef]
- Chen, J.; Li, M.; Zhou, X.; Xie, A.; Cai, Z.; Fu, C.; Peng, Y.; Zhang, H.; Liu, L. Rotenone-Induced Neurodegeneration Is Enabled by a p38-Parkin-ROS Signaling Feedback Loop. J. Agric. Food Chem. 2021, 69, 13942–13952. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, Y.; Wu, C.; Qiu, S.; Chen, X.; Cai, B.; Xie, H. Freeze-thawing impairs the motility, plasma membrane integrity and mitochondria function of boar spermatozoa through generating excessive ROS. BMC Vet. Res. 2021, 17, 127. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, Y.; Zhang, X.; Ullah, R.; Tong, J.; Shen, Y. The role of the PI3K/AKT signalling pathway in the corneal epithelium: Recent updates. Cell Death Dis. 2022, 13, 513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Z.; Lan, J.; Wang, J.; Shen, Z.; Shi, G.; Li, S. Cadmium induces the thymus apoptosis of pigs through ROS-dependent PTEN/PI3K/AKT signaling pathway. Environ. Sci. Pollut. Res. Int. 2021, 28, 39982–39992. [Google Scholar] [CrossRef]


| Target Gene | Forward Primer | Reverse Primer |
|---|---|---|
| CSNK (NC_037333.1) | CCAGGAGCAAAACCAAGAAC | TGCAACTGGTTTCTGTTGGT |
| GAPDH (NC_037332.1) | ACTGGCGTCTTCACCACCAT | AAGGCCATGCCAGTGAGCTT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Qiu, D.; Miao, X.; Yang, W.; He, Q.; Ren, H.; Zhang, L.; Ruan, H.; Zhang, J.; Zhang, N. Zearalenone Depresses Lactation Capacity Through the ROS-Mediated PI3K/AKT Pathway. Animals 2025, 15, 1050. https://doi.org/10.3390/ani15071050
Chen H, Qiu D, Miao X, Yang W, He Q, Ren H, Zhang L, Ruan H, Zhang J, Zhang N. Zearalenone Depresses Lactation Capacity Through the ROS-Mediated PI3K/AKT Pathway. Animals. 2025; 15(7):1050. https://doi.org/10.3390/ani15071050
Chicago/Turabian StyleChen, Hong, Di Qiu, Xue Miao, Wenyue Yang, Qi He, Hao Ren, Luyao Zhang, Hongri Ruan, Jiantao Zhang, and Na Zhang. 2025. "Zearalenone Depresses Lactation Capacity Through the ROS-Mediated PI3K/AKT Pathway" Animals 15, no. 7: 1050. https://doi.org/10.3390/ani15071050
APA StyleChen, H., Qiu, D., Miao, X., Yang, W., He, Q., Ren, H., Zhang, L., Ruan, H., Zhang, J., & Zhang, N. (2025). Zearalenone Depresses Lactation Capacity Through the ROS-Mediated PI3K/AKT Pathway. Animals, 15(7), 1050. https://doi.org/10.3390/ani15071050






