Remote Vital Sensing in Clinical Veterinary Medicine: A Comprehensive Review of Recent Advances, Accomplishments, Challenges, and Future Perspectives
Simple Summary
Abstract
1. Introduction
2. Infrared Thermography
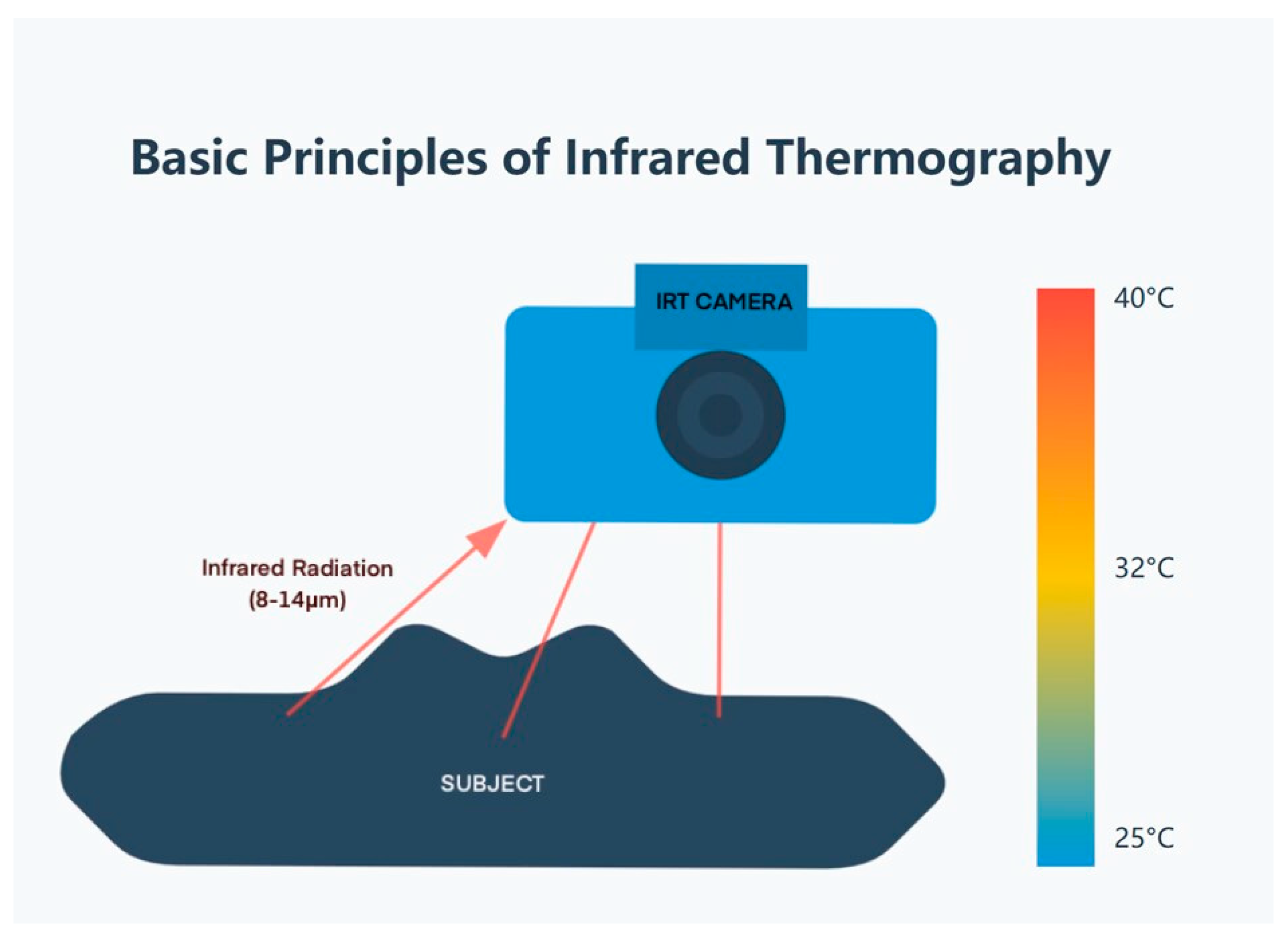
2.1. Principles and Technology
- (1)
- Infrared detector: Usually a focal plane array (FPA) of microbolometers or quantum detectors.
- (2)
- Optical system: Lenses that are cognizant of the infrared radiation onto the detector.
- (3)
- Signal processing unit: Converts detector signals into temperature values.
- (4)
- Display: Presents the thermal image, frequently using a color palette to symbolize temperature variations.
2.2. Applications in Various Species
2.2.1. Cattle
- (1)
- Detection of mastitis: IRT can identify temperature changes in udders associated with clinical and subclinical mastitis, potentially allowing for earlier intervention [18]. Recent work [19] has shown that infrared thermography effectively detects early-stage bovine mastitis infections that show no visible symptoms, allowing for earlier treatment. When detecting mastitis using infrared thermography, affected areas of the udder typically show temperature increases of 1.5–2.5 °C above normal.
- (2)
- Lameness diagnosis: In regard to the issues of hooves and legs, thermal imaging helps find different temperature signs that indicate lameness and foot diseases at an initial stage [20]. Temperature differences greater than 1.0 °C between opposite hooves often indicate disease, with active digital dermatitis (hoof infections) showing temperature increases of 2.3–3.1 °C compared to healthy tissue.
2.2.2. Horses
- (1)
- Musculoskeletal injuries: IRT can aid in revealing inflammation or injury in tendons, ligaments, and muscles before the clinical symptoms manifest [21]. A thorough review [22] has highlighted the growing uses of infrared thermography in horse clinical and sports medicine, especially for finding minor issues that limit performance before they develop into visible lameness.
- (2)
- Saddle-fitting assessment: Saddle-related pressure points manifest as circumscribed hotspots 1.0–2.7 °C above adjacent tissue temperatures. Pressure points and thermal images typical for an improper saddle can be identified with the help of thermal imaging [23].
2.2.3. Small Animals (Dogs and Cats)
- (1)
- Osteoarthritis assessment: When examining osteoarthritis, the affected joints usually show temperature increases of 0.5–1.8 °C compared to healthy joints. IRT helps to diagnose osteoarthritis by capturing temperature changes in the affected joints and assists in treatment [17].
- (2)
- Neoplasia detection: Various neoplasms present characteristic thermal signatures, with many superficial tumors exhibiting temperatures 1.0–3.5 °C higher than surrounding tissues. Abnormal temperature values have been pointed out by thermal imaging for different types of tumors [24].
2.2.4. Zoo Animals and Wildlife Applications
- (1)
- Non-invasive physiological monitoring: Companies permit the monitoring of the temperature and physiological state of free-ranging or confined wildlife without even capturing or restraining them [15].
- (2)
- Stress assessment: Stress responses have also been assessed using thermal imaging where eye temperature variation has been taken in different animals [4].
2.3. Advantages and Limitations
2.3.1. Advantages
- (1)
- (2)
- Real-time imaging: IRT offers the immediate visualization of surface temperature distribution, enabling the rapid assessment of thermal patterns and prompt clinical decision making [14]. This real-time capability enhances diagnostic efficiency in field and clinical settings.
- (3)
- Broad applicability: The technology can be applied across diverse animal species and for various diagnostic objectives regardless of patient size or coat characteristics [26]. This versatility makes it valuable in multiple veterinary disciplines, from companion animal practice to wildlife medicine.
- (4)
- Early detection: IRT can establish relationships with fundamental indicators of health with high predictive capability of subclinical states before symptom manifestations [27].
- (5)
- Non-invasive physiological monitoring: Beyond disease detection, IRT enables the ongoing assessment of physiological status and treatment response without disturbing the animal, making it particularly valuable for critical care and post-surgical monitoring [17].
2.3.2. Limitations
- (1)
- Environmental sensitivity: Expresses a remarkable sensitivity to room temperature, humidity, and airflow conditions [14].
- (2)
- Surface temperature only: This may in fact not read out the internal body temperature or deep tissue conditions perfectly [5].
- (3)
- Fur/feather interference: Heavy pelts of fur or feathers can create a problem for the correct measurement of temperature [15].
- (4)
- Standardization issues: Little consistency between species and conditions [5].
- (5)
- Cost and expertise: Infrared cameras are not cheap and interpreting the images calls for professional experts [26].
2.4. Future Directions
- (1)
- Development of species-specific standardization protocols to establish reliable reference ranges and imaging procedures across diverse animal taxa and clinical conditions [5].
- (2)
- Advancement of automated image analysis through specialized algorithms and artificial intelligence to enhance pattern recognition and diagnostic specificity [16]. This includes the development of machine learning models trained on species-specific thermographic patterns to identify subtle thermal signatures indicative of specific pathologies.
- (3)
- Engineering of specialized portable IRT devices optimized for field veterinary use, including ruggedized designs for farm settings and wildlife applications, with improved battery life and simplified interfaces [28].
- (4)
- Integration with comprehensive digital veterinary platforms to enable longitudinal thermal monitoring and correlation with other clinical data, supporting precision veterinary medicine approaches.
- (5)
- Establishment of validated thermal patterns for differential diagnoses of conditions with similar clinical presentations but distinct thermographic signatures [17].
3. Remote Photoplethysmography (rPPG)
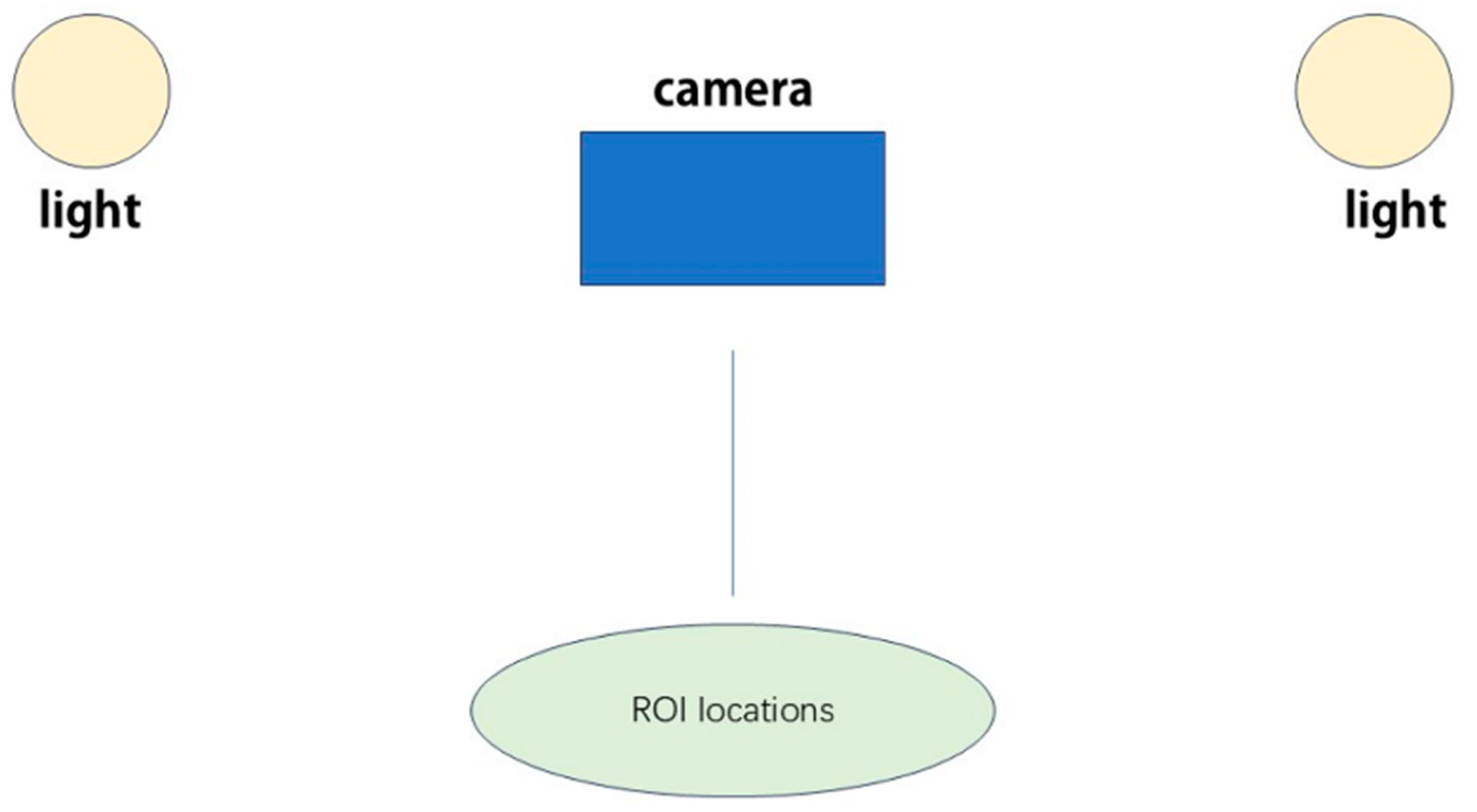
3.1. Technology Overview and Principles
- (1)
- Video Acquisition: The subject is recorded using a digital camera or smartphone that has a view of the skin or bodies with fur [30].
- (2)
- Region of Interest (ROI) Selection: Some regions that potentially experience pulsatile variations are also detected and monitored on the subject’s body by algorithms (Figure 2).
- (3)
- Color Channel Separation: It is typically divided into red–green–blue where green is largely sensitive to the volume change in blood [31].
- (4)
- Signal Extraction: To acquire the rPPG signal, temporal differences in pixel values inside the selected ROI are employed [32].
- (5)
- Signal Processing: Following data acquisition, various post-processing techniques are applied to extract physiological signals from raw video data. These include conventional signal processing approaches such as bandpass filtering (0.7–4 Hz for heart rate), wavelet transforms, and independent component analysis; and specific machine learning algorithms including convolutional neural networks (CNNs) that process spatial–temporal maps of skin color variations to isolate cardiac signals from motion artifacts and environmental noise [33]. These post-processing methods do not alter the data acquisition itself but rather enhance signal extraction from the already acquired video data.
- (6)
- Parameter Estimation: The processed signal affords approximate estimates of various physiological parameters including the heart and respiratory rates of the body [34].
3.2. Application in Various Species
3.2.1. Dogs
3.2.2. Cats
3.2.3. Horses
3.2.4. Cattle
3.2.5. Wildlife
3.3. Challenges and Potential Solutions
- (1)
- Motion Artifacts: One of the main problems in the rPPG study is handling motion artifacts, particularly in unencumbered animals. To overcome this problem, novel motion tracking and compensation methods are being worked on.
- (2)
- Fur Occlusion: Fur in most dogs or cats is a strong issue affecting rPPG in most veterinary patients. Attempts are being made to look for other measurement locations and to devise new algorithms that can handle situations when minimal skin is being exposed to an MRI [1].
- (3)
- Species Variability: The multivaried animal species and distinct physiological attributes and skin/fur morphology also act as barriers to the ideal rPPG solutions. Optimal algorithms and calibration techniques for each species are in the course of being established [39].
- (4)
- Environmental Factors: The fluctuations in light intensity and the background color within veterinary environments may interfere with rPPG signals. Scientists are trying to compute perfect algorithms that take into account lighting conditions [40].
3.4. Future Directions
- (1)
- Development of fur-penetrating imaging technologies using specialized wavelengths and sensor technologies to overcome the significant challenge of coat coverage in most veterinary species.
- (2)
- Creation of species-specific algorithms and calibration techniques tailored to the unique cardiovascular and respiratory physiological characteristics of different animal taxa [39].
- (3)
- Engineering of specialized illumination systems optimized for veterinary clinical environments to improve signal acquisition in varying lighting conditions [40].
- (4)
- Validation studies comparing rPPG measurements against gold-standard techniques across healthy animals and those with specific cardiovascular and respiratory pathologies to establish clinical utility.
- (5)
- Development of miniaturized rPPG systems that can be integrated into existing veterinary clinic infrastructure without requiring specialized facilities or environmental modifications.
4. Radar-Based Sensing
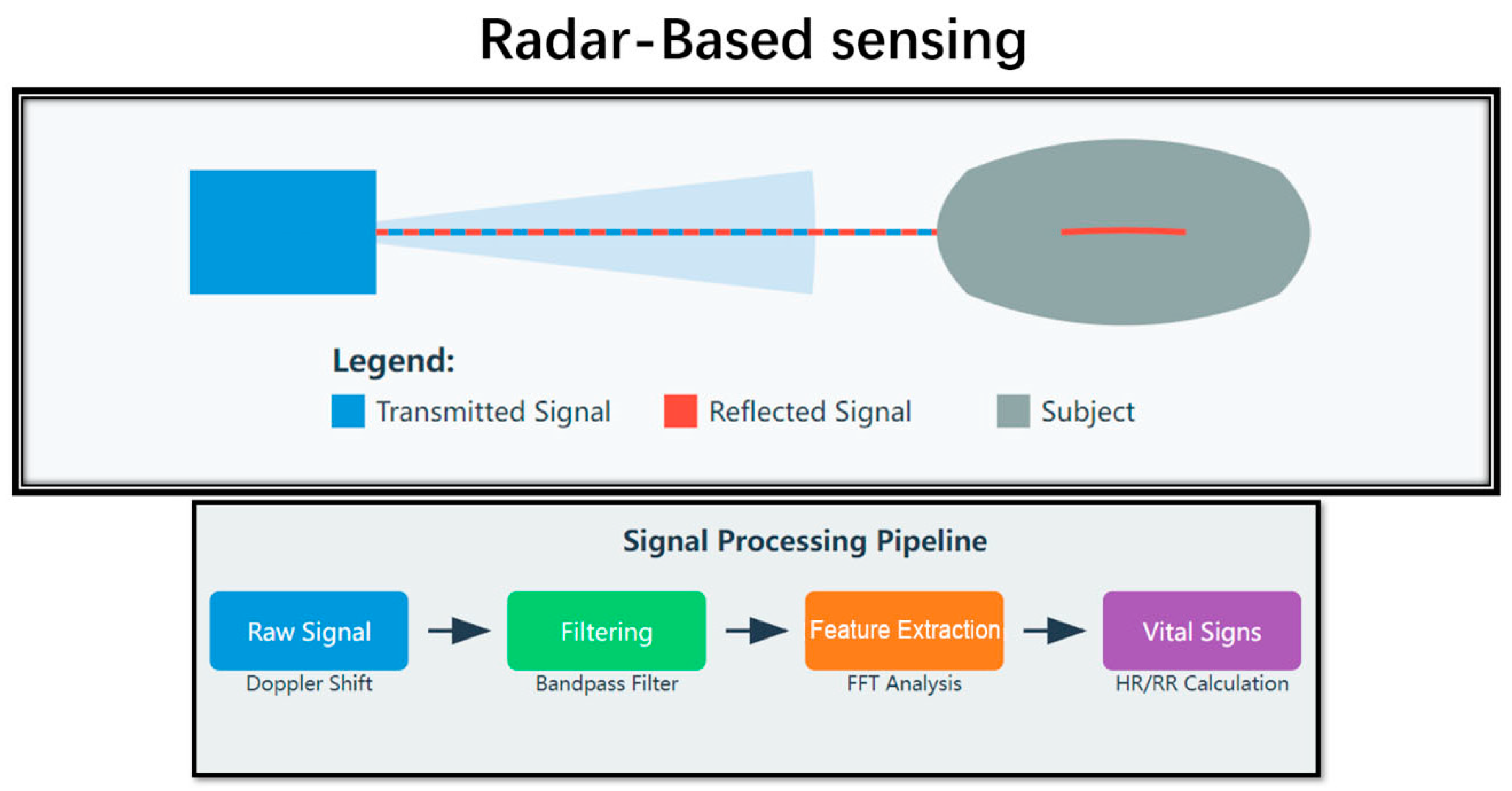
4.1. Principles of Operation
- (1)
- Continuous-Wave (CW) Doppler Radar: This type always transmits a single-frequency signal and determines changes in the frequency of the returned signal. It is non-invasive, time-effective, and affordable although it is vulnerable to patient movement [43].
- (2)
- Frequency-Modulated Continuous-Wave (FMCW) Radar: This type charges and discharges the transmitted signal at different rates, providing finer distance estimation and possibly, enhanced vital sign identification [44].
- (3)
- Ultra-Wideband (UWB) Radar: This type employs extremely brief pulses over a broad spectrum and could provide better penetration through fur and tissue than the previous kind while having a high temporal resolution [45].
4.2. Applications in Various Species
4.2.1. Horses
4.2.2. Cattle
4.2.3. Pigs
4.2.4. Dogs
4.2.5. Poultry
4.3. Advantages and Challenges
4.3.1. Advantages
- (1)
- Non-contact and Non-invasive: This method makes it possible to monitor the vitals without any contact with the animal.
- (2)
- Penetration through Fur and Light Barriers: It can also pass through fur and lighter colors, which makes it ideal for use on animals of different kinds [41].
- (3)
- Continuous Monitoring: This method also offers an opportunity to monitor continuously rather than restraining animals [48].
- (4)
- Multiparameter Measurement: It potentially monitors a number of biophysical variables at once [57].
- (5)
- Operation in Various Lighting Conditions: This kind of therapy is useful where the blood flow is poor, during nighttime, or in the absence of light [58].
4.3.2. Challenges
- (1)
- Motion Artifacts: Movement is often unhandy when the recording is made amid any tiny physiological movements due to its sensitivity to motion artifacts.
- (2)
- Signal Separation: It is still difficult to extract cardiac signals specifically from respiratory signals and other movements of the body [58].
- (3)
- Species-Specific Adaptations: Requires the design of species-specific computations and even, in some cases, specific modifications to molecular hardware.
- (4)
- Limited Research on Long-term Effects: More safety investigations are required on the possible chronic impacts of constant radar illumination on the creatures [41].
- (5)
- Environmental Interference: Interference with signals from other devices or the reflection of surfaces in its vicinity [57].
- (6)
- Cost and Complexity: Modern capable radars are sometimes costly, and may require high-level management [59].
4.4. Future Directions
- (1)
- (2)
- Development of species-adapted radar systems with optimized frequency ranges, power settings, and detection distances calibrated for specific animal groups (large livestock, small mammals, avian species) [47].
- (3)
- Expansion of radar applications beyond vital sign monitoring to include behavioral assessment, gait analysis, and early detection of mobility issues through specialized motion detection algorithms.
- (4)
- Creation of portable, battery-operated radar systems suitable for field veterinary applications in resource-limited settings, with simplified user interfaces accessible to practitioners without specialized technical training.
- (5)
- Long-term safety evaluation studies to conclusively determine the effects of chronic low-level radar exposure on different animal species, particularly for continuous monitoring applications [41].
5. Wearable Sensors
5.1. Types of Wearable Sensors for Veterinary Use
- (1)
- Accelerometers and Inertial Measurement Units (IMUs): These sensors detect movement and position to give details of the activity, walking style, or even standing post. For example, using collar-mounted accelerometers in a study with dogs, the classification accuracy of 91.3% of different activities including, walking, trotting, and galloping was achieved [61].
- (2)
- Heart Rate Monitors: These devices generally employ ECG or PPG that estimates heart rate and heart rate variability. An ECG worn by dogs produced a 0.99 coefficient when compared with conventional ECG readings [62]. Various technologies have been implemented for the heart rate monitoring of livestock comprising chest belt sensors and implantable sensors [63].
- (3)
- Temperature Sensors: Prolonged and consistently high temperatures mirror various diseases early enough; therefore, constant temperature checks are essential. Peculiarly, one method combining the temperature sensors into a collar-worn device for cattle reached an identification accuracy of ±0.1°C higher than the rectal temperature measurement as a standard [64].
- (4)
- GPS Trackers: Primarily used in tracking locations, GPS devices use other sensors in the monitoring process too. GPS collars were used in dogs and were identified to have an average positional error of less than 5 m when tracking movement patterns [65].
- (5)
- Respiratory Rate Monitors: These monitors frequently employ strain gauges or impedance pneumography in monitoring breathing patterns. A respiratory rate-monitoring wearable device in horses had ±2 breaths per minute compared to visual counts of respiratory rates, leading to the conclusion that the wearable device could be used as the standard for respiratory rate counts.
- (6)
- Environmental Sensors: These sensors are, more often than not, part of an interconnected system of other wearable devices used to track the planet’s surroundings. It has been applied in live-stock management for detecting a shift in relative humidity and ammonia level with a study showing its 90 percent efficiency in recognizing environmentally damaging conditions [66].
- (7)
- Biochemical Sensors: One subset of wearable that is starting to receive focus is sensors that bear the responsibility of detecting biochemical indicators in body fluids. For instance, a sweat-cooled wearable sensor for horses was able to determine the electrolyte concentration during the exercise with a difference of ±5% from the actual laboratory results [67].
5.2. Applications in Various Species
5.2.1. Companion Animals
5.2.2. Livestock
5.2.3. Wildlife
5.3. Advantages and Challenges
5.3.1. Advantages
- (1)
- Continuous, Long-term Monitoring: Portable biosensors measure physiological data and activity 24/7 for weeks at a time. This is more informative than mere clinical assessments that are carried out once in a while. Worth noting that the constant data gathering in these cases could help in the diagnosis of early health complications and enhance the welfare of animals [66].
- (2)
- Non-invasive and Well-tolerated: Non-invasive wearable sensors are used in most animal applications and the use of such sensors is normally scratched off veterinary care problems depending on the level of invasiveness. Specifically, this paper reviewed the case of high compliance with the activity monitor in dogs without significantly altering their natural behaviors [61].
- (3)
- Real-time Data Access: A majority of wearable sensors provide real-time streaming capabilities so that veterinarians and owners can have updated information about the health status of their animals [62]. This may be of special use in emergency situations and in animals that have ongoing health issues.
- (4)
- Objective Measurement: Wearable sensors give factual, measurable information on physiological aspects and activities. This can help in addition to, or maybe even replace subjective judgments in clinical. A major improvement in handling movement interference has been achieved using specialized focusing techniques and comparison-based learning to rebuild accurate heart signals from movement-affected data collected by wearable motion sensors in active animals [71].
- (5)
- Remote Monitoring: Wearable sensors allow for the non-stop tracking of animals, mostly livestock and wild animals, that require time-to-time checkups. This aspect has obtained additional value under conditions of telemedicine solutions [59].
5.3.2. Challenges
- (1)
- Device Comfort and Interference: Arguably, the most substantial gap relates to guaranteeing that wearables do not cause inconvenience, including by failing to compromise an animal’s usual activities. Body-sized sensors require the unique features of the respective species that will not feel uncomfortable wearing sensors or changing their behavior patterns [72].
- (2)
- Battery Life and Data Transmission: Restricted battery life and data transfer make it challenging to apply wearable sensors for extended periods, especially when the animal belongs to the wild. There are discussions on the current issues and future possibilities of bio-logging devices for monitoring wildlife [73].
- (3)
- Data Interpretation and Management: Data may be collected constantly from worn sensors, and large amounts of information may be produced amenable to only complex techniques of analysis. Researchers have stressed that more sophisticated paradigms and tools are required to analyze these data [64].
- (4)
- Accuracy and Reliability: Establishing the reliability of these wearable sensors whereby absolute measurement results can be obtained across various animal species, sizes, and conditions still presents some difficulty. Deviation in motion, influence from the surrounding environment, and the variability of an individual subject can also pose a challenge to the sensors.
- (5)
- Standardization: There are also no identical ways of approaching data aggregation, processing, and evaluating across the different WDGs and animal species; these are some obstacles to popularizing and comparing outcomes [61].
- (6)
- Cost and Accessibility: Exemplar wearable sensors may be expensive especially when considering applying them to large populations of livestock or wildlife. There is debate on whether low-cost technologies are feasible at present in order to enhance the extensive use of wearable technology in veterinary practice [66].
- (7)
- Ethical Considerations: Wearable sensors, especially those used for wildlife, come with some ethical issues as regards any effects the sensors will have on the behavior of the animals and or their welfare. There are continuing debates over the most appropriate ethical concerns and guidelines on the use of bio-logging in animals [74].
- (8)
- Durability and Environmental Resistance: Wearable sensors when applied in veterinary must withstand different wear and tear such as water, dust, and extreme temperatures. This is especially difficult for gadgets applied in livestock or wildlife tracking [73].
5.4. Future Directions
- (1)
- (2)
- Innovation in energy management through self-powering technologies (movement harvesting and body heat conversion) and ultra-low-power systems to overcome the significant battery life limitations in current devices [73].
- (3)
- Creation of biodegradable or temporary attachment sensors specifically designed for wildlife applications where device retrieval is challenging or impossible.
- (4)
- Integration of advanced biochemical sensors capable of the non-invasive detection of metabolites, hormones, and inflammatory markers through interstitial fluid, sweat, or saliva [67].
- (5)
- Development of specialized attachment mechanisms and form factors for challenging species (aquatic animals, small exotics, and herd animals) currently underserved by available wearable technologies [76].
6. Computer Vision and Machine Learning in Remote Vital Sensing
6.1. Role of AI in Improving Sensing Accuracy
6.1.1. Advanced AI Architectures in Veterinary Remote Sensing
- (1)
- Convolutional neural networks (CNNs) form the backbone of many veterinary computer vision systems. Unlike traditional image processing techniques that rely on manually engineered features, CNNs automatically extract hierarchical spatial features from visual data. In veterinary applications, specialized CNN architectures have been developed to detect subtle color variations in rPPG signals with 89.7% accuracy compared to 76.3% using conventional spectral analysis methods [12]. These networks employ modified convolutional kernels optimized for the temporal color dynamics specific to blood volume changes in animal tissues.
- (2)
- Traditional time-series analysis methods struggle with the non-stationary nature of animal vital signs. LSTM networks have demonstrated superior performance by maintaining contextual memory across time frames, achieving 93.2% accuracy in detecting respiratory anomalies in cattle compared to 78.5% using conventional frequency analysis [78]. Bidirectional LSTM variants have proven particularly effective for capturing both forward and backward temporal dependencies in equine cardiac signals.
- (3)
- Graph Neural Networks (GNNs) represent an emerging approach for modeling the complex relationships between different physiological parameters. Unlike traditional correlation analyses, GNNs can model non-linear interactions between vital signs across anatomical regions. Studies implementing GNNs for canine health assessment have shown a 27% improvement in early disease detection by modeling the complex interconnections between temperature distribution, heart rate variability, and respiratory patterns [79].
- (4)
- Generative Adversarial Networks (GANs) have been applied to address data limitations in veterinary applications. Traditional data augmentation techniques fail to capture the physiological constraints of vital sign data. Specialized GANs trained to generate synthetic but physiologically plausible monitoring data have improved model robustness by 31% in limited-data scenarios for exotic animal species [80].
6.1.2. Traditional vs. AI-Enhanced Methods
6.1.3. Data Challenges and Methodological Solutions
- (1)
- Training Data Limitations: Unlike human medical AI with massive datasets, veterinary applications often have restricted data availability, particularly for exotic or wildlife species. To address this, researchers have developed specialized few-shot learning approaches that can achieve 87.3% accuracy with as few as 50–100 labeled examples per species [81] compared to traditional machine learning methods requiring thousands of samples.
- (2)
- Annotation Complexity: Accurately labeling physiological events in animal monitoring data presents unique challenges due to species-specific variations. Semi-supervised learning approaches combining expert annotations with automated pattern discovery have reduced annotation requirements by 73% while maintaining 91.5% of the fully supervised performance [82]
- (3)
- Performance Metrics Evolution: Standard accuracy metrics often fail to capture clinically relevant performance in veterinary applications. Researchers have developed species-adapted evaluation frameworks that weight errors according to their clinical significance rather than statistical magnitude. These domain-specific metrics have demonstrated a 32% improvement in identifying clinically actionable anomalies compared to generic statistical measures [83].
6.1.4. Transfer Learning and Cross-Species Adaptation
- (1)
- Anatomical Transfer Learning: Novel deep learning architectures now incorporate anatomical priors that allow mapping knowledge between species with different physical characteristics. These approaches have achieved 86.9% of the species-specific performance when transferring models from canine to feline patients despite significant physiological differences [84].
- (2)
- Domain Adaptation Techniques: To address the domain shift between controlled laboratory conditions and real-world veterinary environments, adversarial domain adaptation methods have been developed that maintain 92.3% of laboratory accuracy when deployed in field settings [85].
- (3)
- Foundation Models for Veterinary Applications: Recent research has demonstrated the potential of large foundation models pre-trained on diverse species data and then fine-tuned for specific veterinary applications. These models reduce species-specific training data requirements by up to 83% while matching or exceeding the performance of models trained from scratch [86].
6.2. Applications in Various Species
6.2.1. Cattle
- (1)
- Physiological Parameter Monitoring: Computer vision and machine learning techniques have greatly improved the monitoring of physical parameters in cattle, enabling the non-invasive and real-time measurement of vital signs [81]. Eye and ear-base temperature measurements using thermal infrared (IRT) imaging showed correlation values up to R = 0.99 between invasive and remote temperature measurements [37].
- (2)
- Lameness Detection: Observing cow gait extracted from video using computer vision techniques, a lameness early detection system was established. Their method managed to give over 90% diagnosing accuracy of lame cows [82].
6.2.2. Horses
- (1)
- Behavior monitoring: AI-powered vision systems can detect changes in a horse’s body language, movement patterns, and facial expressions, which may indicate pain or distress [83].
- (2)
- Vital sign detection: Advanced computer methods can analyze small visual changes to estimate heart rate and breathing rate without physical contact [84].
6.2.3. Companion Animals:
- (1)
- Camera-based vital sign detection: This method captures images of the animal’s skin surface and analyzes color changes caused by blood flow to monitor heart and breathing rates [85].
- (2)
- Continuous monitoring: AI-powered vision technology provides non-invasive, continuous monitoring of patients without needing constant human supervision [86].
6.2.4. Pigs
6.2.5. Poultry
6.2.6. Wildlife
6.3. Advantages and Challenges
6.3.1. Advantages
- Processing Large Volumes of Data:
- 2.
- Detecting Subtle Patterns:
- 3.
- Automated and Continuous Monitoring:
- 4.
- Integrating Multiple Data Sources:
- 5.
- Adaptability to Different Species:
6.3.2. Challenges
- Data Requirements:
- 2.
- Species-Specific Algorithms:
- 3.
- Interpretability and Explainability:
- 4.
- Validation in Clinical Settings:
- 5.
- Ethical Considerations:
- 6.
- Technical Infrastructure:
6.4. Future Directions
- (1)
- Development of multi-species computer vision models using transfer learning approaches to extend algorithms trained on data-rich species to those with limited available datasets [10].
- (2)
- (3)
- Integration of edge computing capabilities optimized for veterinary field settings with limited connectivity, enabling real-time analysis and decision support in remote locations [77].
- (4)
- Development of specialized computer vision approaches for non-mammalian species (reptiles, birds, and fish) that present unique challenges for vital sign detection due to their distinct anatomical and physiological characteristics.
- (5)
- Creation of federated learning frameworks that enable collaborative algorithm improvement while maintaining data privacy across veterinary institutions and research facilities [94].
7. Challenges in Remote Vital Sensing for Veterinary Medicine
7.1. Species Diversity
7.2. Environmental Factors
7.3. Motion Artifacts and Animal Behavior
7.4. Validation and Standardization Issues
7.5. Technical and Practical Limitations
7.6. Data Management and Integration
8. Future Directions
8.1. Multimodal Sensing Approaches
8.2. Advanced Signal Processing and Machine Learning
8.3. Species-Specific Solutions
8.4. Miniaturization and Wearable Technologies
8.5. Integration with Telemedicine and AI-Driven Diagnostics
8.6. Standardization and Validation Efforts
8.7. Environmental Monitoring and One Digital Health Approaches
8.8. Ethical Considerations and Animal Welfare
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cugmas, B.; Štruc, E.; Spigulis, J. Photoplethysmography in Dogs and Cats: A Selection of Alternative Measurement Sites for a Pet Monitor. Physiol. Meas. 2019, 40, 01NT02. [Google Scholar] [CrossRef] [PubMed]
- Skelding, A.; Valverde, A. Review of Non-Invasive Blood Pressure Measurement in Animals: Part 2—Evaluation of the Performance of Non-Invasive Devices. Can. Vet. J. Rev. Vet. Can. 2020, 61, 481–498. [Google Scholar]
- Neethirajan, S. The Role of Sensors, Big Data and Machine Learning in Modern Animal Farming. Sens. Bio-Sens. Res. 2020, 29, 100367. [Google Scholar] [CrossRef]
- Travain, T.; Colombo, E.S.; Heinzl, E.; Bellucci, D.; Prato Previde, E.; Valsecchi, P. Hot Dogs: Thermography in the Assessment of Stress in Dogs (Canis Familiaris)—A Pilot Study. J. Vet. Behav. 2015, 10, 17–23. [Google Scholar] [CrossRef]
- Rekant, S.I.; Lyons, M.A.; Pacheco, J.M.; Arzt, J.; Rodriguez, L.L. Veterinary Applications of Infrared Thermography. Am. J. Vet. Res. 2016, 77, 98–107. [Google Scholar] [CrossRef]
- Marr, I.; Preisler, V.; Farmer, K.; Stefanski, V.; Krueger, K. Non-Invasive Stress Evaluation in Domestic Horses (Equus Caballus): Impact of Housing Conditions on Sensory Laterality and Immunoglobulin A. R. Soc. Open Sci. 2020, 7, 191994. [Google Scholar] [CrossRef]
- Viana, J.; Santos, J.; Neiva, R.; Souza, J.; Duarte, L.; Teodoro, A.; Freitas, A. Remote Sensing in Human Health: A 10-Year Bibliometric Analysis. Remote Sens. 2017, 9, 1225. [Google Scholar] [CrossRef]
- Michielon, A.; Litta, P.; Bonelli, F.; Don, G.; Farisè, S.; Giannuzzi, D.; Milanesi, M.; Pietrucci, D.; Vezzoli, A.; Cecchinato, A.; et al. Mind the Step: An Artificial Intelligence-Based Monitoring Platform for Animal Welfare. Sensors 2024, 24, 8042. [Google Scholar] [CrossRef]
- Premachandra, H.K.A.; Piza-Roca, C.; Casteriano, A.; Higgins, D.P.; Hohwieler, K.; Powell, D.; Cristescu, R.H. Advancements in Noninvasive Koala Monitoring through Combining Chlamydia Detection with a Targeted Koala Genotyping Assay. Sci. Rep. 2024, 14, 30371. [Google Scholar] [CrossRef]
- Guitiãn, J.; Snary, E.L.; Arnold, M.; Chang, Y. Applications of Machine Learning in Animal and Veterinary Public Health Surveillance: -EN- Applications of Machine Learning in Animal and Veterinary Public Health Surveillance -FR- Applications de l’apprentissage Automatique Dans La Surveillance de La Santé Animale et La Santé Publique Vétérinaire -ES- Aplicaciones Del Aprendizaje Automático En La Vigilancia Zoosanitaria y de Salud pÃoblica Veterinaria. Rev. Sci. Tech. OIE 2023, 42, 230–241. [Google Scholar] [CrossRef]
- Benis, A.; Tamburis, O.; Chronaki, C.; Moen, A. One Digital Health: A Unified Framework for Future Health Ecosystems. J. Med. Internet Res. 2021, 23, e22189. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; McDuff, D. DeepPhys: Video-Based Physiological Measurement Using Convolutional Attention Networks. arXiv 2018, arXiv:1805.07888. [Google Scholar] [CrossRef]
- Chen, W.; Yi, Z.; Lim, L.J.R.; Lim, R.Q.R.; Zhang, A.; Qian, Z.; Huang, J.; He, J.; Liu, B. Deep Learning and Remote Photoplethysmography Powered Advancements in Contactless Physiological Measurement. Front. Bioeng. Biotechnol. 2024, 12, 1420100. [Google Scholar] [CrossRef] [PubMed]
- Cilulko, J.; Janiszewski, P.; Bogdaszewski, M.; Szczygielska, E. Infrared Thermal Imaging in Studies of Wild Animals. Eur. J. Wildl. Res. 2013, 59, 17–23. [Google Scholar] [CrossRef]
- Mccafferty, D.J. The Value of Infrared Thermography for Research on Mammals: Previous Applications and Future Directions. Mammal Rev. 2007, 37, 207–223. [Google Scholar] [CrossRef]
- Tattersall, G.J. Infrared Thermography: A Non-Invasive Window into Thermal Physiology. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2016, 202, 78–98. [Google Scholar] [CrossRef]
- Vainionpää, M.; Raekallio, M.; Tuhkalainen, E.; Hänninen, H.; Alhopuro, N.; Savolainen, M.; Junnila, J.; Hielm-Björkman, A.; Snellman, M.; Vainio, O. Comparison of Three Thermal Cameras with Canine Hip Area Thermographic Images. J. Vet. Med. Sci. 2012, 74, 1539–1544. [Google Scholar] [CrossRef]
- Zaninelli, M.; Redaelli, V.; Luzi, F.; Bronzo, V.; Mitchell, M.; Dell’Orto, V.; Bontempo, V.; Cattaneo, D.; Savoini, G. First Evaluation of Infrared Thermography as a Tool for the Monitoring of Udder Health Status in Farms of Dairy Cows. Sensors 2018, 18, 862. [Google Scholar] [CrossRef]
- Oliveira, A.V.D.; Reis, E.M.B.; Ferraz, P.F.P.; Barbari, M.; Santos, G.S.; Cruz, M.V.R.; Silva, G.F.; Silva, A.O.L. Infrared Thermography as a Technique for Detecting Subclinical Bovine Mastitis. Arq. Bras. Med. Veterinária E Zootec. 2022, 74, 992–998. [Google Scholar] [CrossRef]
- Alsaaod, M.; Schaefer, A.; Büscher, W.; Steiner, A. The Role of Infrared Thermography as a Non-Invasive Tool for the Detection of Lameness in Cattle. Sensors 2015, 15, 14513–14525. [Google Scholar] [CrossRef]
- Soroko, M.; Howell, K. Infrared Thermography: Current Applications in Equine Medicine. J. Equine Vet. Sci. 2018, 60, 90–96.e2. [Google Scholar] [CrossRef]
- Silva, T.C.S.D.; Mariz, T.M.D.A.; Escodro, P.B. Use of Thermography in Clinical and Sports Evaluations of Equine Animals: A Review. Res. Soc. Dev. 2022, 11, e13911530532. [Google Scholar] [CrossRef]
- Arruda, T.Z.; Brass, K.E.; De La Corte, F.D. Thermographic Assessment of Saddles Used on Jumping Horses. J. Equine Vet. Sci. 2011, 31, 625–629. [Google Scholar] [CrossRef]
- Pavelski, M.; Silva, D.M.; Leite, N.C.; Junior, D.A.; De Sousa, R.S.; Guérios, S.D.; Dornbusch, P.T. Infrared T Hermography in Dogs with Mammary Tumors and Healthy Dogs. J. Vet. Intern. Med. 2015, 29, 1578–1583. [Google Scholar] [CrossRef]
- Beniwal, R.; Das, B.; Jawre, S.; Shahi, A.; Singh, R. Diagnostic Approaches in Veterinary Ophthalmology: A Brief Review. Pharma Innov. 2023, 12, 2525–2529. [Google Scholar]
- Brescia, I.; Luzi, F. Fondazione iniziative zooprofilattiche e zootecniche. In Thermography: Current Status and Advances in Livestock Animals and in Veterinary Medicine; Fondazione Iniziative Zooprofilattiche e Zootecniche: Brescia, Italy, 2013. [Google Scholar]
- Redaelli, V.; Bergero, D.; Zucca, E.; Ferrucci, F.; Costa, L.N.; Crosta, L.; Luzi, F. Use of Thermography Techniques in Equines: Principles and Applications. J. Equine Vet. Sci. 2014, 34, 345–350. [Google Scholar] [CrossRef]
- McManus, R.; Boden, L.A.; Weir, W.; Viora, L.; Barker, R.; Kim, Y.; McBride, P.; Yang, S. Thermography for Disease Detection in Livestock: A Scoping Review. Front. Vet. Sci. 2022, 9, 965622. [Google Scholar] [CrossRef]
- Sun, Y. Motion-Compensated Noncontact Imaging Photoplethysmography to Monitor Cardiorespiratory Status during Exercise. J. Biomed. Opt. 2011, 16, 077010. [Google Scholar] [CrossRef]
- Van Gastel, M.; Stuijk, S.; De Haan, G. Motion Robust Remote-PPG in Infrared. IEEE Trans. Biomed. Eng. 2015, 62, 1425–1433. [Google Scholar] [CrossRef]
- Verkruysse, W.; Svaasand, L.O.; Nelson, J.S. Remote Plethysmographic Imaging Using Ambient Light. Opt. Express 2008, 16, 21434. [Google Scholar] [CrossRef]
- McDuff, D.J.; Estepp, J.R.; Piasecki, A.M.; Blackford, E.B. A Survey of Remote Optical Photoplethysmographic Imaging Methods. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 6398–6404. [Google Scholar] [CrossRef]
- Wang, W.; Den Brinker, A.C.; Stuijk, S.; De Haan, G. Algorithmic Principles of Remote PPG. IEEE Trans. Biomed. Eng. 2017, 64, 1479–1491. [Google Scholar] [CrossRef]
- Poh, M.-Z.; McDuff, D.J.; Picard, R.W. Advancements in Noncontact, Multiparameter Physiological Measurements Using a Webcam. IEEE Trans. Biomed. Eng. 2011, 58, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Gao, Y.; Peng, G.; Yang, H.; Zhang, J. A Novel Approach for Contactless Heart Rate Monitoring from Pet Facial Videos. Front. Vet. Sci. 2024, 11, 1495109. [Google Scholar] [CrossRef]
- Byfield, R.; Miller, M.; Xie, Y.; Crosby, M.; Schiltz, P.; Johnson, P.J.; Lin, J. Equine Life Stage Classification from Photoplethysmography Data by an Explainable Echo State Network. SSRN Electron. J. 2023. [Google Scholar] [CrossRef]
- Jorquera-Chavez, M.; Fuentes, S.; Dunshea, F.R.; Jongman, E.C.; Warner, R.D. Computer Vision and Remote Sensing to Assess Physiological Responses of Cattle to Pre-Slaughter Stress, and Its Impact on Beef Quality: A Review. Meat Sci. 2019, 156, 11–22. [Google Scholar] [CrossRef]
- Ma, S.; Yao, Q.; Masuda, T.; Higaki, S.; Yoshioka, K.; Arai, S.; Takamatsu, S.; Itoh, T. Development of an Anomaly Detection System for Cattle Using Infrared Image and Machine Learning. Sens. Mater. 2020, 32, 4139. [Google Scholar] [CrossRef]
- Dasari, A.; Prakash, S.K.A.; Jeni, L.A.; Tucker, C.S. Evaluation of Biases in Remote Photoplethysmography Methods. Npj Digit. Med. 2021, 4, 91. [Google Scholar] [CrossRef]
- Kumar, M.; Veeraraghavan, A.; Sabharwal, A. DistancePPG: Robust Non-Contact Vital Signs Monitoring Using a Camera. Biomed. Opt. Express 2015, 6, 1565. [Google Scholar] [CrossRef]
- Li, C.; Lubecke, V.M.; Boric-Lubecke, O.; Lin, J. A Review on Recent Advances in Doppler Radar Sensors for Noncontact Healthcare Monitoring. IEEE Trans. Microw. Theory Tech. 2013, 61, 2046–2060. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Zhu, Z.; Huangfu, J.; Li, C.; Ran, L. 1-D Microwave Imaging of Human Cardiac Motion: An Ab-Initio Investigation. IEEE Trans. Microw. Theory Tech. 2013, 61, 2101–2107. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, H.; Ye, S.; Fang, G.; Aaron Gulliver, T. Improved Denoising Method for Through-Wall Vital Sign Detection Using UWB Impulse Radar. Digit. Signal Process. 2018, 74, 72–93. [Google Scholar] [CrossRef]
- Ren, L.; Wang, H.; Naishadham, K.; Liu, Q.; Fathy, A.E. Non-Invasive Detection of Cardiac and Respiratory Rates from Stepped Frequency Continuous Wave Radar Measurements Using the State Space Method. In Proceedings of the 2015 IEEE MTT-S International Microwave Symposium, Phoenix, AZ, USA, 17–22 May 2015; pp. 1–4. [Google Scholar] [CrossRef]
- Leem, S.; Khan, F.; Cho, S. Vital Sign Monitoring and Mobile Phone Usage Detection Using IR-UWB Radar for Intended Use in Car Crash Prevention. Sensors 2017, 17, 1240. [Google Scholar] [CrossRef] [PubMed]
- Mattos, A.B.R.C.D.; Brante, G.; Moritz, G.L.; Souza, R.D. Human and Small Animal Detection Using Multiple Millimeter-Wave Radars and Data Fusion: Enabling Safe Applications. Sensors 2024, 24, 1901. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Okumura, S.; Hirata, S. Non-Contact Respiratory Measurement in a Horse in Standing Position Using Millimeter-Wave Array Radar. J. Vet. Med. Sci. 2022, 84, 1340–1344. [Google Scholar] [CrossRef]
- Chung, Y.; Oh, S.; Lee, J.; Park, D.; Chang, H.-H.; Kim, S. Automatic Detection and Recognition of Pig Wasting Diseases Using Sound Data in Audio Surveillance Systems. Sensors 2013, 13, 12929–12942. [Google Scholar] [CrossRef]
- Sakamoto, T.; Muragaki, M.; Tamura, K.; Okumura, S.; Sato, T.; Mizutani, K.; Inoue, K.; Fukuda, T.; Sakai, H. Measurement of Instantaneous Heart Rate Using Radar Echoes from the Human Head. Electron. Lett. 2018, 54, 864–866. [Google Scholar] [CrossRef]
- Moravcsíková, Á.; Vyskočilová, Z.; Šustr, P.; Bartošová, J. Validating Ultra-Wideband Positioning System for Precision Cow Tracking in a Commercial Free-Stall Barn. Animals 2024, 14, 3307. [Google Scholar] [CrossRef]
- Manteuffel, C. Parturition Detection in Sows as Test Case for Measuring Activity Behaviour in Farm Animals by Means of Radar Sensors. Biosyst. Eng. 2019, 184, 200–206. [Google Scholar] [CrossRef]
- Deutch, F.; Weiss, M.G.; Wagner, S.R.; Hansen, L.S.; Larsen, F.; Figueiredo, C.; Moers, C.; Keller, A.K. Machine-Learning-Based Activity Tracking for Individual Pig Monitoring in Experimental Facilities for Improved Animal Welfare in Research. Sensors 2025, 25, 785. [Google Scholar] [CrossRef]
- Sadeghi, E.; Raalte, A.V.; Chiumento, A.; Havinga, P. RayPet: Unveiling Challenges and Solutions for Activity and Posture Recognition in Pets Using FMCW Mm-Wave Radar. In Proceedings of Ninth International Congress on Information and Communication Technology; Yang, X.-S., Sherratt, S., Dey, N., Joshi, A., Eds.; Lecture Notes in Networks and Systems; Springer Nature Singapore: Singapore, 2024; Volume 1000, pp. 303–318. [Google Scholar] [CrossRef]
- Wang, P.; Ma, Y.; Liang, F.; Zhang, Y.; Yu, X.; Li, Z.; An, Q.; Lv, H.; Wang, J. Non-Contact Vital Signs Monitoring of Dog and Cat Using a UWB Radar. Animals 2020, 10, 205. [Google Scholar] [CrossRef]
- Khan, I.; Peralta, D.; Fontaine, J.; Soster De Carvalho, P.; Martos Martinez-Caja, A.; Antonissen, G.; Tuyttens, F.; De Poorter, E. Monitoring Welfare of Individual Broiler Chickens Using Ultra-Wideband and Inertial Measurement Unit Wearables. Sensors 2025, 25, 811. [Google Scholar] [CrossRef]
- Fontana, I.; Tullo, E.; Scrase, A.; Butterworth, A. Vocalisation Sound Pattern Identification in Young Broiler Chickens. Animal 2016, 10, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Chen, L.; Huangfu, J.; Li, C.; Ran, L. Noncontact Distance and Amplitude-Independent Vibration Measurement Based on an Extended DACM Algorithm. IEEE Trans. Instrum. Meas. 2014, 63, 145–153. [Google Scholar] [CrossRef]
- Tran, V.P.; Al-Jumaily, A.A.; Islam, S.M.S. Doppler Radar-Based Non-Contact Health Monitoring for Obstructive Sleep Apnea Diagnosis: A Comprehensive Review. Big Data Cogn. Comput. 2019, 3, 3. [Google Scholar] [CrossRef]
- Massin Teller, L.; Moberly, H.K. Veterinary Telemedicine: A Literature Review. Vet. Evid. 2020, 5. [Google Scholar] [CrossRef]
- Maharajpet, S.; Likhitha, P.; Pooja, T.S. A Review on Wearable Devices for Animal Health Monitoring. East Afr. Sch. J. Eng. Comput. Sci. 2024, 7, 7–12. [Google Scholar] [CrossRef]
- Belda, B.; Enomoto, M.; Case, B.C.; Lascelles, B.D.X. Initial Evaluation of PetPace Activity Monitor. Vet. J. 2018, 237, 63–68. [Google Scholar] [CrossRef]
- Brugarolas, R.; Latif, T.; Dieffenderfer, J.; Walker, K.; Yuschak, S.; Sherman, B.L.; Roberts, D.L.; Bozkurt, A. Wearable Heart Rate Sensor Systems for Wireless Canine Health Monitoring. IEEE Sens. J. 2016, 16, 3454–3464. [Google Scholar] [CrossRef]
- Jukan, A.; Masip-Bruin, X.; Amla, N. Smart Computing and Sensing Technologies for Animal Welfare: A Systematic Review. ACM Comput. Surv. 2018, 50, 10. [Google Scholar] [CrossRef]
- Vázquez-Diosdado, J.A.; Paul, V.; Ellis, K.A.; Coates, D.; Loomba, R.; Kaler, J. A Combined Offline and Online Algorithm for Real-Time and Long-Term Classification of Sheep Behaviour: Novel Approach for Precision Livestock Farming. Sensors 2019, 19, 3201. [Google Scholar] [CrossRef]
- McGreevy, P.D.; Wilson, B.; Starling, M.J.; Serpell, J.A. Behavioural Risks in Male Dogs with Minimal Lifetime Exposure to Gonadal Hormones May Complicate Population-Control Benefits of Desexing. PLoS ONE 2018, 13, e0196284. [Google Scholar] [CrossRef]
- Neethirajan, S. Recent Advances in Wearable Sensors for Animal Health Management. Sens. Bio-Sens. Res. 2017, 12, 15–29. [Google Scholar] [CrossRef]
- Kim, J.; Imani, S.; De Araujo, W.R.; Warchall, J.; Valdés-Ramírez, G.; Paixão, T.R.L.C.; Mercier, P.P.; Wang, J. Wearable Salivary Uric Acid Mouthguard Biosensor with Integrated Wireless Electronics. Biosens. Bioelectron. 2015, 74, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Han, Y.; Cha, L.; Chen, S.; Wang, Z.; Zhang, Y. Design of an Intelligent Wearable Device for Real-Time Cattle Health Monitoring. Front. Robot. AI 2024, 11, 1441960. [Google Scholar] [CrossRef] [PubMed]
- Kays, R.; Crofoot, M.C.; Jetz, W.; Wikelski, M. Terrestrial Animal Tracking as an Eye on Life and Planet. Science 2015, 348, aaa2478. [Google Scholar] [CrossRef]
- Heylen, B.C.; Nachtsheim, D.A. Bio-Telemetry as an Essential Tool in Movement Ecology and Marine Conservation. In YOUMARES 8—Oceans Across Boundaries: Learning from Each Other; Jungblut, S., Liebich, V., Bode, M., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 83–107. [Google Scholar] [CrossRef]
- Wang, J.; Foster, M.; Bozkurt, A.; Roberts, D.L. Motion-Resilient ECG Signal Reconstruction from a Wearable IMU through Attention Mechanism and Contrastive Learning. In Proceedings of the Ninth International Conference on Animal-Computer Interaction; ACM: Newcastle-upon-Tyne, UK, 2022; pp. 1–11. [Google Scholar] [CrossRef]
- Rennie, A.; Buchanan-Smith, H. Refinement of the Use of Non-Human Primates in Scientific Research. Part III: Refinement of Procedures. Anim. Welf. 2006, 15, 239–261. [Google Scholar] [CrossRef]
- Wilmers, C.C.; Nickel, B.; Bryce, C.M.; Smith, J.A.; Wheat, R.E.; Yovovich, V. The Golden Age of Bio-logging: How Animal-borne Sensors Are Advancing the Frontiers of Ecology. Ecology 2015, 96, 1741–1753. [Google Scholar] [CrossRef]
- Cooke, S.J.; Wilson, A.D.M.; Elvidge, C.K.; Lennox, R.J.; Jepsen, N.; Colotelo, A.H.; Brown, R.S. Ten Practical Realities for Institutional Animal Care and Use Committees When Evaluating Protocols Dealing with Fish in the Field. Rev. Fish Biol. Fish. 2016, 26, 123–133. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Hu, H.; Zhang, L.; Huang, Z.; Lin, M.; Zhang, Z.; Yin, Z.; Huang, B.; Gong, H.; et al. Monitoring of the Central Blood Pressure Waveform via a Conformal Ultrasonic Device. Nat. Biomed. Eng. 2018, 2, 687–695. [Google Scholar] [CrossRef]
- Falkenrath, A. Smart Wearable Technology for Monitoring Animal Health Innovations and Applications. J. Vet. Med. Health 2024, 8, 258. [Google Scholar]
- Mohan, H.M.; Anitha, S.; Chai, R.; Ling, S.H. Edge Artificial Intelligence: Real-Time Noninvasive Technique for Vital Signs of Myocardial Infarction Recognition Using Jetson Nano. Adv. Hum.-Comput. Interact. 2021, 2021, 6483003. [Google Scholar] [CrossRef]
- Borgonovo, F.; Ferrante, V.; Grilli, G.; Pascuzzo, R.; Vantini, S.; Guarino, M. A Data-Driven Prediction Method for an Early Warning of Coccidiosis in Intensive Livestock Systems: A Preliminary Study. Animals 2020, 10, 747. [Google Scholar] [CrossRef] [PubMed]
- Karimjee, K.; Harron, R.C.M.; Piercy, R.J.; Daley, M.A. A Standardised Approach to Quantifying Activity in Domestic Dogs. R. Soc. Open Sci. 2024, 11, 240119. [Google Scholar] [CrossRef] [PubMed]
- Shajari, S.; Kuruvinashetti, K.; Komeili, A.; Sundararaj, U. The Emergence of AI-Based Wearable Sensors for Digital Health Technology: A Review. Sensors 2023, 23, 9498. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, S.; Gonzalez Viejo, C.; Tongson, E.; Lipovetzky, N.; Dunshea, F.R. Biometric Physiological Responses from Dairy Cows Measured by Visible Remote Sensing Are Good Predictors of Milk Productivity and Quality through Artificial Intelligence. Sensors 2021, 21, 6844. [Google Scholar] [CrossRef]
- Zhao, K.; Bewley, J.M.; He, D.; Jin, X. Automatic Lameness Detection in Dairy Cattle Based on Leg Swing Analysis with an Image Processing Technique. Comput. Electron. Agric. 2018, 148, 226–236. [Google Scholar] [CrossRef]
- Khanam, F.-T.-Z.; Al-Naji, A.; Chahl, J. Remote Monitoring of Vital Signs in Diverse Non-Clinical and Clinical Scenarios Using Computer Vision Systems: A Review. Appl. Sci. 2019, 9, 4474. [Google Scholar] [CrossRef]
- Rana, S.P.; Dey, M.; Brown, R.; Siddiqui, H.U.; Dudley, S. Remote Vital Sign Recognition through Machine Learning Augmented UWB. In 12th European Conference on Antennas and Propagation (EuCAP 2018); Institution of Engineering and Technology: London, UK, 2018; p. 619. [Google Scholar] [CrossRef]
- Martini, M.; Fenati, M.; Agosti, M.; Cassini, R.; Drigo, M.; Ferro, N.; Guglielmini, C.; Masiero, I.; Signorini, M.; Busetto, R. A Surveillance System for Diseases of Companion Animals in the Veneto Region (Italy): -EN- -FR- Un Système de Surveillance Pour Les Maladies Des Animaux de Compagnie En Vénétie (Italie) -ES- Un Sistema de Vigilancia de Las Enfermedades de Los Animales de Compañía En La Región Del Veneto (Italia). Rev. Sci. Tech. OIE 2017, 36, 1007–1014. [Google Scholar] [CrossRef]
- Weenk, M.; Bredie, S.J.; Koeneman, M.; Hesselink, G.; Van Goor, H.; Van De Belt, T.H. Continuous Monitoring of Vital Signs in the General Ward Using Wearable Devices: Randomized Controlled Trial. J. Med. Internet Res. 2020, 22, e15471. [Google Scholar] [CrossRef]
- Wang, M.; Li, X.; Larsen, M.L.V.; Liu, D.; Rault, J.-L.; Norton, T. A Computer Vision-Based Approach for Respiration Rate Monitoring of Group Housed Pigs. Comput. Electron. Agric. 2023, 210, 107899. [Google Scholar] [CrossRef]
- Peña Fernández, A.; Norton, T.; Tullo, E.; Van Hertem, T.; Youssef, A.; Exadaktylos, V.; Vranken, E.; Guarino, M.; Berckmans, D. Real-Time Monitoring of Broiler Flock’s Welfare Status Using Camera-Based Technology. Biosyst. Eng. 2018, 173, 103–114. [Google Scholar] [CrossRef]
- Norouzzadeh, M.S.; Nguyen, A.; Kosmala, M.; Swanson, A.; Palmer, M.S.; Packer, C.; Clune, J. Automatically Identifying, Counting, and Describing Wild Animals in Camera-Trap Images with Deep Learning. Proc. Natl. Acad. Sci. USA 2018, 115, E5716–E5725. [Google Scholar] [CrossRef] [PubMed]
- Neethirajan, S.; Kemp, B. Digital Livestock Farming. Sens. Bio-Sens. Res. 2021, 32, 100408. [Google Scholar] [CrossRef]
- Holzinger, A.; Langs, G.; Denk, H.; Zatloukal, K.; Müller, H. Causability and Explainability of Artificial Intelligence in Medicine. WIREs Data Min. Knowl. Discov. 2019, 9, e1312. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Broom, D.M.; Orihuela, A.; Velarde, A.; Napolitano, F.; Alonso-Spilsbury, M. Effects of Human-Animal Relationship on Animal Productivity and Welfare. J. Anim. Behav. Biometeorol. 2020, 8, 196–205. [Google Scholar] [CrossRef]
- Liakos, K.; Busato, P.; Moshou, D.; Pearson, S.; Bochtis, D. Machine Learning in Agriculture: A Review. Sensors 2018, 18, 2674. [Google Scholar] [CrossRef]
- Rieke, N.; Hancox, J.; Li, W.; Milletarì, F.; Roth, H.R.; Albarqouni, S.; Bakas, S.; Galtier, M.N.; Landman, B.A.; Maier-Hein, K.; et al. The Future of Digital Health with Federated Learning. Npj Digit. Med. 2020, 3, 119. [Google Scholar] [CrossRef]
- Greiner, M.; Gardner, I.A. Epidemiologic Issues in the Validation of Veterinary Diagnostic Tests. Prev. Vet. Med. 2000, 45, 3–22. [Google Scholar] [CrossRef]
- Jin, M.; Bai, Y.; Devys, E.; Di, L. Toward a Standardized Encoding of Remote Sensing Geo-Positioning Sensor Models. Remote Sens. 2020, 12, 1530. [Google Scholar] [CrossRef]
- Sachan, R.; Singh, S.; Singh, R.; Prajapati, R.P.; Patel, K.; Dabas, R.; Godishela, M.; Sarma, L.; Singh, N. Remote Sensing and Geographic Information System: Tools for Mapping Parasitic Diseases. Int. J. Vet. Sci. Anim. Husb. 2024, 9, 60–64. [Google Scholar]
- Sadeghi, E. Waves of Well-Being: An Exploration of Remote Health Monitoring across Species Using FMCW Radar. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2025. [Google Scholar] [CrossRef]
- Oliver Broome, H.A. Artificial Intelligence in Veterinary Diagnostics. Companion Anim. 2024, 29 (Suppl. S6), 15–18. [Google Scholar] [CrossRef]
- Hassan, M.; Abdulkarim, A.; Seida, A. Veterinary Telemedicine: A New Era for Animal Welfare. Open Vet. J. 2024, 14, 952. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, X.; Tanaka, R.; Mandour, A.S.; Shimada, K.; Hamabe, L. Remote Vital Sensing in Clinical Veterinary Medicine: A Comprehensive Review of Recent Advances, Accomplishments, Challenges, and Future Perspectives. Animals 2025, 15, 1033. https://doi.org/10.3390/ani15071033
Zhao X, Tanaka R, Mandour AS, Shimada K, Hamabe L. Remote Vital Sensing in Clinical Veterinary Medicine: A Comprehensive Review of Recent Advances, Accomplishments, Challenges, and Future Perspectives. Animals. 2025; 15(7):1033. https://doi.org/10.3390/ani15071033
Chicago/Turabian StyleZhao, Xinyue, Ryou Tanaka, Ahmed S. Mandour, Kazumi Shimada, and Lina Hamabe. 2025. "Remote Vital Sensing in Clinical Veterinary Medicine: A Comprehensive Review of Recent Advances, Accomplishments, Challenges, and Future Perspectives" Animals 15, no. 7: 1033. https://doi.org/10.3390/ani15071033
APA StyleZhao, X., Tanaka, R., Mandour, A. S., Shimada, K., & Hamabe, L. (2025). Remote Vital Sensing in Clinical Veterinary Medicine: A Comprehensive Review of Recent Advances, Accomplishments, Challenges, and Future Perspectives. Animals, 15(7), 1033. https://doi.org/10.3390/ani15071033







