A Comprehensive Overview of Respiratory Compliance in Dogs Under General Anesthesia: Clinical Factors and Future Perspectives
Simple Summary
Abstract
1. Introduction
2. Purpose
3. Methods
4. Breed and Size
| Mean Weight (kg) | Weight Range (kg) | Respiratory Compliance Range (ml/cm H2O) | Mean Respiratory-Compliance-to-Bodyweight Ratio (CT/BW) | Measurement | Reference | |
|---|---|---|---|---|---|---|
| Non-specific breeds | 1.6–50.0 | 25.38 ± 20 * | Ventilator (Calculated via P-V loops) | [16] | ||
| 11.8–26.4 | 117 | [19] | ||||
| 26.8 | 1.9–45.0 | 32.831 * | [21] | |||
| 19.8 | 2.22 | [18] | ||||
| Specific Breeds | ||||||
| Border collie | 19.77 | 83.3 ± 5 | 3.31 | Ventilator (Calculated via P-V loops) | [18] | |
| German shepherd | 31.0 | 121.0 ± 14.4 | 2.94 | |||
| Labrador retriever | 27.5 | 77.6 ± 3.2 | 1.93 | |||
| Rottweiler | 42.0 | 81.3 ± 7.3 | 1.33 | |||
5. Recumbency
6. Diaphragm Movement
7. Underlying Disorders
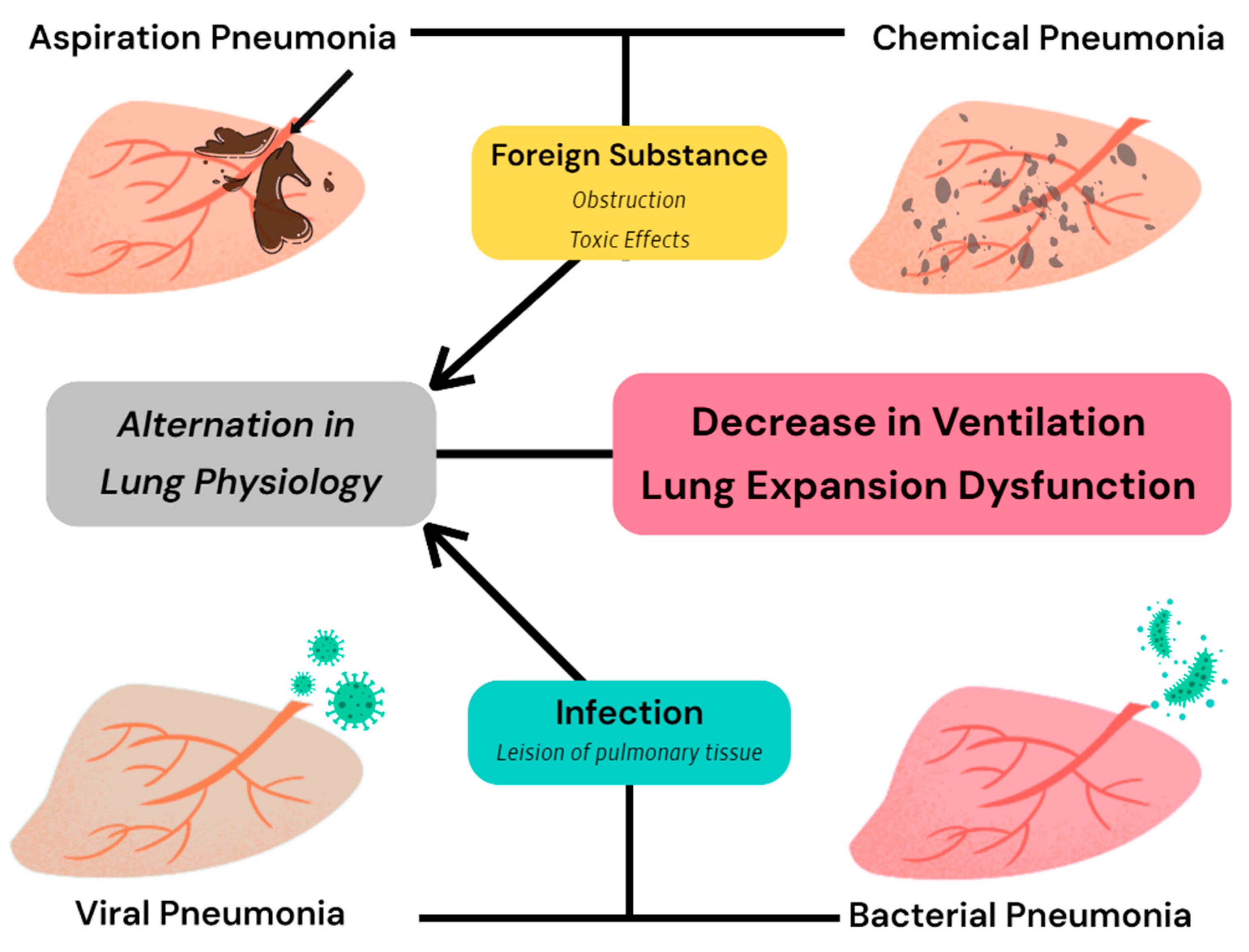
7.1. Restrictive Ventilation Disorder
7.2. Obstructive Ventilation Disorder
| Mechanism | Effect on Respiratory Compliance | Reference | |
|---|---|---|---|
| Restrictive ventilation disorder | |||
| Pneumonia | Lung expansion inhibition Chest volume restriction Surfactant reduction | Reduce | [33,34,35] |
| Pulmonary edema | [36,37,38] | ||
| Pleural effusion | [39] | ||
| Thoracic anomalies | [40,41,42] | ||
| Obstructive ventilation disorder | |||
| Tracheal collapse | Reduced tracheal diameter Serious bronchi obstruction Decreased ventilation Increased airway resistance | Reduce | [43,44,45,46,47] |
| COPD * | [48,49,50] | ||
| Emphysema | [51,52] | ||
| Asthma | [54] | ||
| BAS ** | [55,56,57,58] | ||
| Abdominal disease | |||
| Abdominal organs ectopic | Chest volume restriction Restricted movement of the diaphragm | Possibly reduce | [59] |
| Ascites | [60,61] | ||
7.3. Abdominal Disease
7.4. Obesity
8. Pharmacological Influences
| Specific Medicines | Mechanism | Potential Effect on Respiratory Compliance | Reference | |
|---|---|---|---|---|
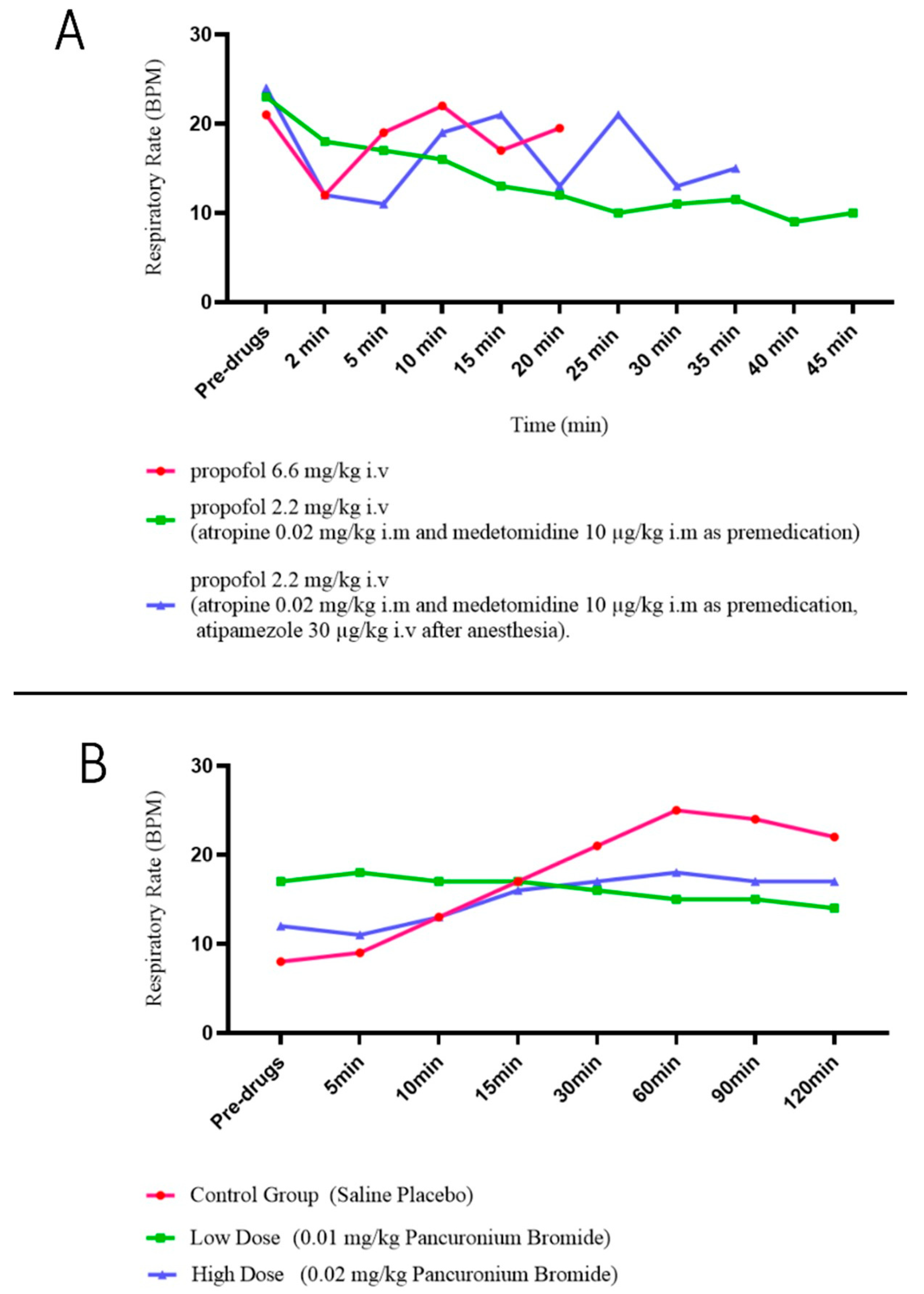
| Anesthetics | Propofol | Respiratory inhibition | Reduce | [65,66] |
| Desflurane | Reduce respiratory resistance | Enhance or reduce (Dependent on MAC) | [68] | |
| Analgesics | Morphine | Respiratory inhibition | [70,71,72] | |
| Sufentanil | [74] | |||
| Remifentanil | [75] | |||
| Muscle relaxants | Pancuronium | Respiratory muscle depression | [77,78] | |
| Bronchodilators | Salbutamol | Dilatation of trachea Increase ventilation Inotropic effects on diaphragms | [80,81,82] | |
| Aminophylline | [84,85,86] | |||
| Antithrombotics | Rivaroxaban | Respiratory depression | [88] | |
| Antineoplastics | Doxorubicin | Side effects (pulmonary edema, interstitial fibrosis) | [89] | |
| Bleomycin | [90] | |||
| Lomustine | [91] |
| Respiratory Inhibition Caused by Different Doses of Morphine in Dogs | ||||||
|---|---|---|---|---|---|---|
| Preinj Control * | 5 min | 10 min | 30 min | 60 min | 120 min | |
| End Tidal CO2 (mm Hg) | ||||||
| 0.9% NaCl (Control) | 35.17 | 25.83 | 35.83 | 35.33 | ||
| 0.25 mg/kg Morphine | 34.83 | 26.83 | 29.00 | 30.67 | ||
| 0.5 mg/kg Morphine | 36.33 | 18.83 | 18.83 | 22.83 | ||
| 1.0 mg/kg Morphine | 36.33 | 16.67 | 14.83 | 22.50 | ||
| Tidal Volume (mL/kg) | ||||||
| 0.9% NaCl (Control) | 11.33 | 12.31 | 11.78 | 10.81 | ||
| 0.25 mg/kg Morphine | 12.52 | 9.52 | 9.00 | 8.47 | ||
| 0.5 mg/kg Morphine | 12.32 | 6.92 | 6.35 | 6.64 | ||
| 1.0 mg/kg Morphine | 11.15 | 5.21 | 4.55 | 5.99 | ||
| Respiratory Rate (breaths/min, BPM) | ||||||
| L. K.Cullen, M. R. Raffe et al. [70] | ||||||
| 0.9% NaCl (Control) | 19.84 | 17.69 | 19.07 | 18.68 | ||
| 0.25 mg/kg Morphine | 18.34 | 42.34 | 43.31 | 35.27 | ||
| 0.5 mg/kg Morphine | 19.59 | 91.54 | 99.56 | 60.51 | ||
| 1.0 mg/kg Morphine | 18.99 | 116.36 | 134.81 | 69.32 | ||
| Kamata M., Nagahama S. et al. [71] | ||||||
| 0.3 × 10−3 mg/kg Morphine | 30 ± 3 | 30 ± 6 | 31 ± 9 | 31 ± 3 | 32 ± 3 | 30 ± 4 |
| 0.6 × 10−3 mg/kg Morphine | 37 ± 7 | 28 ± 3 | 32 ± 10 | 25 ± 3 | 31 ± 6 | 30 ± 2 |
| 1.2 × 10−3 mg/kg Morphine | 34 ± 5 | 26 ± 7 | 24 ± 3 | 26 ± 3 | 23 ± 5 | 27 ± 5 |
| 2.4 × 10−3 mg/kg Morphine | 30 ± 5 | 26 ± 2 | 25 ± 2 | 20 ± 4 | 22 ± 3 | 26 ± 2 |
| Peak Expiratory Flow (L/min) | ||||||
| 0.9% NaCl (Control) | 18.31 | 15.84 | 14.94 | 17.86 | ||
| 0.25 mg/kg Morphine | 17.81 | 21.80 | 18.85 | 16.98 | ||
| 0.5 mg/kg Morphine | 17.77 | 30.51 | 30.28 | 20.81 | ||
| 1.0 mg/kg Morphine | 16.53 | 28.93 | 31.25 | 19.64 | ||
| Ventilatory Response to Salbutamol in Dogs | ||
|---|---|---|
| Control | Salbutamol | |
| Minute Ventilation (L/min) | 10 | 16 |
| Minute Volume per kg Body Weight (L/kg/min) | ||
| Tidal Ventilation (mL) | 370 | 480 |
| Tidal Volume per kg Body Weight (mL/kg) | ||
| Respiratory Rate (BPM) | 27.6 | 34.9 |
| Inspiratory Time (s) | 1.06 | 0.94 |
9. Surgical Procedures
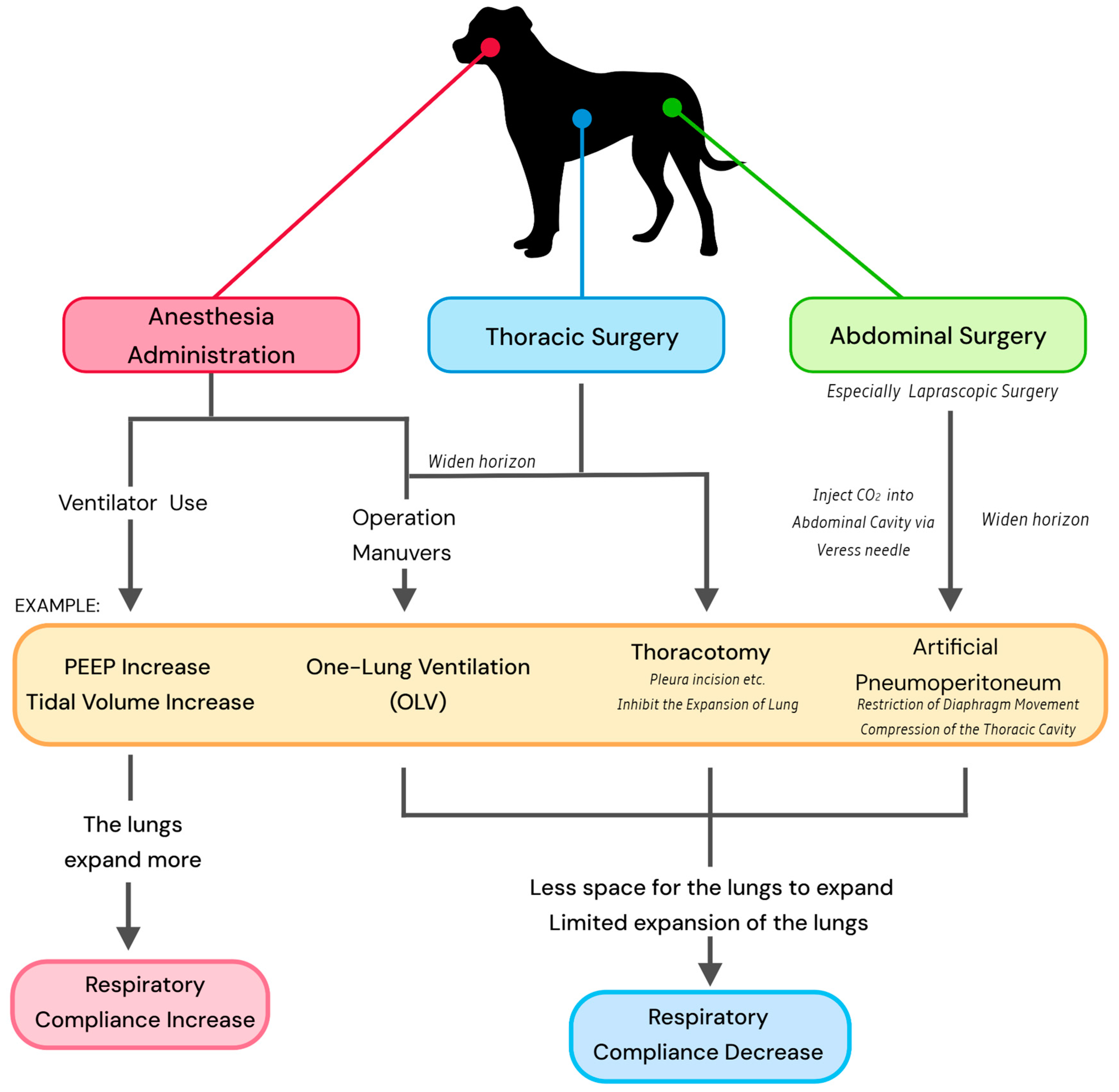
9.1. Anesthesia
9.2. Thoracic Surgery
9.3. Abdominal Surgery
10. Current Applications and Prospects
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edwards, Z.; Annamaraju, P. Physiology, Lung Compliance. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Zersen, K.M. Setting the optimal positive end-expiratory pressure: A narrative review. Front. Vet. Sci. 2023, 10, 1083290. [Google Scholar] [CrossRef] [PubMed]
- Venegas, J.G.; Harris, R.S.; Simon, B.A. A comprehensive equation for the pulmonary pressure-volume curve. J. Appl. Physiol. 1998, 84, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Robinson, N.E.; Gillespie, J.R.; Berry, J.D.; Simpson, A. Lung compliance, lung volumes, and single-breath diffusing capacity in dogs. J. Appl. Physiol. 1972, 33, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Desai, J.P.; Moustarah, F. Pulmonary Compliance. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Harris, R.S.; Hess, D.R.; Venegas, J.G. An objective analysis of the pressure-volume curve in the acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2000, 161 Pt 1, 432–439. [Google Scholar] [CrossRef]
- Lucangelo, U.; Bernabé, F.; Blanch, L. Respiratory mechanics derived from signals in the ventilator circuit. Respir. Care 2005, 50, 55–67. [Google Scholar]
- Nikischin, W.; Gerhardt, T.; Everett, R.; Bancalari, E. A new method to analyze lung compliance when pressure-volume relationship is nonlinear. Am. J. Respir. Crit. Care Med. 1998, 158, 1052–1060. [Google Scholar] [CrossRef]
- Suter, P.M.; Fairley, B.; Isenberg, M.D. Optimum end-expiratory airway pressure in patients with acute pulmonary failure. N. Engl. J. Med. 1975, 292, 284–289. [Google Scholar] [CrossRef]
- Pascoe, P.J.; Mayhew, P.D. Anesthesia for thoracoscopy. In Small Animal laparoscopy and Thoracoscopy; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2022; pp. 309–327. [Google Scholar]
- Canfrán, S.; Gómez de Segura, I.A.; Cediel, R.; García-Fernández, J. Effects of a stepwise lung recruitment manoeuvre and positive end-expiratory pressure on lung compliance and arterial blood oxygenation in healthy dogs. Vet. J. 2012, 194, 89–93. [Google Scholar] [CrossRef]
- Raillard, M.; Levionnois, O.; Mosing, M. A Survey on the Use of Spirometry in Small Animal Anaesthesia and Critical Care. Anim. Open Access J. 2022, 12, 239. [Google Scholar] [CrossRef]
- McKiernan, B.C.; Johnson, L.R. Clinical pulmonary function testing in dogs and cats. Vet. Clin. North America. Small Anim. Pract. 1992, 22, 1087–1099. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Tong, C.W. Clinical Application of Pulmonary Function Testing in Small Animals. Vet. Clin. North America. Small Anim. Pract. 2020, 50, 273–294. [Google Scholar] [CrossRef]
- King, L.G.; Drobatz, K.J.; Hendricks, J.C. Static thoracic compliance as a measurement of pulmonary function in dogs. Am. J. Vet. Res. 1991, 52, 1597–1601. [Google Scholar] [CrossRef] [PubMed]
- Nagai, A.; Hamaoka, S.; Ebata, K.; Fujioka, S.; Fujioka, T. The usefulness of pressure-volume curve (PV loop) as an anesthetic monitor and the evaluation of lung-thoracic compliance in dogs. J. Anim. Clin. Med. 2008, 17, 37–43. [Google Scholar]
- Asorey, I.; Pellegrini, L.; Canfrán, S.; Ortiz-Díez, G.; Aguado, D. Factors affecting respiratory system compliance in anaesthetised mechanically ventilated healthy dogs: A retrospective study. J. Small Anim. Pract. 2020, 61, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, B.M. Static respiratory compliance in normal dogs. J. Small Anim. Pract. 1991, 32, 438–442. [Google Scholar] [CrossRef]
- Clark, W.T.; Jones, B.R.; Clark, J. Dynamic pulmonary compliance as a measurement of lung function in dogs. Vet. Rec. 1977, 101, 497–499. [Google Scholar]
- Choi, S.Y.; Lee, I.; Jeong, W.C.; Heng, H.G.; Lee, Y.W.; Choi, H.J. Quantitative CT evaluation for lung volume and density in dogs. J. Vet. Clin. 2014, 31, 376–381. [Google Scholar] [CrossRef]
- Bradbrook, C.A.; Clark, L.; Dugdale, A.H.; Burford, J.; Mosing, M. Measurement of respiratory system compliance and respiratory system resistance in healthy dogs undergoing general anaesthesia for elective orthopaedic procedures. Vet. Anaesth. Analg. 2013, 40, 382–389. [Google Scholar] [CrossRef]
- Larsson, A.; Gilbert, J.T.; Bunegin, L.; Gelineau, J.; Smith, R.B. Pulmonary effects of body position, PEEP, and surfactant depletion in dogs. Acta Anaesthesiol. Scand. 1992, 36, 38–45. [Google Scholar] [CrossRef]
- De Troyer, A.; Leduc, D.; Cappello, M.; Gevenois, P.A. Mechanics of the canine diaphragm in pleural effusion. J. Appl. Physiol. 2012, 113, 785–790. [Google Scholar] [CrossRef][Green Version]
- Yilmaz, C.; Dane, D.M.; Tustison, N.J.; Song, G.; Gee, J.C.; Hsia, C.C. In vivo imaging of canine lung deformation: Effects of posture, pneumonectomy, and inhaled erythropoietin. J. Appl. Physiol. 2020, 128, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Clercx, C.; Van den Brom, W.E.; De Vries, H.W. Effect of posture and anaesthesia on the distribution of pulmonary perfusion and lung configuration in beagle dogs. Res. Vet. Sci. 1989, 47, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Margulies, S.S.; Farkas, G.A.; Rodarte, J.R. Effects of body position and lung volume on in situ operating length of canine diaphragm. J. Appl. Physiol. 1990, 69, 1702–1708. [Google Scholar] [CrossRef]
- Leevers, A.M.; Road, J.D. Effect of lung inflation and upright posture on diaphragmatic shortening in dogs. Respir. Physiol. 1991, 85, 29–40. [Google Scholar] [CrossRef]
- Brunson, D.B.; Johnson, R.A. Respiratory disease. In Canine and Feline Anesthesia and Co-Existing Disease; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 55–70. [Google Scholar]
- Zumla, A.; Yew, W.W.; Hui, D.S. Emerging respiratory infections in the 21st century. Preface. Infect. Dis. Clin. North Am. 2010, 24, xiii–xvi. [Google Scholar] [CrossRef] [PubMed]
- Faisal, A.; Alghamdi, B.J.; Ciavaglia, C.E.; Elbehairy, A.F.; Webb, K.A.; Ora, J.; Neder, J.A.; O’Donnell, D.E. Common mechanisms of dyspnea in chronic interstitial and obstructive lung disorders. Am. J. Respir. Crit. Care Med. 2016, 193, 299–309. [Google Scholar] [CrossRef]
- Heyder, J.; Takenaka, S. Long-term canine exposure studies with ambient air pollutants. Eur. Respir. J. 1996, 9, 571–584. [Google Scholar] [CrossRef]
- Dear, J.D. Bacterial pneumonia in dogs and cats. Vet. Clin. N. Am. Small Anim. Pract. 2014, 44, 143–159. [Google Scholar] [CrossRef][Green Version]
- Dear, J.D. Bacterial Pneumonia in Dogs and Cats: An Update. Vet. Clin. N. Am. Small Anim. Pract. 2020, 50, 447–465. [Google Scholar] [CrossRef]
- Vieson, M.D.; Piñeyro, P.; LeRoith, T. A review of the pathology and treatment of canine respiratory infections. Vet. Med. 2012, 3, 25–39. [Google Scholar] [CrossRef]
- Quan, S.F.; Witten, M.L.; Grad, R.; Ray, C.G.; Lemen, R.J. Changes in lung mechanics and histamine responsiveness after sequential canine adenovirus 2 and canine parainfluenza 2 virus infection in beagle puppies. Pediatr. Pulmonol. 1991, 10, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Snapper, J.R. Lung mechanics in pulmonary edema. Clin. Chest Med. 1985, 6, 393–412. [Google Scholar] [CrossRef]
- Johnson, L.R. Canine and Feline Respiratory Medicine; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Akella, A.; Deshpande, S.B. Pulmonary surfactants and their role in pathophysiology of lung disorders. Indian J. Exp. Biol. 2013, 51, 5–22. [Google Scholar] [PubMed]
- Dechman, G.; Sato, J.; Bates, J.H. Effect of pleural effusion on respiratory mechanics, and the influence of deep inflation, in dogs. Eur. Respir. J. 1993, 6, 219–224. [Google Scholar] [CrossRef]
- Ryan, R.; Gutierrez-Quintana, R.; Ter Haar, G.; De Decker, S. Prevalence of thoracic vertebral malformations in French bulldogs, Pugs and English bulldogs with and without associated neurological deficits. Vet. J. 2017, 221, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Komsta, R.; Osiński, Z.; Dębiak, P.; Twardowski, P.; Lisiak, B. Prevalence of pectus excavatum (PE), pectus carinatum (PC), tracheal hypoplasia, thoracic spine deformities and lateral heart displacement in thoracic radiographs of screw-tailed brachycephalic dogs. PLoS ONE 2019, 14, e0223642. [Google Scholar] [CrossRef]
- Mitze, S.; Barrs, V.R.; Beatty, J.A.; Hobi, S.; Bęczkowski, P.M. Brachycephalic obstructive airway syndrome: Much more than a surgical problem. Vet. Q. 2022, 42, 213–223. [Google Scholar] [CrossRef]
- Hlastala, M.P.; Berger, A.J. Physiology of Respiration; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Buback, J.L.; Boothe, H.W.; Hobson, H.P. Surgical treatment of tracheal collapse in dogs: 90 cases (1983–1993). J. Am. Vet. Med. Assoc. 1996, 208, 380–384. [Google Scholar] [CrossRef]
- Kim, M.R.; Kim, S.H.; Ryu, M.O.; Youn, H.Y.; Choi, J.H.; Seo, K.W. A retrospective study of tracheal collapse in small-breed dogs: 110 cases (2022–2024). Front. Vet. Sci. 2024, 11, 1448249. [Google Scholar] [CrossRef]
- Tangner, C.H.; HOBSON, H.P. A retrospective study of 20 surgically managed cases of collapsed trachea. Vet. Surg. 1982, 11, 146–149. [Google Scholar] [CrossRef]
- Tappin, S.W. Canine tracheal collapse. J. Small Anim. Pract. 2016, 57, 9–17. [Google Scholar] [CrossRef]
- Mondal, D.; Gupta, A.; Pradhan, S. Recent Advances in Chronic Obstructive Pulmonary Disease (COPD) in Animals and Human. Adv. Res. Vet. Sci. 2021, 22, 1. [Google Scholar]
- Upadhyay, P.; Wu, C.W.; Pham, A.; Zeki, A.A.; Royer, C.M.; Kodavanti, U.P.; Takeuchi, M.; Bayram, H.; Pinkerton, K.E. Animal models and mechanisms of tobacco smoke-induced chronic obstructive pulmonary disease (COPD). J. Toxicol. Environ. Health Part B Crit. Rev. 2023, 26, 275–305. [Google Scholar] [CrossRef]
- Tanner, L.; Single, A.B. Animal Models Reflecting Chronic Obstructive Pulmonary Disease and Related Respiratory Disorders: Translating Pre-Clinical Data into Clinical Relevance. J. Innate Immun. 2020, 12, 203–225. [Google Scholar] [CrossRef]
- Freed, A.N. Models and mechanisms of exercise-induced asthma. Eur. Respir. J. 1995, 8, 1770–1785. [Google Scholar] [CrossRef]
- Sánchez Jiménez, C.; Schofield, I.; Plested, M. Pulmonary interstitial emphysema and spontaneous pneumomediastinum are more prevalent in sighthounds than other dog breeds undergoing thoracic CT. Vet. Radiol. Ultrasound Off. J. Am. Coll. Vet. Radiol. Int. Vet. Radiol. Assoc. 2024, 65, 392–399. [Google Scholar] [CrossRef]
- Boushey, H.A.; Fahy, J.V. Basic mechanisms of asthma. Environ. Health Perspect. 1995, 103 (Suppl. S6), 229–233. [Google Scholar]
- Papandrinopoulou, D.; Tzouda, V.; Tsoukalas, G. Lung compliance and chronic obstructive pulmonary disease. Pulm. Med. 2012, 2012, 542769. [Google Scholar] [CrossRef] [PubMed]
- Ekenstedt, K.J.; Crosse, K.R.; Risselada, M. Canine Brachycephaly: Anatomy, Pathology, Genetics and Welfare. J. Comp. Pathol. 2020, 176, 109–115. [Google Scholar] [CrossRef]
- Crosse, K.R.; Bray, J.P.; Orbell, G.; Preston, C.A. Histological evaluation of the soft palate in dogs affected by brachycephalic obstructive airway syndrome. N. Z. Vet. J. 2015, 63, 319–325. [Google Scholar] [CrossRef]
- Beitler, J.R.; Malhotra, A.; Thompson, B.T. Ventilator-induced Lung Injury. Clin. Chest Med. 2016, 37, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.S.; Abelson, A.L.; Lindsey, J.C.; Wetmore, L.A. Postoperative regurgitation and respiratory complications in brachycephalic dogs undergoing airway surgery before and after implementation of a standardized perianesthetic protocol. J. Am. Vet. Med. Assoc. 2020, 256, 899–905. [Google Scholar] [CrossRef]
- Canola, P.A.; Johnson, P.J. Intra-abdominal hypertension in horses. Equine Vet. Educ. 2013, 25, 189–195. [Google Scholar] [CrossRef]
- Leduc, D.; Cappello, M.; Gevenois, P.A.; De Troyer, A. Mechanics of the canine diaphragm in ascites: A CT study. J. Appl. Physiol. 2008, 104, 423–428. [Google Scholar] [CrossRef]
- Worth, A.J.; Machon, R.G. Traumatic diaphragmatic herniation: Pathophysiology and management. Compend Contin Educ Pract. Vet. 2005, 27, 178–191. [Google Scholar]
- Pelosi, P.; Croci, M.; Ravagnan, I.; Vicardi, P.; Gattinoni, L. Total respiratory system, lung, and chest wall mechanics in sedated-paralyzed postoperative morbidly obese patients. Chest 1996, 109, 144–151. [Google Scholar] [CrossRef]
- Dixon, A.E.; Bhatawadekar, S.A. Obesity, Lung Function, and lung disease. In Handbook of Obesity; CRC Press: Boca Raton, FL, USA, 2024; Volume 1, pp. 548–555. [Google Scholar]
- Saraswat, V. Effects of anaesthesia techniques and drugs on pulmonary function. Indian J. Anaesth. 2015, 59, 557–564. [Google Scholar] [CrossRef]
- Muir, W.W., 3rd; Gadawski, J.E. Respiratory depression and apnea induced by propofol in dogs. Am. J. Vet. Res. 1998, 59, 157–161. [Google Scholar] [CrossRef]
- Lin, C.C.; Shyr, M.H.; Tan, P.P.; Chien, C.S.; Pan, S.L.; Wang, C.C.; Chiu, C.T.; Yang, C.M. Mechanisms underlying the inhibitory effect of propofol on the contraction of canine airway smooth muscle. Anesthesiology 1999, 91, 750–759. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, K.; Li, Y.; Yang, L.; Lu, J.; Qing, E. The effect of propofol on respiratory index and chest-lung compliance in patients undergoing direct vision cardiac surgery. Chin. J. Compr. Clin. Med. 2007, 23, 48–50. [Google Scholar]
- Mutoh, T.; Kanamaru, A.; Tsubone, H.; Nishimura, R.; Sasaki, N. Respiratory Reflexes in Response to Upper-Airway Administration of Sevoflurane and Isoflurane in Anesthetized, Spontaneously Breathing Dogs. Vet. Surg. 2001, 30, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Dikmen, Y.; Eminoglu, E.; Salihoglu, Z.; Demiroluk, S. Pulmonary mechanics during isoflurane, sevoflurane and desflurane anaesthesia. Anaesthesia 2003, 58, 745–748. [Google Scholar] [CrossRef]
- Cullen, L.K.; Raffe, M.R.; Randall, D.A.; Bing, D.R. Assessment of the respiratory actions of intramuscular morphine in conscious dogs. Res. Vet. Sci. 1999, 67, 141–148. [Google Scholar] [CrossRef]
- Kamata, M.; Nagahama, S.; Kakishima, K.; Sasaki, N.; Nishimura, R. Comparison of behavioral effects of morphine and fentanyl in dogs and cats. J. Vet. Med. Sci. 2012, 74, 231–234. [Google Scholar] [CrossRef]
- Maiante, A.A.; Teixeira Neto, F.J.; Beier, S.L.; Corrente, J.E.; Pedroso, C.E.B.P. Comparison of the cardio-respiratory effects of methadone and morphine in conscious dogs. J. Vet. Pharmacol. Ther. 2009, 32, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Hurlé, M.A.; Mediavilla, A.; Flórez, J. Differential respiratory patterns induced by opioids applied to the ventral medullary and dorsal pontine surfaces of cats. Neuropharmacology 1985, 24, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Latasch, L.; Freye, E. Sufentanil-related respiratory depression and antinociception in the dog. Mediation by different receptor types. Arzneim. Forsch. 2002, 52, 870–876. [Google Scholar] [CrossRef]
- Palkovic, B.; Mustapic, S.; Saric, I.; Stuth, E.A.E.; Stucke, A.G.; Zuperku, E.J. Changes in pontine and preBötzinger/Bötzinger complex neuronal activity during remifentanil-induced respiratory depression in decerebrate dogs. Front. Physiol. 2023, 14, 1156076. [Google Scholar] [CrossRef]
- Ihara, T.; Shannon, R.P.; Komamura, K.; Pasipoularides, A.; Patrick, T.; Shen, Y.T.; Vatner, S.F. Effects of anaesthesia and recent surgery on diastolic function. Cardiovasc. Res. 1994, 28, 325–336. [Google Scholar] [CrossRef]
- Lee, D.D.; Meyer, R.E.; Sullivan, T.C.; Davidson, M.G.; Swanson, C.R.; Hellyer, P.W. Respiratory depressant and skeletal muscle relaxant effects of low-dose pancuronium bromide in spontaneously breathing, isoflurane-anesthetized dogs. Vet. Surg. VS 1998, 27, 473–479. [Google Scholar] [CrossRef]
- Sullivan, T.C.; Hellyer, P.W.; Lee, D.D.; Davidson, M.G. Respiratory function and extraocular muscle paralysis following administration of pancuronium bromide in dogs. Vet. Ophthalmol. 1998, 1, 125–128. [Google Scholar] [CrossRef]
- Keegan, R.D. Muscle Relaxants and Neuromuscular Blockade. In Veterinary Anesthesia and Analgesia; Grimm, K.A., Lamont, L.A., Tranquilli, W.J., Greene, S.A., Robertson, S.A., Eds.; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar] [CrossRef]
- Authier, S.; Legaspi, M.; Gauvin, D.; Chaurand, F.; Fournier, S.; Troncy, E. Validation of respiratory safety pharmacology models: Conscious and anesthetized beagle dogs. J. Pharmacol. Toxicol. Methods 2008, 57, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Easton, P.A.; Katagiri, M.; Johnson, M.W.; Rothwell, B.C.; Holroyde, M.C.; Kusuhara, N. Effect of salbutamol on respiratory muscle function and ventilation in awake canines. Respir. Physiol. Neurobiol. 2008, 161, 253–260. [Google Scholar] [CrossRef]
- Han, B.K.; Im, J.G.; Kim, H.S.; Koo, J.M.; Kim, H.D.; Yeon, K.M. Airway reactivity to bronchoconstrictor and bronchodilator: Assessment using thin-section and volumetric three-dimensional CT. Korean J. Radiol. 2000, 1, 127–134. [Google Scholar] [CrossRef][Green Version]
- Akhtar, S.; Mazzeo, A.J.; Cheng, E.Y.; Bosnjak, Z.; Kampine, J.P. Differential bronchodilatory effects of terbutaline, diltiazem, and aminophylline in canine intraparenchymal airways. Crit. Care Med. 1999, 27, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Suneby Jagers, J.V.; Ji, M.; Rothwell, B.; Easton, P.A. Aminophylline increases parasternal muscle action in awake canines. Pulm. Pharmacol. Ther. 2019, 56, 1–7. [Google Scholar] [CrossRef]
- Jagers, J.V.; Hawes, H.G.; Easton, P.A. Aminophylline increases ventilation and diaphragm contractility in awake canines. Respir. Physiol. Neurobiol. 2009, 167, 273–280. [Google Scholar] [CrossRef]
- Gayan-Ramirez, G.; Palecek, F.; Chen, Y.; Janssens, S.; Decramer, M. Inotropic effects of aminophylline on canine diaphragm are enhanced by hyperinflation. J. Appl. Physiol. 1994, 76, 39–44. [Google Scholar] [CrossRef]
- Yu, T.J.; Liu, Y.C.; Chu, C.M.; Hu, H.C.; Kao, K.C. Effects of theophylline therapy on respiratory muscle strength in patients with prolonged mechanical ventilation: A retrospective cohort study. Medicine 2019, 98, e13982. [Google Scholar] [CrossRef]
- Yang, V.K.; Cunningham, S.M.; Rush, J.E.; de Laforcade, A. The use of rivaroxaban for the treatment of thrombotic complications in four dogs. J. Vet. Emerg. Crit. Care 2016, 26, 729–736. [Google Scholar] [CrossRef]
- Minchin, R.F.; Johnston, M.R.; Schuller, H.M.; Aiken, M.A.; Boyd, M.R. Pulmonary toxicity of doxorubicin administered by in situ isolated lung perfusion in dogs. Cancer 1988, 61, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.R.; Baker, J.R.; Fleischman, R.W.; Rosenkrantz, H.; Schaeppi, U.H.; Cooney, D.A.; Davis, R.D. Preclinical toxicologic evaluation of bleomycin (NSC 125 066), a new antitumor antibiotic. Toxicol. Appl. Pharmacol. 1972, 22, 544–555. [Google Scholar] [CrossRef]
- Van Meervenne, S.A.E.; de Vos, J.P.; Van Ham, L.; Bavegems, V. Comparative aspects of pulmonary toxicity induced by cytotoxic agents with emphasis on lomustine, and a veterinary case report. Vlaams Diergeneeskd. Tijdschr. 2008, 77, 248–255. [Google Scholar] [CrossRef]
- Aparna Datta, A. Comparison of Anesthetic Potency and Cardiopulmonary Effects of Halothane and Isoflurane Anesthesia in Dogs. Ph.D. Thesis, Chattogram Veterinary and Animal Sciences University, Chattogram, Bangladesh, 2020. [Google Scholar]
- Raillard, M.; Mosing, M.; Raisis, A.; Auckburally, A.; Beaumont, G.; Downing, F.; Heselton, C.; MacFarlane, P.; Portier, K.; Robertson, J.; et al. Characterization of dynamic compliance of the respiratory system in healthy anesthetized dogs. Front. Vet. Sci. 2024, 11, 1490494. [Google Scholar] [CrossRef] [PubMed]
- Webb, H.H.; Tierney, D.F. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am. Rev. Respir. Dis. 1974, 110, 556–565. [Google Scholar]
- Andrade, F.S.R.M.; Ambrósio, A.M.; Rodrigues, R.R.; Faccó, L.L.; Gonçalves, L.A.; Garcia Filho, S.G.; Santos, R.T.D.; Rossetto, T.C.; Pereira, M.A.A.; Fantoni, D.T. The optimal PEEP after alveolar recruitment maneuver assessed by electrical impedance tomography in healthy horses. Front. Vet. Sci. 2022, 9, 1024088. [Google Scholar] [CrossRef] [PubMed]
- Araos, J.; Cruces, P.; Martin-Flores, M.; Donati, P.; Gleed, R.D.; Boullhesen-Williams, T.; Perez, A.; Staffieri, F.; Retamal, J.; Vidal Melo, M.F.; et al. Distribution and Magnitude of Regional Volumetric Lung Strain and Its Modification by PEEP in Healthy Anesthetized and Mechanically Ventilated Dogs. Front. Vet. Sci. 2022, 9, 839406. [Google Scholar] [CrossRef]
- Peterson, N.W.; Buote, N.J.; Barr, J.W. The impact of surgical timing and intervention on outcome in traumatized dogs and cats. J. Vet. Emerg. Crit. Care 2015, 25, 63–75. [Google Scholar] [CrossRef]
- Baudouin, S.V. Lung injury after thoracotomy. Br. J. Anaesth. 2003, 91, 132–142. [Google Scholar] [CrossRef]
- Tillson, D.M. Thoracic surgery; important considerations and practical steps. Vet. Clin. North Am. Small Anim. Pract. 2015, 45, 489–506. [Google Scholar] [CrossRef]
- Mayhew, P.D.; Friedberg, J.S. Video-assisted thoracoscopic resection of noninvasive thymomas using one-lung ventilation in two dogs. Vet. Surg. 2008, 37, 756–762. [Google Scholar] [CrossRef]
- Rawley, M.; Harris, E.; Pospishil, L.; Thompson, J.A.; Falyar, C. Assessing Provider Adherence To A Lung Protective Ventilation Protocol In Patients Undergoing Thoracic Surgery Using One-Lung Ventilation. AANA J. 2022, 90, 439–445. [Google Scholar]
- Broaddus, K.; Tillson, M. Patent ductus arteriosus in dogs. Compendium 2010, 32, E3. [Google Scholar]
- Summer, W.R.; Permutt, S.O.L.B.E.R.T.; Sagawa, K.I.I.C.H.I.; Shoukas, A.A.; Bromberger-Barnea, B.A.R.U.C.H. Effects of spontaneous respiration on canine left ventricular function. Circ. Res. 1979, 45, 719–728. [Google Scholar] [CrossRef]
- Bouferrache, K.; Vieillard-Baron, A. Acute respiratory distress syndrome, mechanical ventilation, and right ventricular function. Curr. Opin. Crit. Care 2011, 17, 30–35. [Google Scholar] [CrossRef]
- Nakazawa, Y.; Ohshima, T.; Kitagawa, M.; Nuruki, T.; Fujiwara-Igarashi, A. Relationship between Respiratory Rate, Oxygen Saturation, and Blood Test Results in Dogs with Chronic or Acute Respiratory Disease: A Retrospective Study. Vet. Sci. 2024, 11, 27. [Google Scholar]
- Mayhew, P. Fundamentals of Laparoscopy and Thoracoscopy. In Veterinary Surgery. Small Animal, 2nd ed.; Elsevier Inc.: St. Louis, MO, USA, 2018; pp. 317–324. [Google Scholar]
- Scott, J.; Singh, A.; Valverde, A. Pneumoperitoneum in veterinary laparoscopy: A review. Vet. Sci. 2020, 7, 64. [Google Scholar] [CrossRef]
- Mama, K.; de Rezende, M.L. Anesthesia management of dogs and cats for laparoscopy. In Small Animal Laparoscopy and Thoracoscopy; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 73–80. [Google Scholar]
- Di Bella, C.; Vicenti, C.; Araos, J.; Lacitignola, L.; Fracassi, L.; Stabile, M.; Grasso, S.; Crovace, A.; Staffieri, F. Effects of two alveolar recruitment maneuvers in an “open-lung” approach during laparoscopy in dogs. Front. Vet. Sci. 2022, 9, 904673. [Google Scholar] [CrossRef]
- Scales, C.; Simpson, K. Anaesthesia for the Laparoscopic Patient. Anaesthesia 2003. Available online: https://articles.burtonsveterinary.com/articles/anaesthesia-for-the-laparoscopic-patient (accessed on 2 October 2023).
- Matthay, M.A.; Bhattacharya, S.; Gaver, D.; Ware, L.B.; Lim, L.H.; Syrkina, O.; Eyal, F.; Hubmayr, R. Ventilator-induced lung injury: In vivo and in vitro mechanisms. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 283, L678–L682. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ahmed, F.; Shafeeq, A.M.; Moiz, J.A.; Geelani, M.A. Comparison of effects of manual versus ventilator hyperinflation on respiratory compliance and arterial blood gases in patients undergoing mitral valve replacement. Heart Lung J. Crit. Care 2010, 39, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Anđelić, N.; Uvelin, A.; Stokić, E.; Popović, R.; Zdravković, R.; Preveden, A.; Zornić, N. The Effect of Recruitment Maneuver on Static Lung Compliance in Patients Undergoing General Anesthesia for Laparoscopic Cholecystectomy: A Single-Centre Prospective Clinical Intervention Study. Medicina 2024, 60, 666. [Google Scholar] [CrossRef]
- Li, B.; Li, D.; Huang, W.; Che, Y. Effect of lung recruitment on blood gas index, hemodynamics, lung compliance, and rehabilitation index in children with acute respiratory distress syndrome. Transl. Pediatr. 2020, 9, 795. [Google Scholar] [CrossRef]
| Static Compliance (Cst) | Dynamic Compliance (Cdyn) | |
|---|---|---|
| Calculation Method | ||
| Unit of Measurement | Milliliters per centimeter of water column (ml/cm H2O) Liters per centimeter of water column (L/cm H2O) | |
| Measurement Conditions | Ventilation pauses under anesthesia | Mechanical ventilation under anesthesia. |
| Reflects | Elasticity of the lungs Compliance of lung tissue | Ventilation states |
| Main Influencing Factors | Lung tissue elasticity Thoracic compliance Respiratory muscle function | Airway resistance Mechanical properties of the airway Ventilator settings and ventilation modalities |
| Differences in Tissue and Air Volume in the Lungs Due to Different Body Positions of Dogs | ||
|---|---|---|
| Supine | Prone | |
| Air Volume (mL/kg) | ||
| L cranial | 13.7 ± 4.1 | 13.3 ± 3.4 |
| L middle | 8.8 ± 3.0 | 8.6 ± 2.9 |
| L caudal | 27.6 ± 5.1 | 27.9 ± 4.5 |
| L lung | 50.1 ± 11.9 | 49.8 ± 10.5 |
| Whole lung | 118.7 ± 26.4 | 118 ± 24.5 |
| Tissue Volume (mL/kg) | ||
| L cranial | 1.17 ± 0.30 | 1.35 ± 0.34 |
| L middle | 0.74 ± 0.17 | 0.93 ± 0.29 |
| L caudal | 2.37 ± 0.43 | 2.60 ± 0.53 |
| L lung | 4.28 ± 0.81 | 4.88 ± 1.08 |
| Whole lung | 10.16 ± 1.83 | 11.57 ± 2.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, T.; Mandour, A.S.; Shimada, K.; Hamabe, L.; Tanaka, R. A Comprehensive Overview of Respiratory Compliance in Dogs Under General Anesthesia: Clinical Factors and Future Perspectives. Animals 2025, 15, 746. https://doi.org/10.3390/ani15050746
Xu T, Mandour AS, Shimada K, Hamabe L, Tanaka R. A Comprehensive Overview of Respiratory Compliance in Dogs Under General Anesthesia: Clinical Factors and Future Perspectives. Animals. 2025; 15(5):746. https://doi.org/10.3390/ani15050746
Chicago/Turabian StyleXu, Tingfeng, Ahmed S. Mandour, Kazumi Shimada, Lina Hamabe, and Ryou Tanaka. 2025. "A Comprehensive Overview of Respiratory Compliance in Dogs Under General Anesthesia: Clinical Factors and Future Perspectives" Animals 15, no. 5: 746. https://doi.org/10.3390/ani15050746
APA StyleXu, T., Mandour, A. S., Shimada, K., Hamabe, L., & Tanaka, R. (2025). A Comprehensive Overview of Respiratory Compliance in Dogs Under General Anesthesia: Clinical Factors and Future Perspectives. Animals, 15(5), 746. https://doi.org/10.3390/ani15050746









