Evidence of Morphological and Morphometric Differences in the Sella Turcica of Pteronotus mesoamericanus and P. mexicanus
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
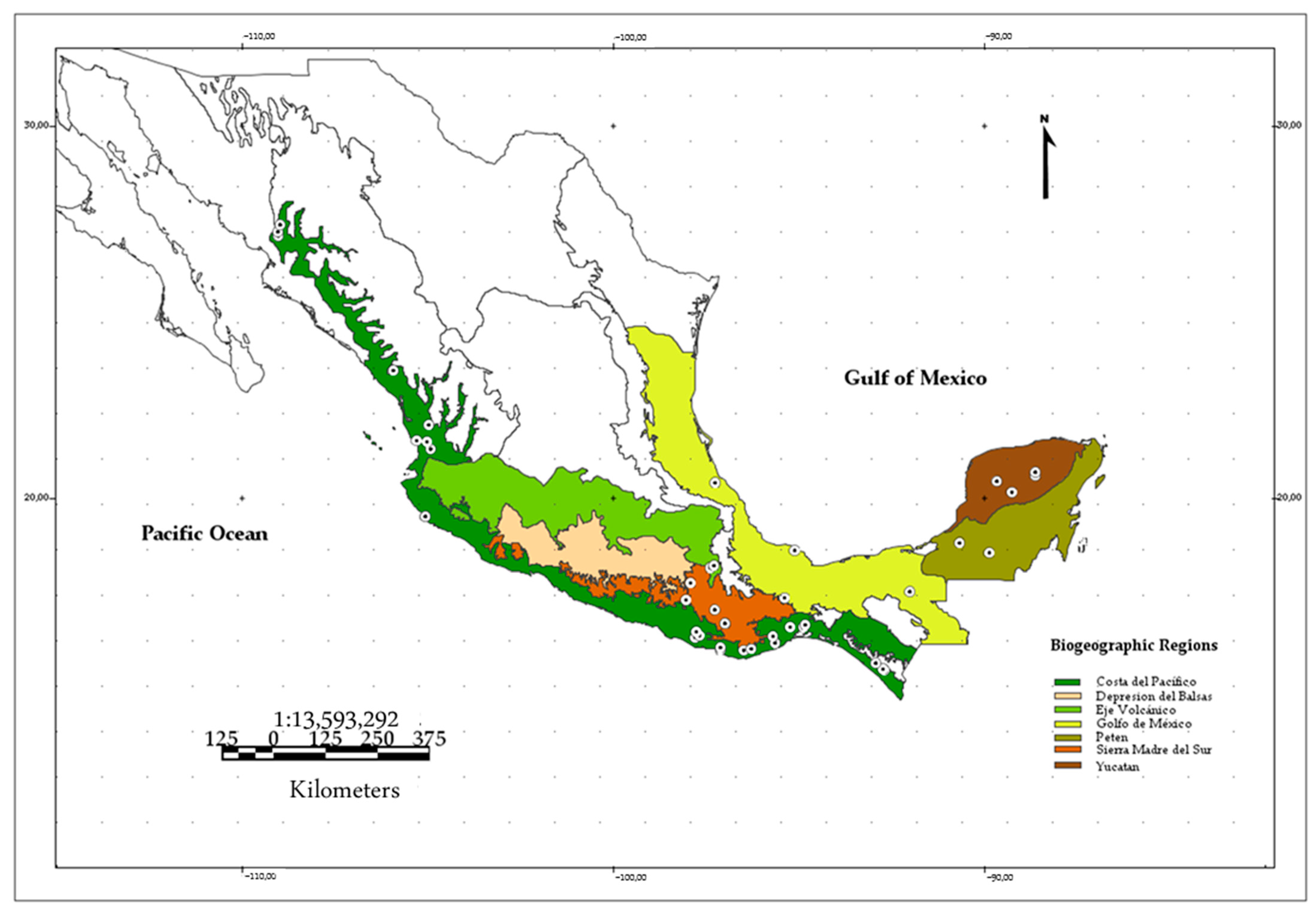
2.1. Specimen Review
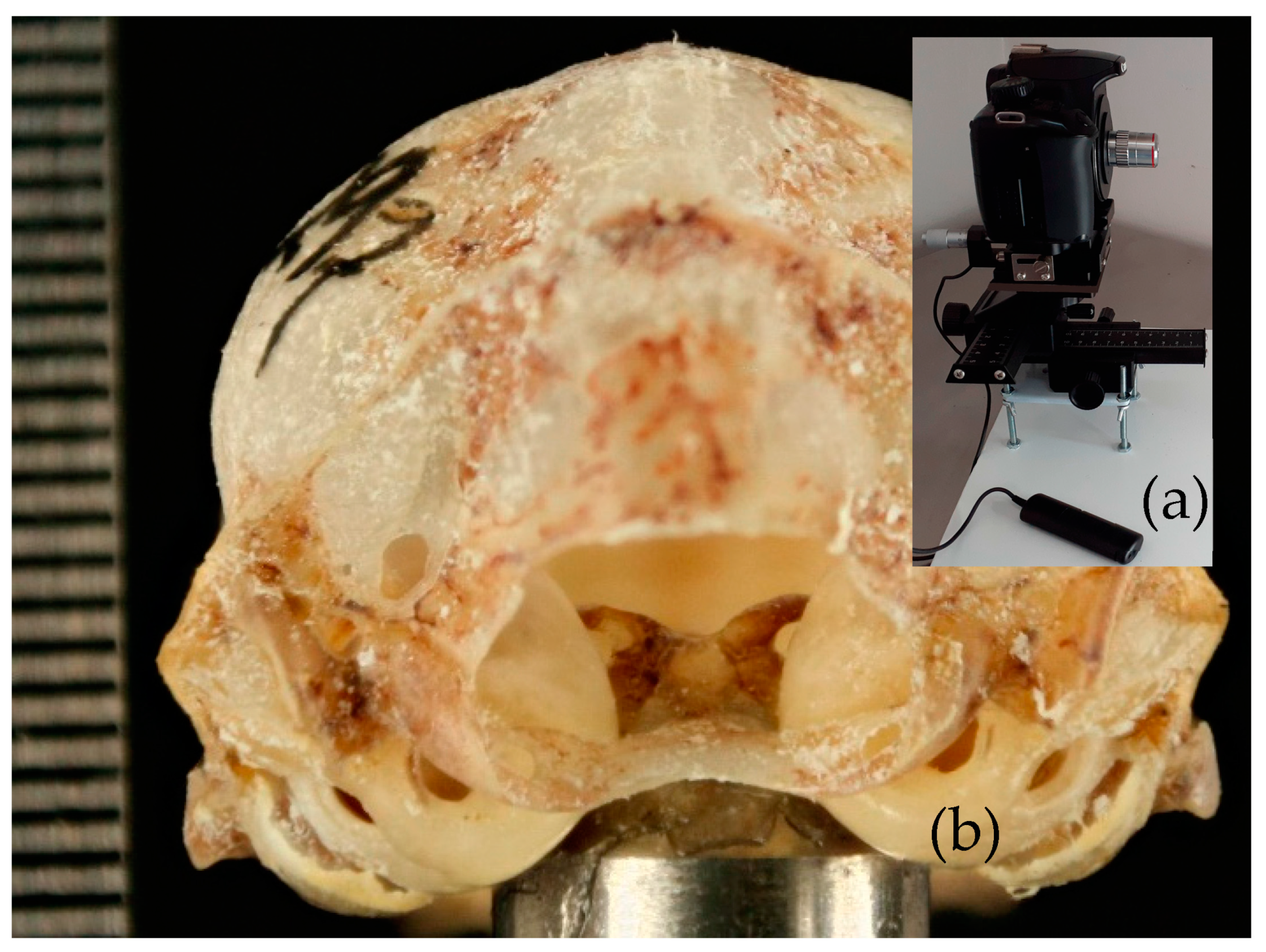
2.2. Photographs
2.3. Measurements
3. Results
3.1. General Description
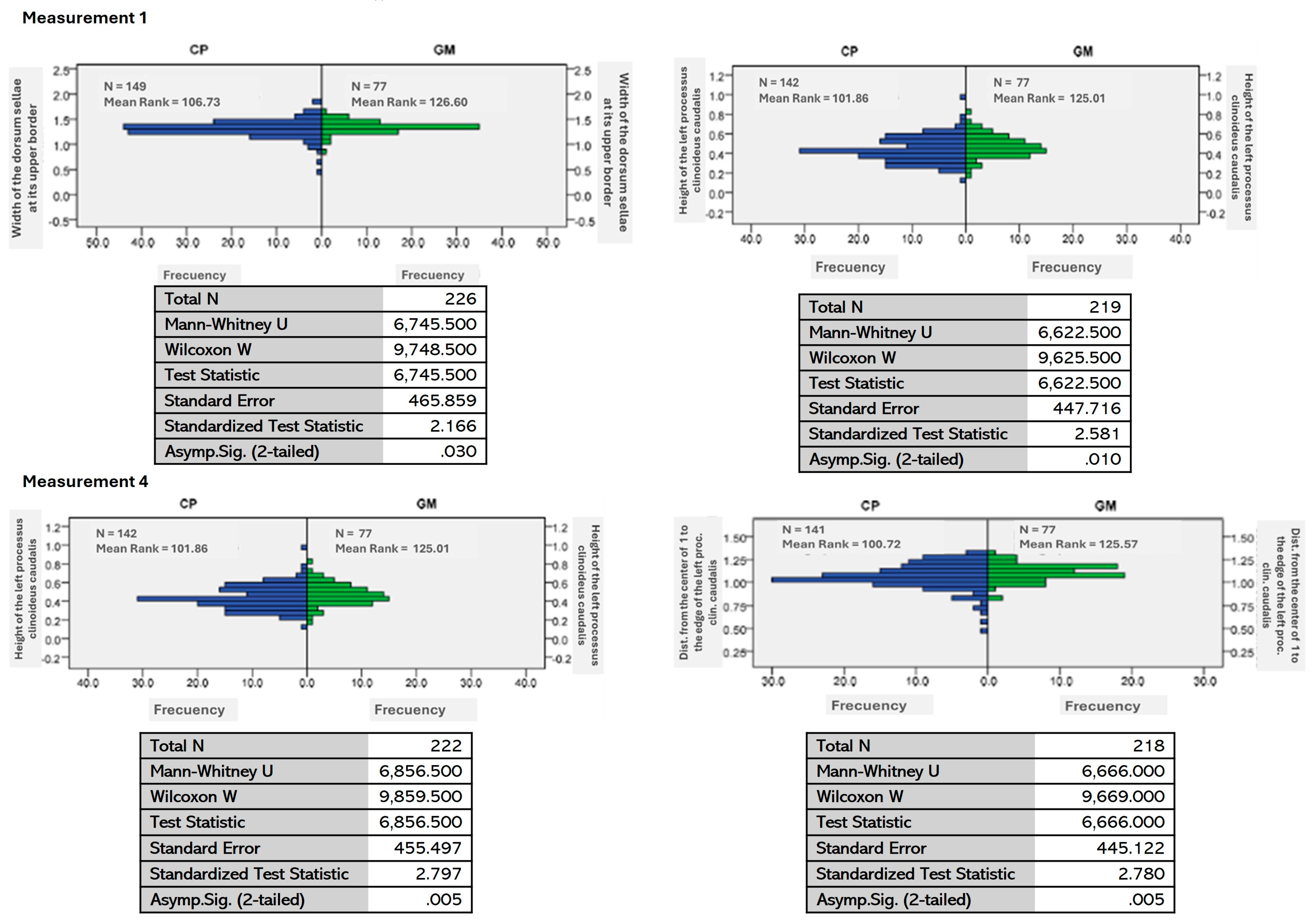
3.2. Morphometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Locality | State | Biogeographic Regions (BIOGEO) | Lat. | Lon. | N |
|---|---|---|---|---|---|
| Chamela | Jalisco | Costa del Pacífico | 19.5 | −105.04 | 24 |
| Compostela | Nayarit | Costa del Pacífico | 21.31 | −104.9 | 2 |
| El Venado | Nayarit | Costa del Pacífico | 21.96 | −104.95 | 1 |
| San Blas | Nayarit | Costa del Pacífico | 21.54 | −105.28 | 2 |
| Tepic | Nayarit | Costa del Pacífico | 21.51 | −104.99 | 1 |
| La Venta, Santo Domingo Ingenio | Oaxaca | Costa del Pacífico | 16.53 | −94.84 | 4 |
| Magdalena Tacotepec | Oaxaca | Costa del Pacífico | 16.53 | −95.2 | 2 |
| Pluma Hidalgo | Oaxaca | Costa del Pacífico | 15.9 | −96.45 | 1 |
| San Francisco Huamelula | Oaxaca | Costa del Pacífico | 16.1 | −95.61 | 1 |
| San José de las Flores | Oaxaca | Costa del Pacífico | 16.4 | −97.73 | 2 |
| San Pedro Mixtepec | Oaxaca | Costa del Pacífico | 15.92 | −97.08 | 12 |
| Santa María Petatengo | Oaxaca | Costa del Pacífico | 15.94 | −96.03 | 6 |
| Santiago Jamiltepec | Oaxaca | Costa del Pacífico | 16.24 | −97.77 | 1 |
| Santiago Tetepec | Oaxaca | Costa del Pacífico | 16.3 | −97.65 | 2 |
| Copala | Sinaloa | Costa del Pacífico | 23.42 | −105.91 | 25 |
| Álamos | Sonora | Costa del Pacífico | 27.04 | −109.01 | 40 |
| Mina San Alberto, Mexiquillo | Sonora | Costa del Pacífico | 27.35 | −108.96 | 18 |
| Constitución Mexicana | Oaxaca | Golfo de México | 17.31 | −95.35 | 1 |
| Estación Los Tuxtlas | Veracruz | Golfo de México | 18.59 | −95.08 | 4 |
| Chichen-itza | Yucatán | Yucatán | 20.6 | −88.59 | 7 |
| Ticul-Muna | Yucatán | Yucatán | 20.45 | −89.63 | 14 |
| Ticum | Yucatán | Yucatán | 20.15 | −89.22 | 11 |
| Ayoquezco de Aldama | Oaxaca | Sierra Madre del Sur | 16.67 | −96.87 | 1 |
| San Juan Mazatlán | Oaxaca | Sierra Madre del Sur | 17.71 | −97.89 | 1 |
| San Miguel Cuevas | Oaxaca | Sierra Madre del Sur | 17.26 | −98.01 | 3 |
| San Miguel Piedras | Oaxaca | Sierra Madre del Sur | 17 | −97.23 | 2 |
| San Sebastian de las Grutas | Oaxaca | Sierra Madre del Sur | 16.62 | −96.95 | 7 |
| Tepelmeme, Villa de Morelos | Oaxaca | Sierra Madre del Sur | 18.12 | −97.36 | 1 |
| San José Miahuatlán | Puebla | Eje volcánico | 18.17 | −97.26 | 1 |
| Number of individuals | 243 |
| Measures | Null Hypothesis | Significance |
|---|---|---|
| 1 | The distribution of the dorsum width is the same among BIOGEO categories. | 0.030 |
| 3 | The distribution of the perpendicular height of the left clinoid process is the same among BIOGEO categories. | 0.010 |
| 4 | The distribution of the length of the right clinoid process from the center of width 1 is the same among BIOGEO categories. | 0.005 |
| 5 | The length distribution of the left clinoid process from the center of the width is the same among BIOGEO categories. | 0.005 |
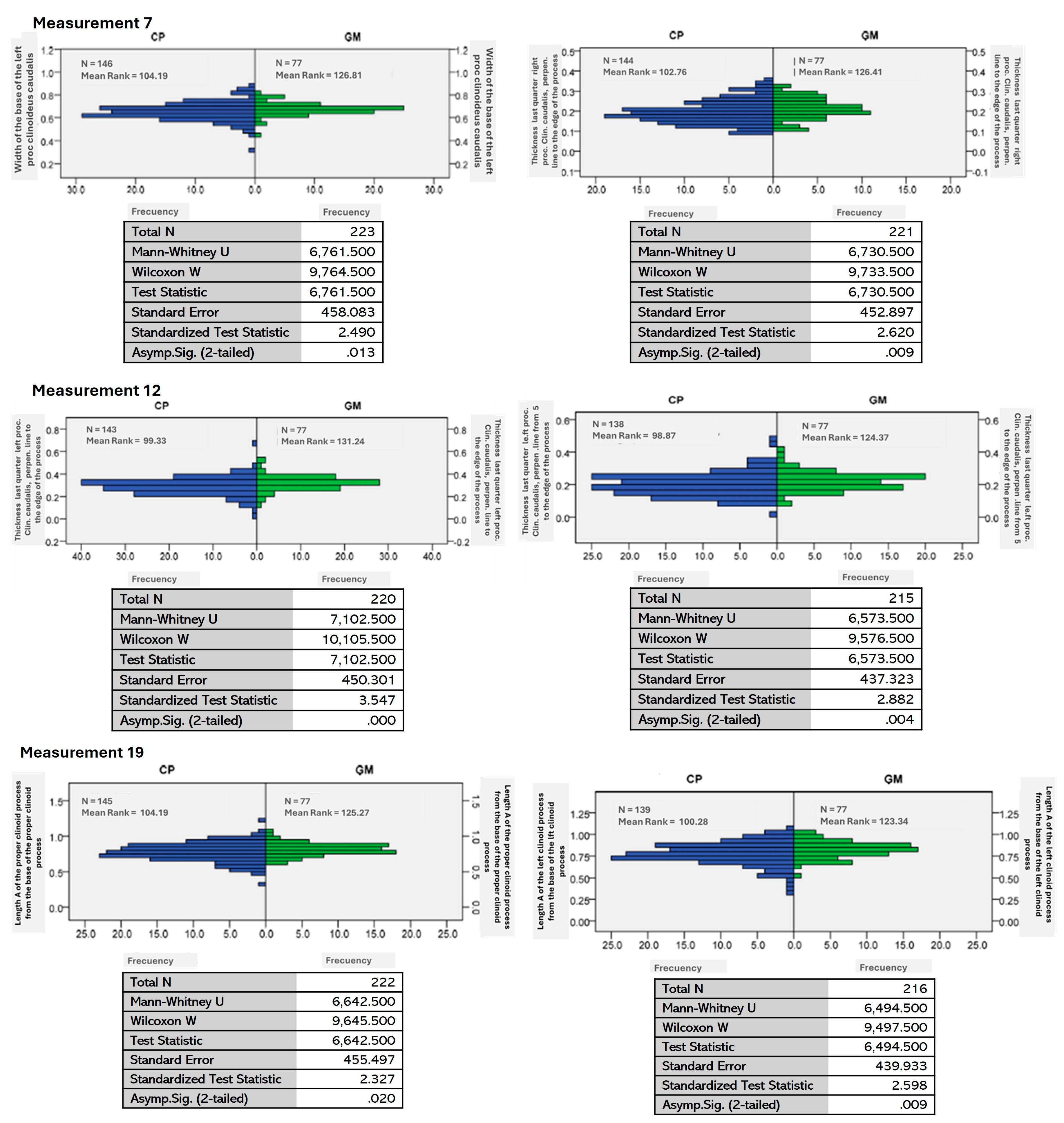
| 7 | The distribution of the base of the left clinoid process is the same between BIOGEO categories. | 0.013 |
| 11 | The width 11 distribution of the right clinoid process is the same between BIOGEO categories. | 0.009 |
| 12 | The width 12 distribution of the left clinoid process is the same between BIOGEO categories. | 0.000 |
| 15 | The width 15 distribution of the left clinoid process is the same between BIOGEO categories. | 0.004 |
| 19 | The length 19 distribution of the right clinoid process from the base of the right clinoid process is the same between BIOGEO categories. | 0.020 |
| 20 | The length 20 distribution of the left clinoid process from the base of the left clinoid process is the same between BIOGEO categories. | 0.009 |
References
- Evin, A.; Baylac, M.; Ruedi, M.; Mucedda, M.; Pons, J.-M. Taxonomy, skull diversity and evolution in a species complex of Myotis Chiroptera:Vespertilionidae: A geometric morphometric appraisal. Biol. J. Linn. Soc. 2008, 95, 529–538. [Google Scholar] [CrossRef]
- Noftz, L.A.; Calede, J.J.M. Multivariate analyses of skull morphology inform the taxonomy and evolution of geomyoid rodents. Curr. Zool. 2023, 69, 456–474. [Google Scholar] [CrossRef] [PubMed]
- Lala, K. Understanding niche construction and phenotypic plasticity as causes of natural selection. Paleontology 2024, 67, 1–10. [Google Scholar] [CrossRef]
- Stanchack, K.E.; Arbour, J.H.; Santana, S.E. Anatomical diversification of a skeletal novelty in bat feet. Evolution 2019, 73, 1591–1603. [Google Scholar] [CrossRef] [PubMed]
- Infante Contreras, C. Fundamentos para la Evaluación del Crecimiento, Desarrollo y Función Craneofacial; Universidad Nacional de Colombia: Bogotá, Colombia, 2009; Available online: https://repositorio.unal.edu.co/bitstream/handle/unal/2386/9789584442864.08.pdf (accessed on 9 July 2023).
- Magat, S.G.; Sener, O. Morphometric analysis of the sella turcica in Turkish individuals with different dentofacial skeletal patterns. Folia Morphol. 2018, 77, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Pulido, J.; González-Ruiz, N.; Gardner, A.L.; Arroyo-Cabrales, J. List of Recent Land Mammals of Mexico; Biodiversity Heritage Library: Washington, DC, USA, 2014; Volume 63, pp. 1–69. [Google Scholar]
- López-Wilchis, R.; Flores-Romero, M.; Guevara-Chumacero, L.M.; Serrato-Díaz, A.; Díaz-Larrea, J.; Salgado-Mejia, F.; Ibañez, C.; Salles, L.O.; Juste, J. Evolutionary scenarios associated with the Pteronotus parnellii cryptic species-complex (Chiroptera: Mormoopidae). Acta Chiropterologica 2016, 18, 91–116. [Google Scholar] [CrossRef]
- Pavan, A.C.; Marroig, G. Integrating multiple evidences in taxonomy: Species diversity and phylogeny of mustached bats (Mormoopidae: Pteronotus). J. Mol. Evol. 2016, 103, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Pavan, A.C.; Marroig, G. Timing and patterns of diversification in the Neotropical bat genus Pteronotus (Mormoopidae). Mol. Phylogenetics Evol. 2017, 108, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, D.S.; Eick Geeta, N.; Corrie, M.S.; Matthee, C.A. Cryptic species in an insectivorous bat, Scotophilus dinganii. J. Mammal. 2006, 87, 161–170. [Google Scholar] [CrossRef]
- Santos, H.; Juste, J.; Ibáñez, C.; Palmeirim, J.M.; Godinho, R.; Amorim, F.; Alves, P.; Costa, H.; De Paz, O.; Pérez-Suarez, G.; et al. Influences of ecology and biogeography on shaping the distributions of cryptic species: Three bat tales in Iberia. Biol. J. Linn. Soc. 2014, 112, 150–162. [Google Scholar] [CrossRef]
- Filippi-Codaccioni, O.; Beugin, M.P.; de Vienne, D.M.; Portanier, E.; Fouchet, D.; Kaerle, C.; Muselet, L.; Queney, G.; Petit, E.J.; Regis, C.; et al. Coexistence of two sympatric cryptic bat species in French Guiana: Insights from genetic, acoustic and ecological data. BMC Evol. Biol. 2018, 18, 175. [Google Scholar] [CrossRef] [PubMed]
- International Committee on Veterinary Gross Anatomical Nomenclature. Nomina Anatomica Veterinaria, 6th ed.; Editorial Committee: Hanover, Germany; Ghent, Belgium; Columbia, MO, USA; Rio de Janeiro, Brazil, 2017; p. 12. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to Image J: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- IBM Corp. IBM SPSS Statistics for Windows, Version 21.0; IBM Corp: Armonk, NY, USA, 2012. [Google Scholar]
- Velazco, P.M. Morphological Phylogeny of the Bat Genus Platyrrhinus Saussure, 1860 (Chiroptera: Phyllostomidae) with the Description of Four New Species. In FIELDIANA: Zoology, New Series; Field Museum of Natural History: Chicago, IL, USA, 2005; Volume 105, pp. 1–54. [Google Scholar]
- Simmons, N.B.; Conway, T.M. Phylogenetic relationships of mormoopid bats (Chiroptera: Mormoopidae) based on morphological data. Bull. Am. Mus. Nat. Hist. 2001, 258, 1–100. [Google Scholar] [CrossRef]
- Velayudhan, V.; Luttrull, M.D.; Naidich, T.P. Sella Turcica and Pituitary Gland. In Imaging of the Brain; Naidich, T.P., Castillo, M., Cha, S., Smirniotopoulos, J.G., Eds.; Elsevier: Philadelphia, PA, USA, 2013; pp. 272–293. [Google Scholar]
- Kumar, T.S.; Govindraju, P. Relationship between the morphological variation of sella turcica with age and gender: A digital radiographic study. J. Indian Acad. Oral Med. Radiol. 2017, 29, 164–169. [Google Scholar] [CrossRef]
- Nagaraj, T.; Shruthi, R.; James, L.; Keerthi, I.; Balraj, L.; Goswami, R.D. The size and morphology of sella turcica: A lateral cephalometric study. J. Med. Radiol. Pathol. Surg. 2015, 1, 3–7. [Google Scholar] [CrossRef]
- Davis, S.W.; Ellsworth, B.S.; Millan, M.I.P.; Gergics, P.; Schade, V.; Foyouzi, N.; Brinkmeier, M.L.; Mortensen, A.H.; Camper, S.A. Pituitary Gland Development and Disease: From Stem Cell to Hormone Production. Curr. Top. Dev. Biol. 2013, 106, 1–47. [Google Scholar] [PubMed]
- Berger, P.E.; Harwood-Nash, D.C.; Fitz, C.R. The Dorsum Sellae in Infancy and Childhood. Pediat. Radiol. 1976, 4, 214–220. [Google Scholar] [CrossRef] [PubMed]
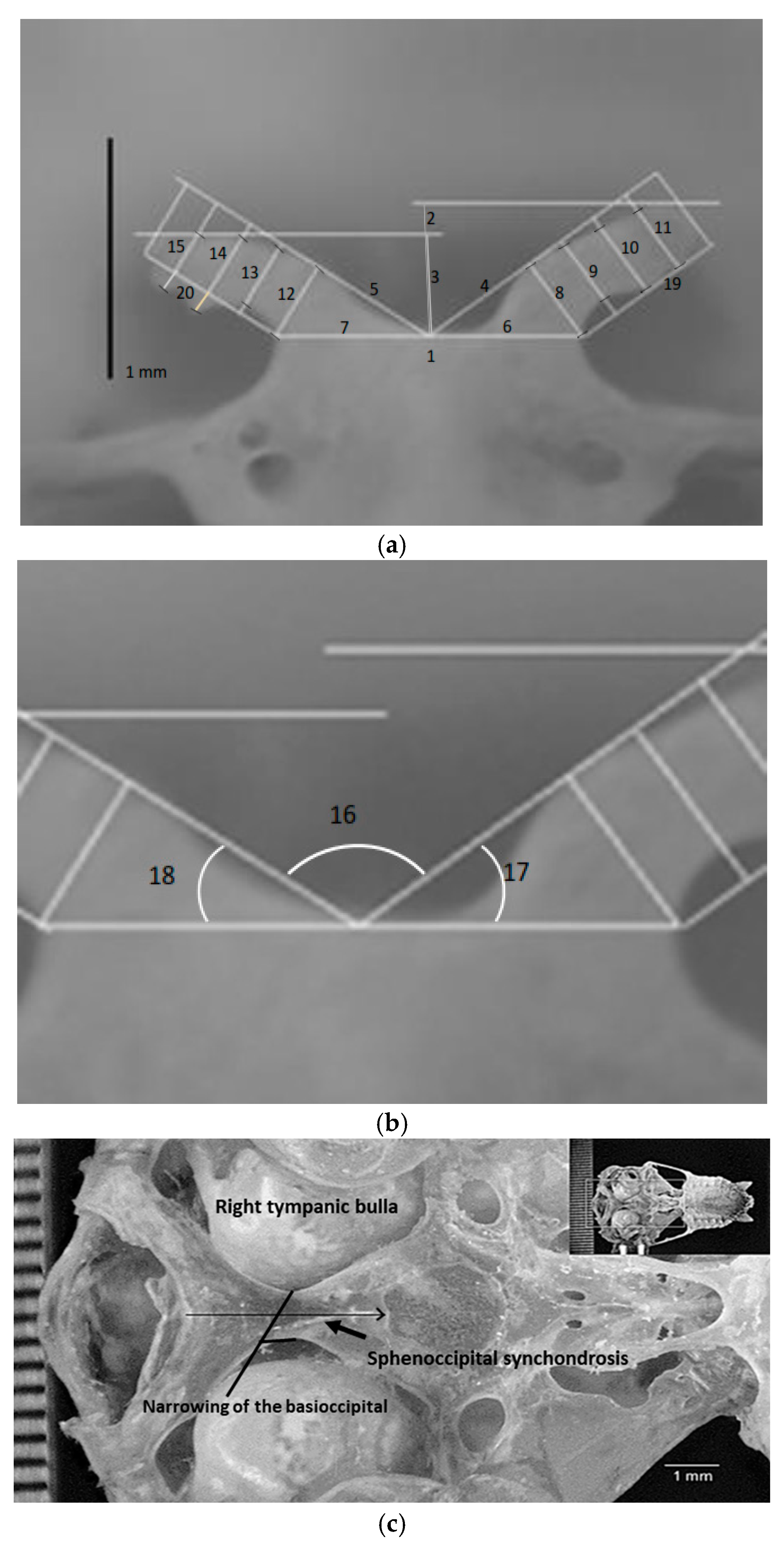
| No. | Description of Measurement |
|---|---|
| 1 | Width of the dorsum sellae; |
| 2 | Height of the right processus clinoideus caudalis; |
| 3 | Height of the left processus clinoideus caudalis; |
| 4 | Distance from the center of measurement 1 to the edge of the right processus clinoideus caudalis; the line must touch the edge of the clinoid process; |
| 5 | Distance from the center of the width of the dorsum sellae to the edge of the left processus clinoideus caudalis; |
| 6 | Width of the base of the right processus clinoideus caudalis; |
| 7 | Width of the base of the left processus clinoideus caudalis; |
| 8 | Thickness of the first quarter of the right processus clinoideus caudalis; |
| 9 | Thickness of the second quarter of the right processus clinoideus caudalis; |
| 10 | Thickness of the third quarter of the right processus clinoideus caudalis; |
| 11 | Thickness of the last quarter of the right processus clinoideus caudalis; |
| 12 | Thickness of the first quarter of the left processus clinoideus caudalis; |
| 13 | Thickness of the second quarter of the left processus clinoideus caudalis; |
| 14 | Thickness of the third quarter of the left processus clinoideus caudalis; |
| 15 | Thickness of the last quarter of the left processus clinoideus caudalis; |
| 16 | Angle between the lines of measurements 4 and 5; |
| 17 | Angle between the lines of measurements 4 and 6; |
| 18 | Angle between measurement lines 5 and 7; |
| 19 | Length A of the proper clinoid process from the base of the proper clinoid process; |
| 20 | Length A of the left clinoid process from the base of the left clinoid process. |
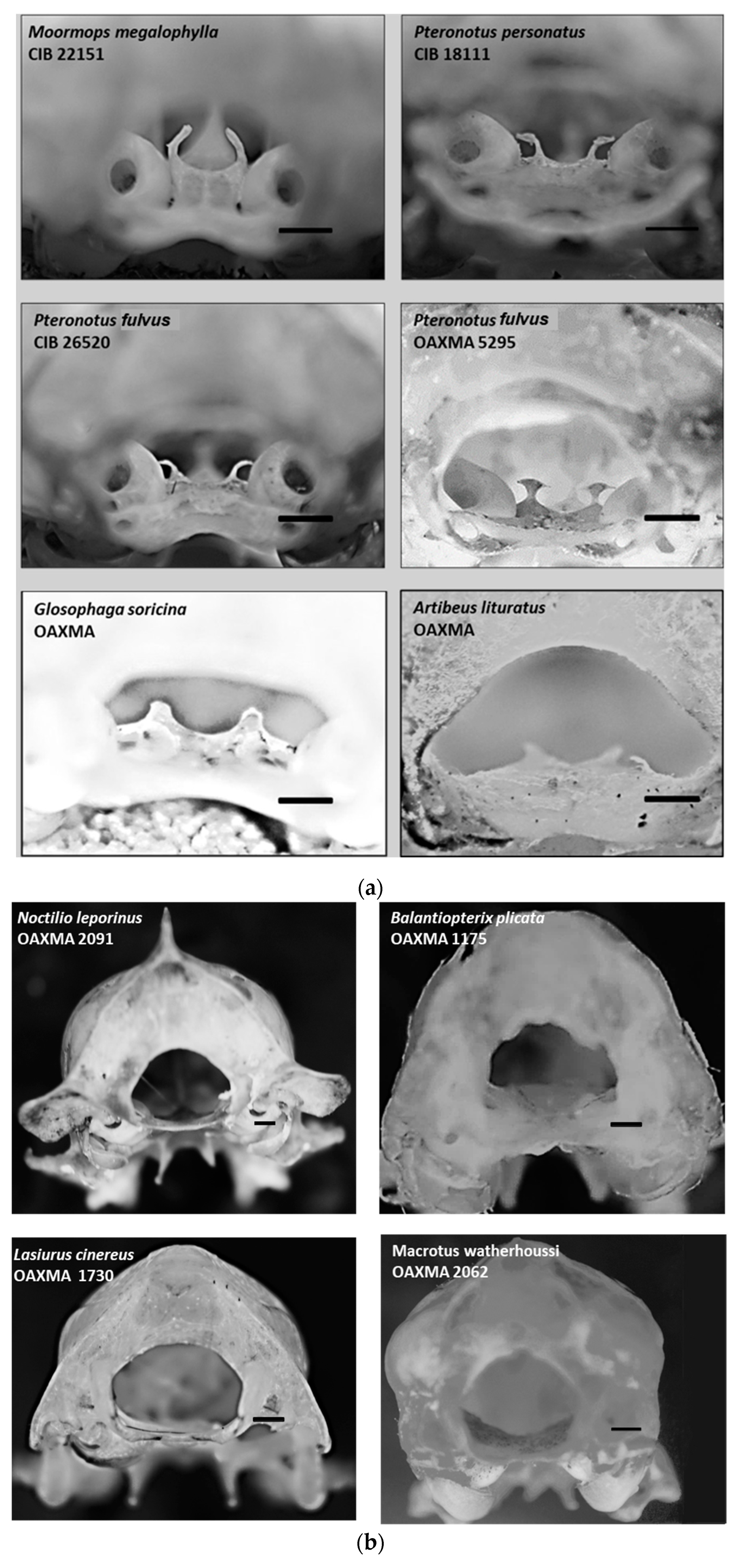
| Species | Presence | Species | Presence |
|---|---|---|---|
| Pteronotus fulvus | P | Rhogeesa parvula | N |
| Pteronotus psilotis | P | Lasiurus frantzii | N |
| Mormoops megalophylla | P | Aeroestes cinereus | N |
| Macrotus waterhousii | N | Plecotus mexicanus | N |
| Balantiopterix plicata | N | Corynorhinus townsendii | N |
| Noctilio leporinus | N | Eptesicus fuscus | N |
| Tadarida brasiliensis | N | Myotis californicus | N |
| Molossus rufus | N | Myotis keysi | N |
| Nyctinomops macrotis | N | Myotis thysanodes | N |
| Promops centralis | N | Myotis vellifer | N |
| Molossus molosus | N | Myotis nigricans | N |
| Rhogeesa alleni | N | Glossophaga soricina | P |
| Artibeus lituratus | P |
| Measure | Average | Minimum Value | Maximum Value | Standard Deviation | Variance | Significance |
|---|---|---|---|---|---|---|
| 1 | 1.32 | 0.48 | 1.82 | 0.15 | 0.02 | 0.03 |
| 2 | 0.42 | 0.11 | 1.006 | 0.12 | 0.015 | 0.01 |
| 3 | 0.44 | 0.12 | 0.96 | 0.12 | 0.01 | 0.01 |
| 4 | 1.07 | 0.47 | 1.52 | 0.12 | 0.01 | 0.01 |
| 5 | 1.07 | 0.46 | 1.34 | 0.13 | 0.01 | 0.01 |
| 6 | 0.66 | 0.30 | 0.95 | 0.07 | 0.005 | |
| 7 | 0.66 | 0.30 | 0.89 | 0.07 | 0.005 | 0.01 |
| 8 | 0.29 | 0.07 | 0.5 | 0.07 | 0.005 | |
| 9 | 0.21 | 0.08 | 0.39 | 0.04 | 0.002 | |
| 10 | 0.21 | 0.07 | 0.38 | 0.05 | 0.003 | |
| 11 | 0.20 | 0.09 | 0.37 | 0.05 | 0.003 | 0.01 |
| 12 | 0.29 | 0 | 0.65 | 0.07 | 0.006 | 0.00 |
| 13 | 0.21 | 0 | 0.34 | 0.05 | 0.003 | |
| 14 | 0.21 | 0.004 | 0.44 | 0.06 | 0.004 | |
| 15 | 0.20 | 0.004 | 0.47 | 0.07 | 0.004 | 0.004 |
| 16 | 119.04 | 26.96 | 180 | 15.10 | 228.01 | |
| 17 | 29.86 | 12.13 | 50.66 | 7.17 | 51.53 | |
| 18 | 30.92 | 0.31 | 61.98 | 7.33 | 53.80 | |
| 19 | 0.78 | 0.33 | 1.22 | 0.12 | 0.01 | 0.02 |
| 20 | 0.79 | 0.31 | 1.08 | 0.12 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peralta-Pérez, M.A.; Briones-Salas, M. Evidence of Morphological and Morphometric Differences in the Sella Turcica of Pteronotus mesoamericanus and P. mexicanus. Animals 2025, 15, 519. https://doi.org/10.3390/ani15040519
Peralta-Pérez MA, Briones-Salas M. Evidence of Morphological and Morphometric Differences in the Sella Turcica of Pteronotus mesoamericanus and P. mexicanus. Animals. 2025; 15(4):519. https://doi.org/10.3390/ani15040519
Chicago/Turabian StylePeralta-Pérez, M. A., and M. Briones-Salas. 2025. "Evidence of Morphological and Morphometric Differences in the Sella Turcica of Pteronotus mesoamericanus and P. mexicanus" Animals 15, no. 4: 519. https://doi.org/10.3390/ani15040519
APA StylePeralta-Pérez, M. A., & Briones-Salas, M. (2025). Evidence of Morphological and Morphometric Differences in the Sella Turcica of Pteronotus mesoamericanus and P. mexicanus. Animals, 15(4), 519. https://doi.org/10.3390/ani15040519






