Environmental Exposure to the Common Trunk of Mammalian Appeasing Pheromone Modulates Social Behavior and Reduces Fight Wounds in Male Mice
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
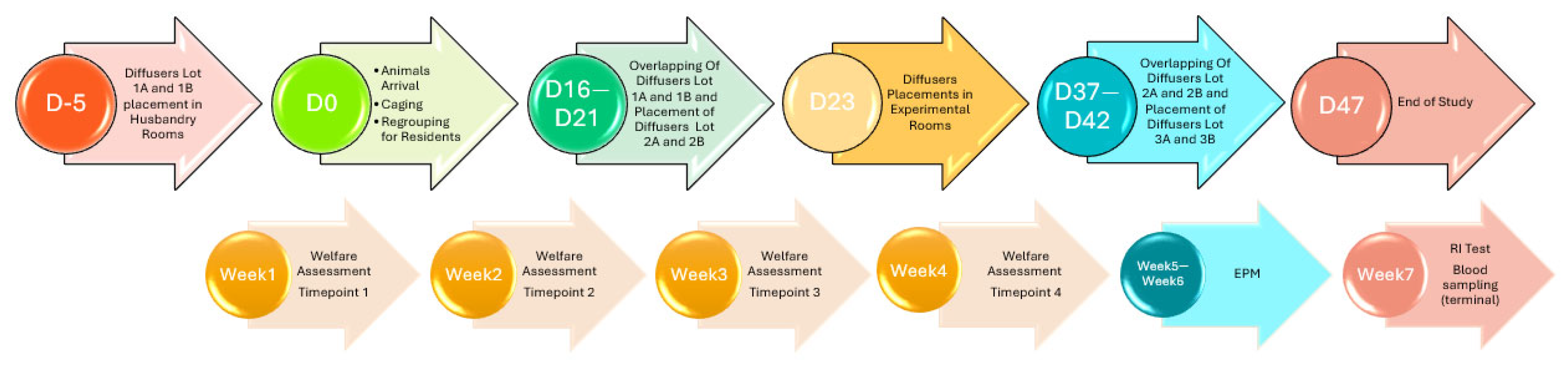
2.2. Treatment, Groups, and Timeline
2.3. Video Analysis
2.4. Welfare Assessment
2.5. Elevated Plus Maze (EPM) Test
2.6. Resident–Intruder (RI) Test
2.7. Hematobiochemical Analysis
2.8. Inclusion, Exclusion and Endpoint Criteria
2.9. Statistical Analysis
3. Results
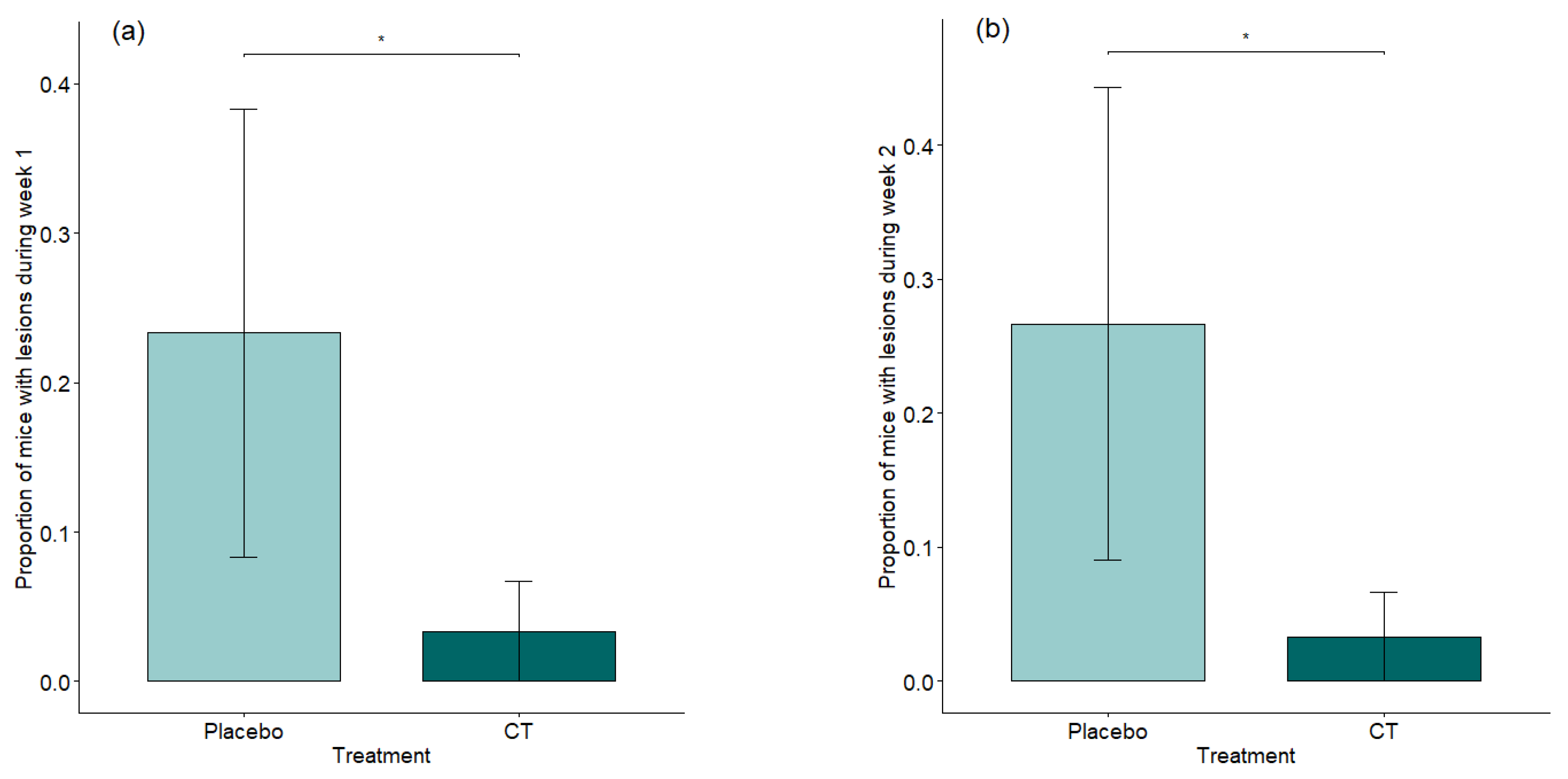
3.1. Welfare Assessment
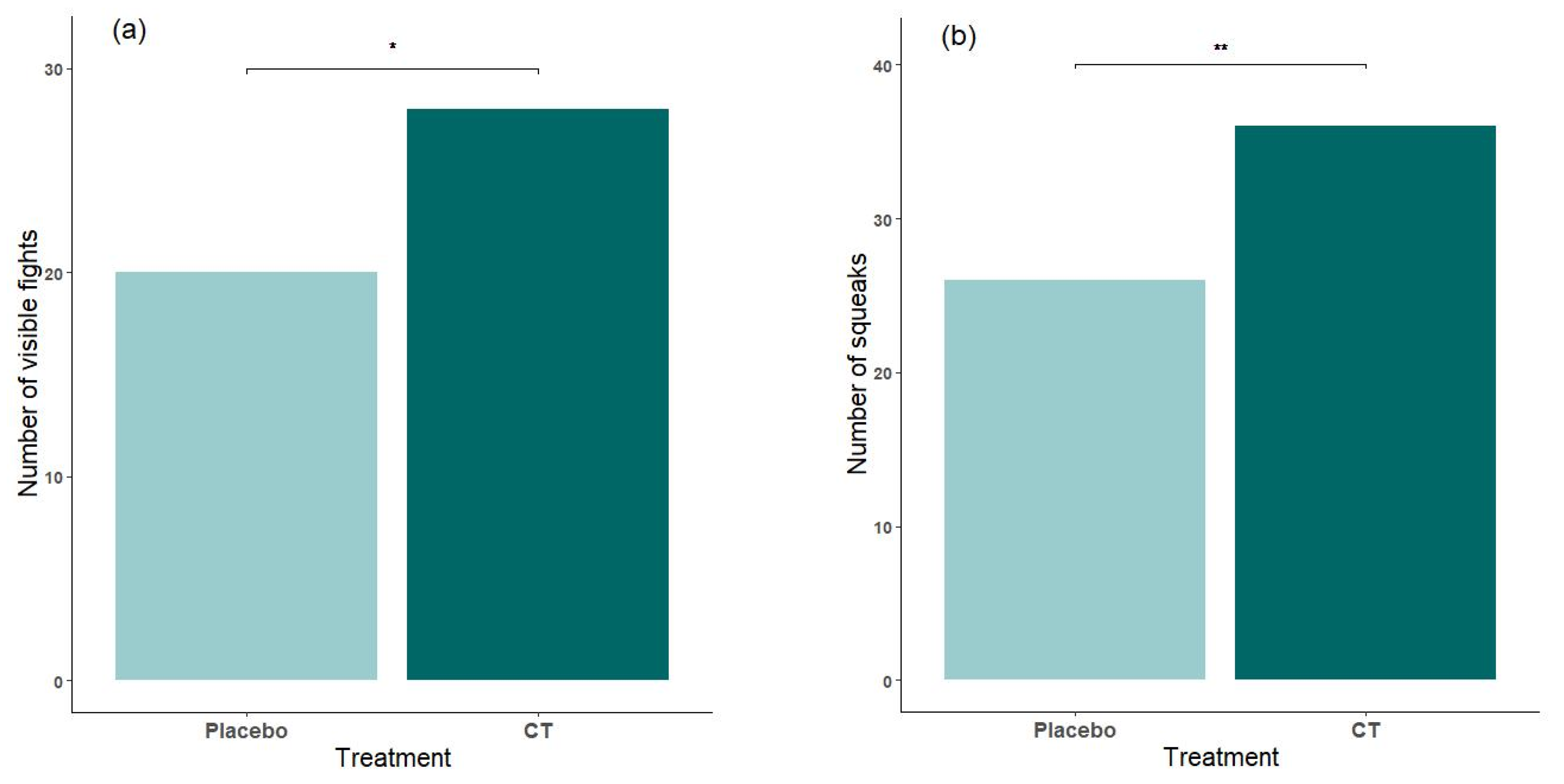
3.2. Effect of Treatment on Regrouping
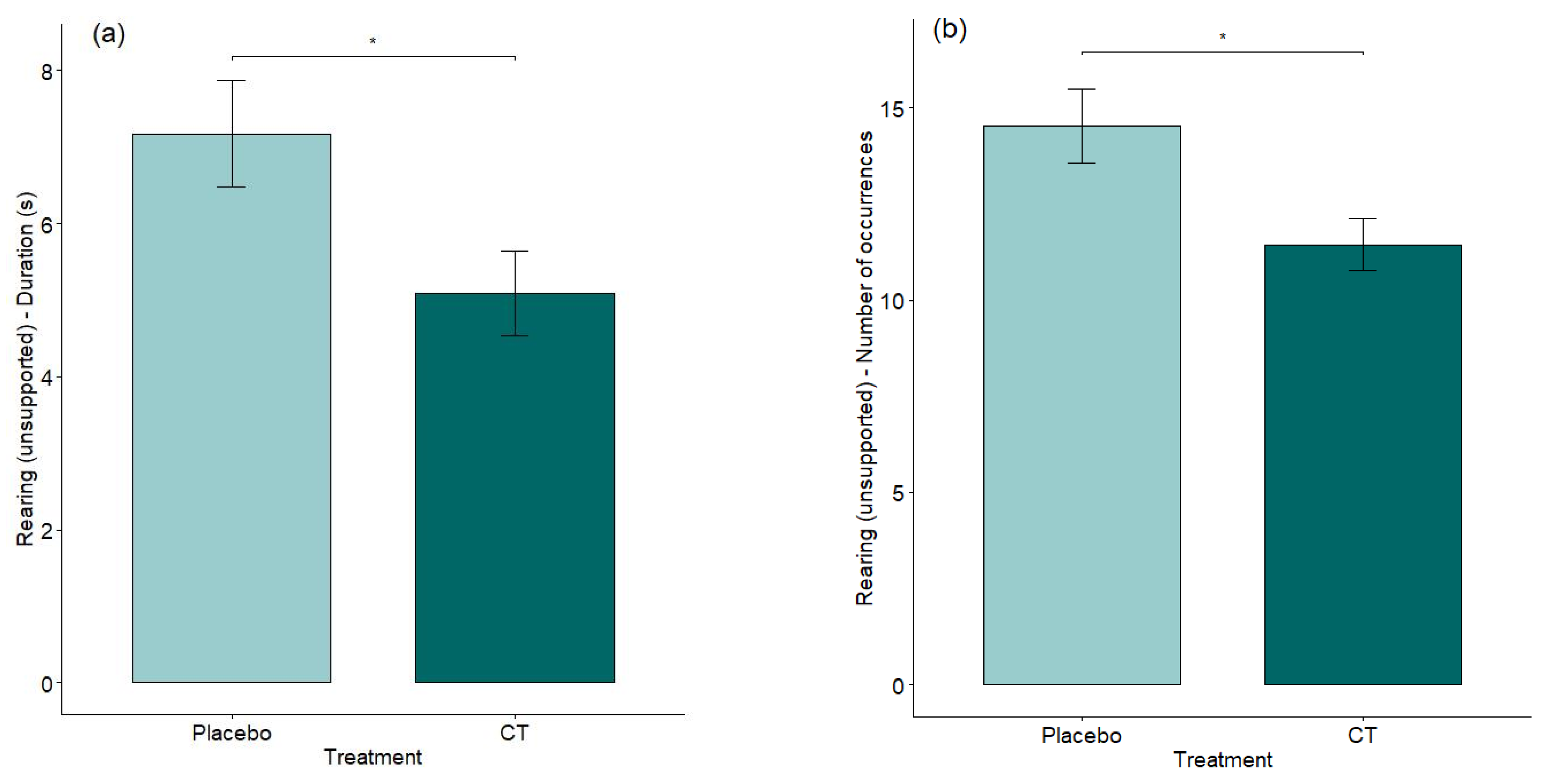
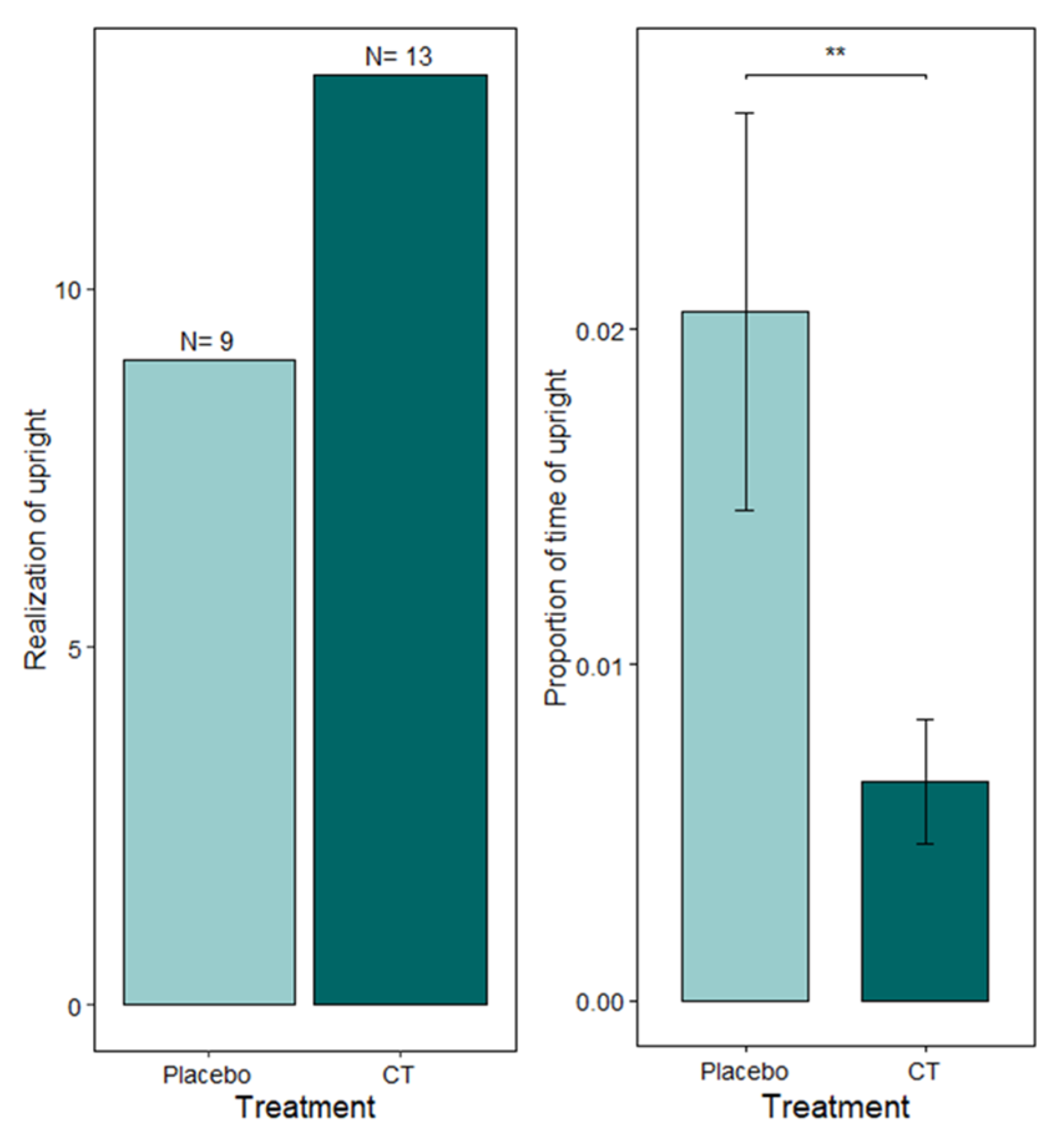
3.3. EPM and RI Test
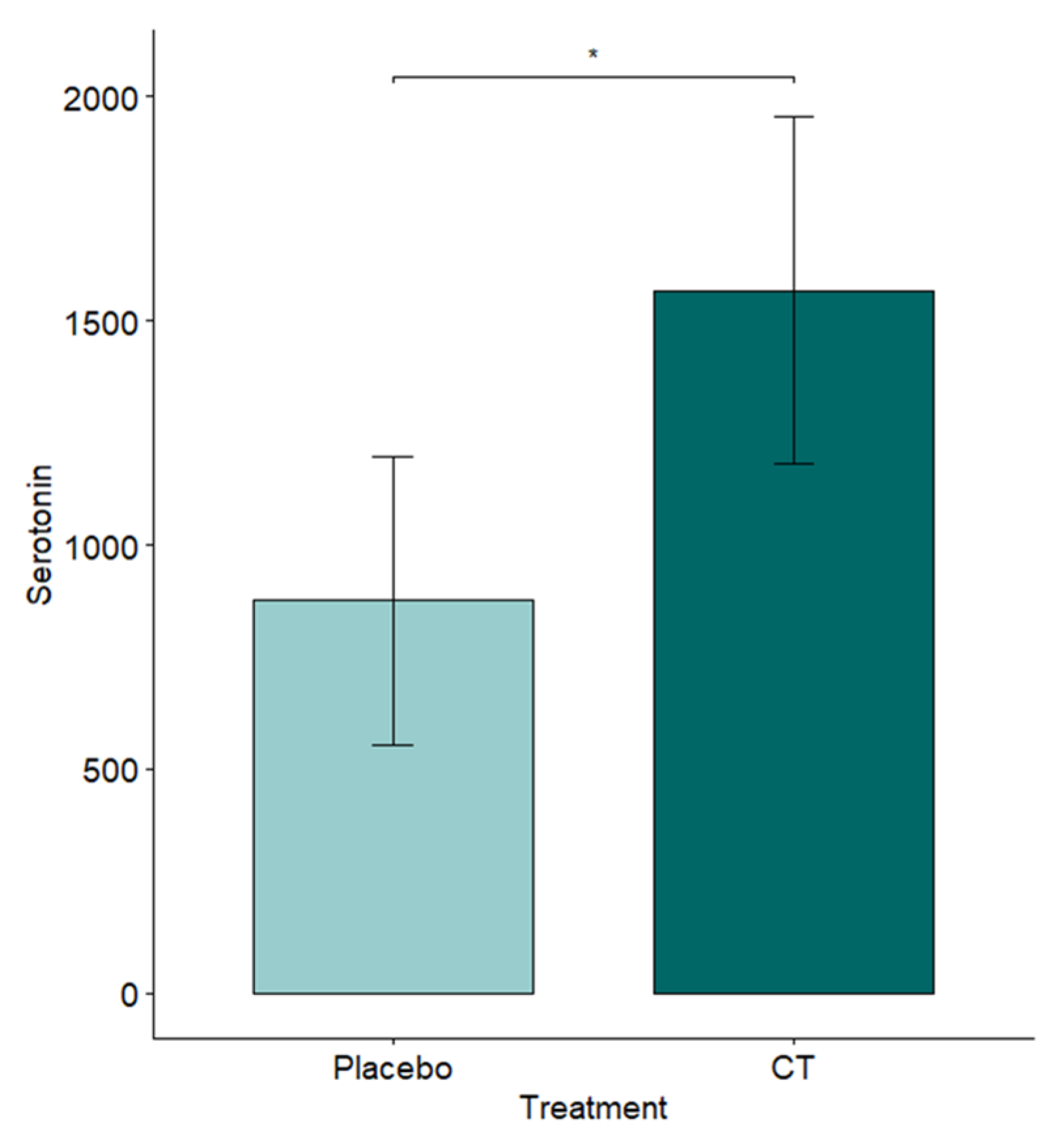
3.4. Hematobiochemistry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rault, J.-L. Be kind to others: Prosocial behaviours and their implications for animal welfare. Appl. Anim. Behav. Sci. 2019, 210, 113–123. [Google Scholar] [CrossRef]
- Wispé, L.G. Positive forms of social behavior: An overview. J. Soc. News. 1972, 28, 1–19. [Google Scholar] [CrossRef]
- Boissy, A.; Manteuffel, G.; Jensen, M.B.; Moe, R.O.; Spruijt, B.; Keeling, L.J.; Winckler, C.; Forkman, B.; Dimitrov, I.; Langbein, J.; et al. Assessment of positive emotions in animals to improve their welfare. Physiol. Behav. 2007, 92, 375–397. [Google Scholar] [CrossRef]
- DePorter, T.L.; Bledsoe, D.L.; Beck, A.; Ollivier, E. Evaluation of the efficacy of an appeasing pheromone diffuser product vs placebo for management of feline aggression in multi-cat households: A pilot study. J. Feline Med. Surg. 2019, 21, 293–305. [Google Scholar] [CrossRef]
- Pageat, P.; Gaultier, E. Current Research in Canine and Feline Pheromones. Vet. Clin. N. Am. Small Anim. Pract. 2003, 33, 187–211. [Google Scholar] [CrossRef]
- Asproni, P.; Codecasa, E.; Marcet-Rius, M.; Demellier, J.; Descout, E.; Verbaere, M.; Vinck, O.; Pageat, P.; Cozzi, A. Helping Rabbits Cope with Veterinary Acts and Vaccine-Related Stress: The Effects of the Rabbit Appeasing Pheromone (RAP). Animals 2024, 14, 3549. [Google Scholar] [CrossRef]
- Marcet-Rius, M.; Mendonça, T.; Pageat, P.; Arroub, S.; Bienboire-Frosini, C.; Chabaud, C.; Teruel, E.; Cozzi, A. Effect of wither application of an analogue of pig appeasing pheromone on encounters between unfamiliar mini-pigs. Porc. Health Manag. 2022, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Piccinni, A.; Veltri, A.; Marazziti, D.; Mucci, F.; Cozzi, A.; Pageat, P. Human Appeasing Pheromone (HAP) influence on behavior and psychopathological residual symptoms of patients with complex psychiatric disorders. Clin. Case Rep. 2018, 6, 664–668. [Google Scholar] [CrossRef]
- Kiyokawa, Y.; Tamogami, S.; Ootaki, M.; Kahl, E.; Mayer, D.; Fendt, M.; Nagaoka, S.; Tanikawa, T.; Takeuchi, Y. An appeasing pheromone ameliorates fear responses in the brown rat (Rattus norvegicus). iScience 2023, 26, 107081. [Google Scholar] [CrossRef]
- Commission Staff Working Document Summary Report on the Statistics on the Use of Animals for Scientific Purposes in the Member States of the European Union and Norway in 2022; European Commission: Brussels, Belgium, 2024; Available online: https://data.consilium.europa.eu/doc/document/ST-12496-2024-INIT/en/pdf (accessed on 22 May 2025).
- Kappel, S.; Hawkins, P.; Mendl, M.T. To Group or Not to Group? Good Practice for Housing Male Laboratory Mice. Animals 2017, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Theil, J.H.; Ahloy-Dallaire, J.; Weber, E.M.; Gaskill, B.N.; Pritchett-Corning, K.R.; Felt, S.A.; Garner, J.P. The epidemiology of fighting in group-housed laboratory mice. Sci. Rep. 2020, 10, 16649. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.M.; Zidar, J.; Ewaldsson, B.; Askevik, K.; Udén, E.; Svensk, E.; Törnqvist, E. Aggression in Group-Housed Male Mice: A Systematic Review. Animals 2023, 13, 143. [Google Scholar] [CrossRef]
- Riddell, P.; Paris, M.C.J.; Joonè, C.J.; Pageat, P.; Paris, D.B.B.P. Appeasing Pheromones for the Management of Stress and Aggression during Conservation of Wild Canids: Could the Solution Be Right under Our Nose? Animals 2021, 11, 1574. [Google Scholar] [CrossRef]
- Pageat, P. Animal Appeasing Pheromones. U.S. Patent 6384252B1, 7 May 2025. [Google Scholar]
- Lezak, K.R.; Missig, G.; Carlezon, W.A., Jr. Behavioral methods to study anxiety in rodents. Dialogues Clin. Neurosci. 2017, 19, 181–191. [Google Scholar] [CrossRef]
- Garner, J.P. The Mouse Ethogram: Investigating Stretched Attend. Available online: https://med.stanford.edu/mousebehavior/ethogram/active-behaviors/general-activity/exploratory-behavior/investigate/stretched-attend.html (accessed on 22 May 2025).
- Ducottet, C.; Belzun. Correlations between behaviours in the elevated plus-maze and sensitivity to unpredictable subchronic mild stress: Evidence from inbred strains of mice. Behav. Brain Res. 2005, 156, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Espejo, E. Structure of the mouse behaviour on the elevated plus-maze test of anxiety. Behav. Brain Res. 1997, 86, 105–112. [Google Scholar] [CrossRef]
- Zalaquett, C.; Thiessen, D. The effects of odors from stressed mice on conspecific behavior. Physiol. Behav. 1991, 50, 221–227. [Google Scholar] [CrossRef]
- Sturman, O.; Germain, P.L.; Bohacek, J. Exploratory rearing: A context- and stress-sensitive behavior recorded in the open-field test. Stress 2018, 21, 443–452. [Google Scholar] [CrossRef]
- Berridge, K.C.; Aldridge, J.W.; Houchard, K.R.; Zhuang, X. Sequential super-stereotypy of an instinctive fixed action pattern in hyper-dopaminergic mutant mice: A model of obsessive compulsive disorder and Tourette’s. BMC Biol. 2005, 3, 4. [Google Scholar] [CrossRef]
- Koolhaas, J.M.; Coppens, C.M.; de Boer, S.F.; Buwalda, B.; Meerlo, P.; Timmermans, P.J. The resident-intruder paradigm: A standardized test for aggression, violence and social stress. J. Vis. Exp. JoVE 2013, 77, e4367. [Google Scholar] [CrossRef]
- Lumley, L.A.; Charles, R.F.; Charles, R.C.; Hebert, M.A.; Morton, D.M.; Meyerhoff, J.L. Effects of social defeat and of diazepam on behavior in a resident-intruder test in male DBA/2 mice. Pharmacol. Biochem. Behav. 2000, 67, 433–447. [Google Scholar] [CrossRef]
- Kwiatkowski, C.C.; Akaeze, H.; Ndlebe, I.; Goodwin, N.; Eagle, A.L.; Moon, K.; Bender, A.R.; Golden, S.A.; Robison, A.J. Quantitative standardization of resident mouse behavior for studies of aggression and social defeat. Neuropsychopharmacology 2021, 46, 1584–1593. [Google Scholar] [CrossRef]
- Bartolomucci, A.; Palanza, P.; Parmigiani, S. Group housed mice: Are they really stressed? Ethol. Ecol. Evol. 2002, 14, 341–350. [Google Scholar] [CrossRef]
- Banks, E.M. A Time and Motion Study of Prefighting Behavior in Mice. J. Genet. Psychol. 1962, 101, 165–183. [Google Scholar] [CrossRef]
- Miczek, K.A.; Maxson, S.C.; Fish, E.W.; Faccidomo, S. Aggressive Behavioral Phenotypes in Mice. Behav. Brain Res. 2001, 125, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Brain, P.; Poole, A. Some studies on the use of “standard opponents” in intermale aggression testing in TT albino mice. Behaviour 1974, 50, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.-H.; Wang, W.; Wang, Z.-S.; Zhang, J.-X. The Common Fatty Acids Have a Pheromone-like Effect, Improving the Emotion in Mice. Res. Sq. Prepr. 2022. [Google Scholar] [CrossRef]
- Lever, C.; Burton, S.; O’Keefe, J. Rearing on hind legs, environmental novelty, and the hippocampal formation. Rev. Neurosci. 2006, 17, 111–133. [Google Scholar] [CrossRef]
- Komada, M.; Takao, K.; Miyakawa, T. Elevated Plus Maze for Mice. J. Vis. Exp. 2008, 22, e1088. [Google Scholar] [CrossRef]
- Fink, G. Stress, definition mechanisms and effects outlined: Lessons from anxiety. In Stress: Concepts, Cognition, Emotion, and Behavior: Handbook of Stress; Fink, G., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 3–9. [Google Scholar]
- Grant, E.C.; Mackintosh, J.H. A comparison of the social postures of some common laboratory rodents. Behaviour 1963, 21, 246–259. [Google Scholar] [CrossRef]
- Kimbrell, G.M. Relationship of the upright agonistic posture in the foot shock situation to dominance-submission in male C57BL/6 mice. Psychon. Sci. 1969, 16, 167–168. [Google Scholar] [CrossRef]
- Chasles, M.; Marcet-Rius, M.; Chou, J.Y.; Teruel, E.; Pageat, P.; Cozzi, A. Cutaneous application of SecurePig® FLASH, a Pig appeasing pheromone analogue, facilitates adaptation and manages social behavior during feeding in semi-extensive conditions. Porc. Health Manag. 2024, 10, 13. [Google Scholar] [CrossRef]
- Guy, J.; Burns, S.; Barker, J.; Edwards, S. Reducing post-mixing aggression and skin lesions in weaned pigs by application of a synthetic maternal pheromone. Anim. Welf. 2009, 18, 249–255. [Google Scholar] [CrossRef]
- Weber, E.M.; Dallaire, J.A.; Gaskill, B.N.; Pritchett-Corning, K.R.; Garner, J.P. Aggression in group-housed laboratory mice: Why can’t we solve the problem? Lab. Anim. 2017, 46, 157–161. [Google Scholar] [CrossRef]
- Caramaschi, D.; de Boer, S.F.; Koolhaas, J.M. Differential role of the 5-HT1A receptor in aggressive and non-aggressive mice: An across-strain comparison. Physiol. Behav. 2007, 90, 590–601. [Google Scholar] [CrossRef]
- Mosienko, V.; Bert, B.; Beis, D.; Matthes, S.; Fink, H.; Bader, M.; Alenina, N. Exaggerated Aggression and Decreased Anxiety in Mice Deficient in Brain Serotonin. Transl. Psychiatry 2012, 2, e122. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, P.F.; Palanza, P.; Parmigiani, S.; de Almeida, R.M.; Miczek, K.A. Serotonin and aggressive behavior in rodents and nonhuman primates: Predispositions and plasticity. Eur. J. Pharmacol. 2005, 526, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Ursinus, W.W.; Van Reenen, C.G.; Reimert, I.; Bolhuis, J.E. Tail biting in pigs: Blood serotonin and fearfulness as pieces of the puzzle? PloS ONE 2014, 9, e107040. [Google Scholar] [CrossRef] [PubMed]
- León, M.; Rosado, B.; García-Belenguer, S.; Chacón, G.; Villegas, A.; Palacio, J. Assessment of serotonin in serum, plasma, and platelets of aggressive dogs. J. Vet. Behav. 2012, 7, 348–352. [Google Scholar] [CrossRef]
- Cozzi, A.; Sighieri, C.; Gazzano, A.; Nicol, C.J.; Baragli, P. Post-conflict friendly reunion in a permanent group of horses (Equus caballus). Behav. Process. 2010, 85, 185–190. [Google Scholar] [CrossRef]
- Bertoni, V.; Spiezio, C.; Regaiolli, B.; Cozzi, A.; Valsecchi, P.; Normando, S. Group Reunion in Zoo European Wildcats Using Cat Appeasing Pheromone (CAP) and Gradual Release of the Animals in the Exhibit—A Case Study. Animals 2022, 12, 1302. [Google Scholar] [CrossRef] [PubMed]
- Åhlgren, J.; Voikar, V. Housing mice in the individually ventilated or open cages-Does it matter for behavioral phenotype? Genes Brain Behav. 2019, 18, e12564. [Google Scholar] [CrossRef]
- Logge, W.; Kingham, J.; Karl, T. Behavioural consequences of IVC cages on male and female C57BL/6J mice. Neuroscience 2013, 237, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Suckow, M.A.; Tirado-Muñiz, N. Seasonal Variation of Laboratory Animals as a Consideration for Research Reproducibility. Compar. Med. 2023, 73, 255–259. [Google Scholar] [CrossRef]
- Meyer, L.; Caston, J.; Mensah-Nyagan, A. Seasonal Variation of the Impact of a Stressful Procedure on Open Field Behaviour and Blood Corticosterone in Laboratory Mice. Behav. Brain Res. 2006, 167, 342–348. [Google Scholar] [CrossRef]
- Van Loo, P.L.; Van Zutphen, L.F.; Baumans, V. Male management: Coping with aggression problems in male laboratory mice. Lab. Anim. 2003, 37, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Haug, M.; Mandel, P. Sex differences in aggressive behaviour in various strains of mice. Aggress. Behav. 1978, 4, 353–364. [Google Scholar] [CrossRef]
- Poole, T.B.; Morgan, H.D. Aggressive behaviour of male mice (Mus musculus) towards familiar and unfamiliar opponents. Anim. Behav. 1975, 23, 470–479. [Google Scholar] [CrossRef]
- Parmigiani, S.; Brain, P.F.; Mainardi, D.; Brunoni, V. Different patterns of biting attack employed by lactating female mice (Mus domesticus) in encounters with male and female conspecific intruders. J. Comp. Psychol. 1988, 102, 287–293. [Google Scholar] [CrossRef]
- Aubry, A.V.; Joseph Burnett, C.; Goodwin, N.L.; Li, L.; Navarrete, J.; Zhang, Y.; Tsai, V.; Durand-de Cuttoli, R.; Golden, S.A.; Russo, S.J. Sex differences in appetitive and reactive aggression. Neuropsychopharmacology 2022, 47, 1746–1754. [Google Scholar] [CrossRef]
- Oizumi, H.; Kuriyama, N.; Imamura, S.; Tabuchi, M.; Omiya, Y.; Mizoguchi, K.; Kobayashi, H. Influence of Aging on the Behavioral Phenotypes of C57BL/6J Mice After Social Defeat. PLoS ONE 2019, 14, e0222076. [Google Scholar] [CrossRef]
- Morel, C.; Parise, L.F.; Van der Zee, Y.Y.; Issler, O.; Cai, M.; Browne, C.J.; Blando, A.; LeClair, K.B.; Aubry, A.V.; Haynes, S.; et al. Male and Female Behavioral Variability and Morphine Response in C57BL/6J, DBA/2J, and Their BXD Progeny Following Chronic Stress Exposure. Sci. Rep. 2024, 14, 30785. [Google Scholar] [CrossRef] [PubMed]
- Dow, H.C.; Kreibich, A.S.; Kaercher, K.A.; Sankoorikal, G.M.; Pauley, E.D.; Lohoff, F.W.; Ferraro, T.N.; Li, H.; Brodkin, E.S. Genetic Dissection of Intermale Aggressive Behavior in BALB/cJ and A/J Mice. Genes Brain Behav. 2011, 10, 57–68. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rutsch, A.; Iachizzi, M.; Kirundi, J.; Kantsjö, J.B.; Planchette, A.L.; Müller, T.; Schmitz, W.; Radenovic, A.; Doenlen, R.; de Agüero, M.G.; et al. Assessment of Neurobehavioural Traits under Gnotobiotic Conditions: An Approach for Multiple Analyses in the Same Mouse. Brain Behav. Immun. 2025, 130, 106084. [Google Scholar] [CrossRef] [PubMed]
- Uzan-Yulzari, A.; Turjeman, S.; Moadi, L.; Getselter, D.; Sharon, E.; Rautava, S.; Isolauri, E.; Khatib, S.; Elliott, E.; Koren, O. A Gut Reaction? The Role of the Microbiome in Aggression. Brain Behav. Immun. 2024, 122, 301–312. [Google Scholar] [CrossRef]

| Category | Behavior | Measured As | Description/Notes |
|---|---|---|---|
| Space use | Entries in maze areas | Occurrence | Number of entries into Open arms (O), Closed arms (C), and Platform (P); counted when hind paws cross the zone. |
| Time in maze areas | Duration (s) | Time spent in Open arms (O), Closed arms (C), and Platform (P). | |
| Latency to enter the open arm | Latency (s) | Time before first entry into an open arm; 600 s noted if never entered. | |
| Stress indicators | Stretched attend posture, unprotected and protected | Occurrence and duration | Investigatory posture with head extended. uSAP = in open arms, pSAP = in closed arms/platform. |
| Head dipping | Occurrence | Mouse dips its head over the maze edges. uDip = in open arms, pDip = in closed arms/platform. | |
| Rearing | Occurrence and duration | Mouse stands on hindlegs. sR = supported by wall, uR = unsupported. | |
| Grooming | Occurrence and duration | Mouse licks, scratches, or rubs fur, often in a sitting position and a mix of grooming actions. |
| Category | Behavior | Measured As | Description/Notes |
|---|---|---|---|
| Social investigation | Social investigation | Occurrence and duration | Sniffing/touching the opponent’s body, including the anogenital area. |
| Agonistic behavior | Attack | Binary, latency, occurrence and duration | Latency to first attack (300 s if absent); forward motion with contact. |
| Under attack | Occurrence and duration | The resident being attacked by the intruder. | |
| Offensive upright posture | Occurrence and duration | Stands on hindlimbs, pushes with forepaws, head pulled back | |
| Mounting | Occurrence and duration | Forelegs on opponent’s body; may include pelvic thrusting | |
| Threat | Occurrence | Lunging without contact or sideways rotation with piloerection | |
| Tail rattling | Occurrence | Rapid tail vibration, stiff posture; high arousal. | |
| Vocalizations | Squeaking | Occurrence | High-pitched vocalizations; may indicate submission. |
| Endpoint | Blood (lesion presence) | Binary | Presence of bleeding; test endpoint. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuochi, S.; Bienboire-Frosini, C.; Descout, E.; Marcet-Rius, M.; Pageat, P.; Cozzi, A. Environmental Exposure to the Common Trunk of Mammalian Appeasing Pheromone Modulates Social Behavior and Reduces Fight Wounds in Male Mice. Animals 2025, 15, 3278. https://doi.org/10.3390/ani15223278
Fuochi S, Bienboire-Frosini C, Descout E, Marcet-Rius M, Pageat P, Cozzi A. Environmental Exposure to the Common Trunk of Mammalian Appeasing Pheromone Modulates Social Behavior and Reduces Fight Wounds in Male Mice. Animals. 2025; 15(22):3278. https://doi.org/10.3390/ani15223278
Chicago/Turabian StyleFuochi, Sara, Cecile Bienboire-Frosini, Estelle Descout, Miriam Marcet-Rius, Patrick Pageat, and Alessandro Cozzi. 2025. "Environmental Exposure to the Common Trunk of Mammalian Appeasing Pheromone Modulates Social Behavior and Reduces Fight Wounds in Male Mice" Animals 15, no. 22: 3278. https://doi.org/10.3390/ani15223278
APA StyleFuochi, S., Bienboire-Frosini, C., Descout, E., Marcet-Rius, M., Pageat, P., & Cozzi, A. (2025). Environmental Exposure to the Common Trunk of Mammalian Appeasing Pheromone Modulates Social Behavior and Reduces Fight Wounds in Male Mice. Animals, 15(22), 3278. https://doi.org/10.3390/ani15223278







