Assessment of Habitat Suitability for Amphioxus in the Changli Marine Reserve and Adjacent Coastal Waters, Hebei Province
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Screening of Habitat Indicators
2.4. Modeling of Habitat Fitness Curve
2.5. Calculation of Habitat Suitability Index (HSI)
3. Results
3.1. Analysis of Amphioxus Abundance
3.2. Habitat Indicator Factor Analysis
3.3. Construction of Habitat Suitability Curves
3.4. Habitat Suitability Index (HSI) and Its Relationship with Amphioxus Abundance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, H.; Ma, M.; Liang, B.; Bao, C. Temporal and spatial dynamics of amphioxus population (Branchiostoma belcheri tsingtaneuse) and its influential factors in Luan River Estuary, China. Ecol. Evol. 2014, 4, 3027–3037. [Google Scholar] [CrossRef]
- Brasó-Vives, M.; Marlétaz, F.; Echchiki, A.; Mantica, F.; Acemel, R.D.; Gómez-Skarmeta, J.L.; Hartasánchez, D.A.; Le Targa, L.; Pontarotti, P.; Tena, J.J.; et al. Parallel evolution of amphioxus and vertebrate small-scale gene duplications. Genome Biol. 2022, 23, 243. [Google Scholar] [CrossRef]
- D’Aniello, S.; Bertrand, S.; Escriva, H. Amphioxus as a model to study the evolution of development in chordates. eLife 2023, 12, e87028. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Chen, J.; Chan, B.K.K.; Shao, K. The interplay of sediment characteristics, depth, water temperature, and ocean currents shaping the biogeography of lancelets (Subphylum Cephalochordata) in the NW Pacific waters. Mar. Ecol. 2015, 36, 780–793. [Google Scholar] [CrossRef]
- Yasui, K.; Igawa, T.; Kaji, T.; Henmi, Y. Stable Aquaculture of the Japanese Lancelet Branchiostoma japonicum for 7 Years. J. Exp. Zool. B Mol. Dev. Evol. 2013, 320, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Sakaki, K.; Samakura, H. Mass recruitment of the lancelet Branchiostoma japonicum at a sandbank in the western Seto Inland Sea in 2002 and subsequent age structure of the population. Plankton Benthos Res. 2024, 19, 17–25. [Google Scholar] [CrossRef]
- Maghsoudlou, A. Iranian Branchiostoma species (Cephalochordata, Branchiostomatidae) inhabiting Chabahar Bay (Gulf of Oman), with remarks on habitat preferences. Turk. J. Zool. 2018, 42, 179–186. [Google Scholar] [CrossRef]
- Desdevise, Y.; Maillet, V.; Fuentes, M.; Escriva, H. A snapshot of the population structure of Branchiostoma lanceolatum in the Racou Beach, France, during its spawning season. PLoS ONE 2011, 6, e18520. [Google Scholar] [CrossRef]
- Vergara, M.; Oliva, M.E.; Riascos, J.M. Population dynamics of the amphioxus Branchiostoma elongatum from northern Chile. J. Mar. Biol. Assoc. UK 2012, 92, 591–599. [Google Scholar] [CrossRef]
- Chen, Y.; Cheung, S.; Shin, P. A baseline study of benthic community associated with Amphioxus Sand in subtropical Hong Kong. Mar. Pollut. Bull. 2013, 72, 274–280. [Google Scholar] [CrossRef]
- Rao, Y.; Cai, L.; Li, W.; Chen, X.; Yang, D. Spatiotemporal variations of benthic macrofaunal community in the Xiamen Amphioxus Nature Reserve, eastern South China Sea. Acta Oceanol. Sin. 2020, 39, 10–18. [Google Scholar] [CrossRef]
- Dubois, S.; Gelpi, C.G.; Condrey, R.E.; Grippo, M.A.; Fleeger, J.W. Diversity and composition of macrobenthic community associated with sandy shoals of the Louisiana continental shelf. Biodivers. Conserv. 2009, 18, 3759–3784. [Google Scholar] [CrossRef]
- Saito, H.; Mimura, K.; Kawai, K.; lmabayashi, H. Temporal and spatial dynamics of a lower-intertidal lancelet population in the Seto Inland Sea, Japan. Zool. Sci. 2009, 26, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Campos-Dávila, L.; Pérez-Estrada, C.J.; Rodríguez-Estrella, R.; Morales-Bojórquez, E.; Brun-Murillo, F.G.; Balart, E.F. Seagrass Halodule wrightii as a new habitat for the amphioxus Branchiostoma californiense (Cephalochordata, Branchiostomidae) in the southern Gulf of California, Mexico. ZooKeys 2019, 873, 113–131. [Google Scholar] [CrossRef]
- Nielsen, S.E.; Bone, Q.; Bond, P.; Harper, G. On particle filtration by amphioxus (Branchiostoma lanceolatum). J. Mar. Biol. Assoc. UK 2007, 87, 983–989. [Google Scholar] [CrossRef]
- Zhang, Q.-L.; Zhang, G.-L.; Xiong, Y.; Li, H.-W.; Guo, J.; Wang, F.; Deng, X.-Y.; Chen, J.-Y.; Wang, Y.-J.; Lin, L.-B. Genome-wide gene expression analysis reveals novel insights into the response to nitrite stress in gills of Branchiostoma belcheri. Chemosphere 2019, 218, 609–615. [Google Scholar] [CrossRef]
- Au, M.F.F.; Au, H.M.; Chu, W.K.V.; Kwok, C.K.; Cheung, S.G.; Leung, K.M.Y.; Qiu, J.-W. Spatial distribution, abundance, seasonality and environmental relationship of amphioxus in subtropical Hong Kong waters. Reg. Stud. Mar. Sci. 2023, 57, 102726. [Google Scholar] [CrossRef]
- Weng, Z.; Xie, Y.; Fang, Q.; Li, J.; Liu, J.; Wang, J.; Huang, L.; Qu, T.; Xie, W. Current population, habitat status and species of amphioxus in Dongshan Bay, Fujian Province. J. Ocean Univ. China 2025, 24, 417–426. [Google Scholar] [CrossRef]
- Rota, E.; Perra, G.; Focardi, S. The European lancelet Branchiostoma lanceolatum (Pallas) as an indicator of environmental quality of Tuscan Archipelago (Western Mediterranean Sea). Chem. Ecol. 2009, 25, 61–69. [Google Scholar] [CrossRef]
- Rodríguez-Uribe, M.C.; Chávez-Dagostino, R.M.; Del Moral-Flores, L.F.; Bravo-Olivas, M.L. First record of amphioxus Branchiostoma californiense (Amphioxiformes: Branchiostomatidae) adjacent to a shallow submarine hydrothermal system at Banderas Bay (Mexico). Diversity 2019, 11, 227. [Google Scholar] [CrossRef]
- Renzi, M.; Blašković, A.; Fastelli, P.; Marcelli, M.; Guerranti, C.; Cannas, S.; Barone, L.; Massara, F. Is the microplastic selective according to the habitat? Records in amphioxus sands, Mäerl bed habitats and Cymodocea nodosa habitats. Mar. Pollut. Bull. 2018, 130, 179–183. [Google Scholar] [CrossRef] [PubMed]
- GB 17378.7-2007; The Specification for Marine Monitoring—Part 7: Ecological Survey for Offshore Pollution and Biological Monitoring. General Administration of Quality Supervision. Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2007.
- Li, F.; Cai, Q.; Fu, X.; Liu, J. Construction of habitat suitability models (HSMs) for benthic macroinvertebrate and their applications to instream environmental flows: A case study in Xiangxi River of Three Gorges Reservoir region, China. Prog. Nat. Sci. 2008, 18, 1417–1427. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, T.; Zhuang, P.; Hou, J.-L.; Wang, Y.; Song, C.; Zhang, L.-Z. Preliminary assessment of habitat of juvenile Collichthys lucidus in the Yangtze estuary. Chin. J. Appl. Ecol. 2014, 25, 2418–2424. [Google Scholar]
- Saaty, T.L. The Analytic Hierarchy Process: Planning, Priority Setting, Resource Allocation; McGraw-Hill: New York, NY, USA, 1980. [Google Scholar]
- Wu, H.-Y.; Fu, S.-F.; Wu, J.; Cai, X.-Q.; Chen, Q.-H. Spatiotemporal variation of benthic biodiversity under persistent and extreme human disturbances in the Xiamen sea area, China. Ocean Coast. Manag. 2021, 207, 105556. [Google Scholar] [CrossRef]
- Hargrave, B.T. Empirical relationships describing benthic impacts of salmon aquaculture. Aquac. Environ. Interact. 2010, 1, 33–46. [Google Scholar] [CrossRef]
- Kondo, R.; Mori, Y.; Sakami, T. Comparison of sulphate-reducing bacterial communities in Japanese fish farm sediments with different levels of organic enrichment. Microbes Environ. 2012, 27, 193–199. [Google Scholar] [CrossRef]
- Zhu, Y.; Feng, J.; Zhao, L. Analysis of potential habitat of Branchiostoma belcheri tsingtauense in the Bohai Sea and Yellow Sea based on MaxEnt. Mar. Sci. Bull. 2025, 44, 47–56. [Google Scholar]
- Zhang, S.; Yi, M. Amphioxus endostyle and origin of vertebrate thyroid. Acta Oceanol. Sin. 2025, 44, 127–137. [Google Scholar] [CrossRef]
- Markos, A.; Kubovciak, J.; Mikula Mrstakova, S.; Zitova, A.; Paces, J.; Machacova, S.; Kozmik, Z., Jr.; Kozmik, Z.; Kozmikova, I. Cell type and regulatory analysis in amphioxus illuminates evolutionary origin of the vertebrate head. Nat. Commun. 2024, 15, 8859. [Google Scholar] [CrossRef]
- Guarneri, I.; Bozzo, M.; Criado, N.P.; Serafini, E.; Manfè, G.; Tagliapietra, D.; Fiorin, R.; Scapin, L.; Povero, P.; Bellitto, D.; et al. Amphioxus (Branchiostoma lanceolatum) in the North Adriatic Sea: Ecological observations and spawning behavior. Integr. Zool. 2025, 20, 331–343. [Google Scholar] [CrossRef]

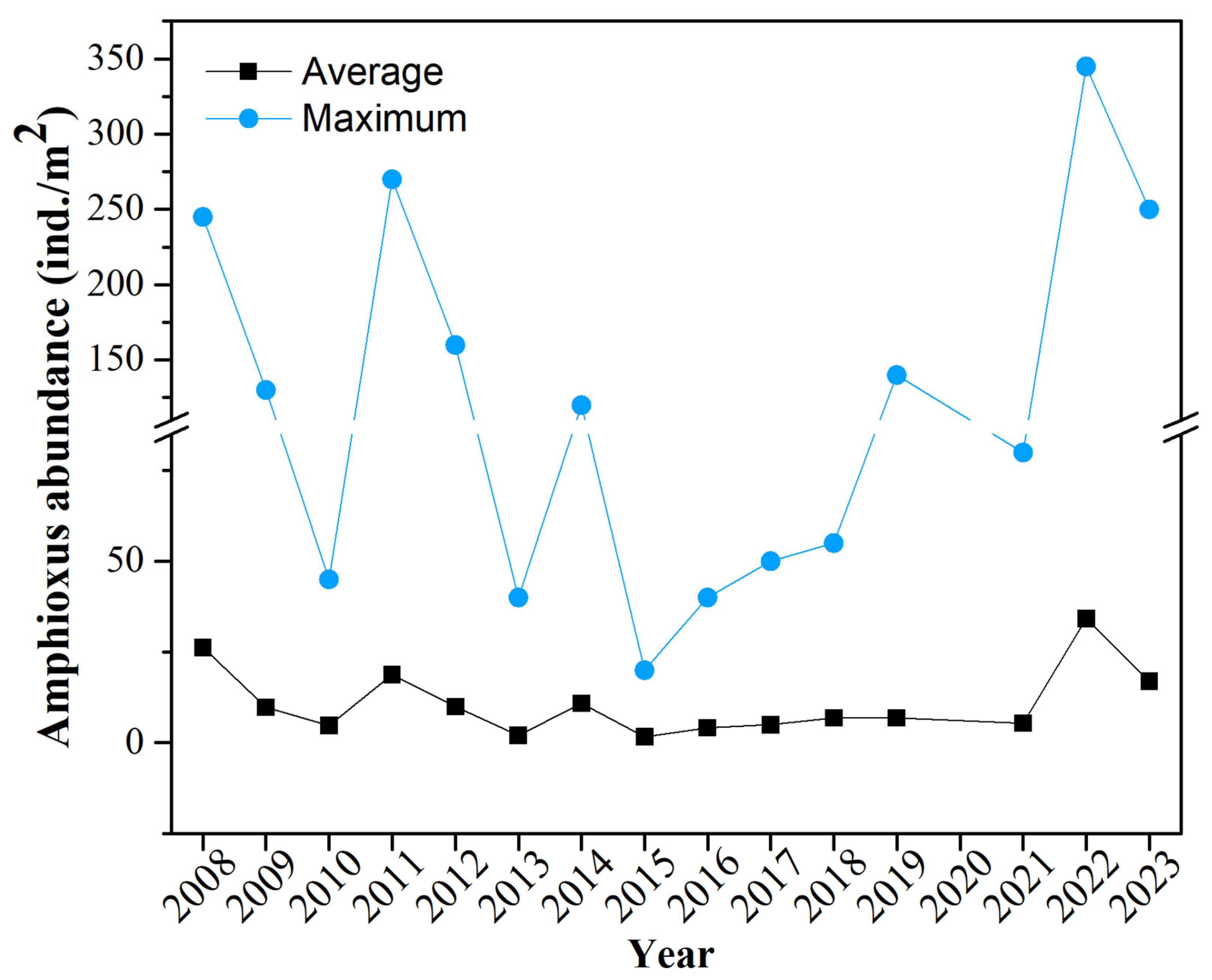
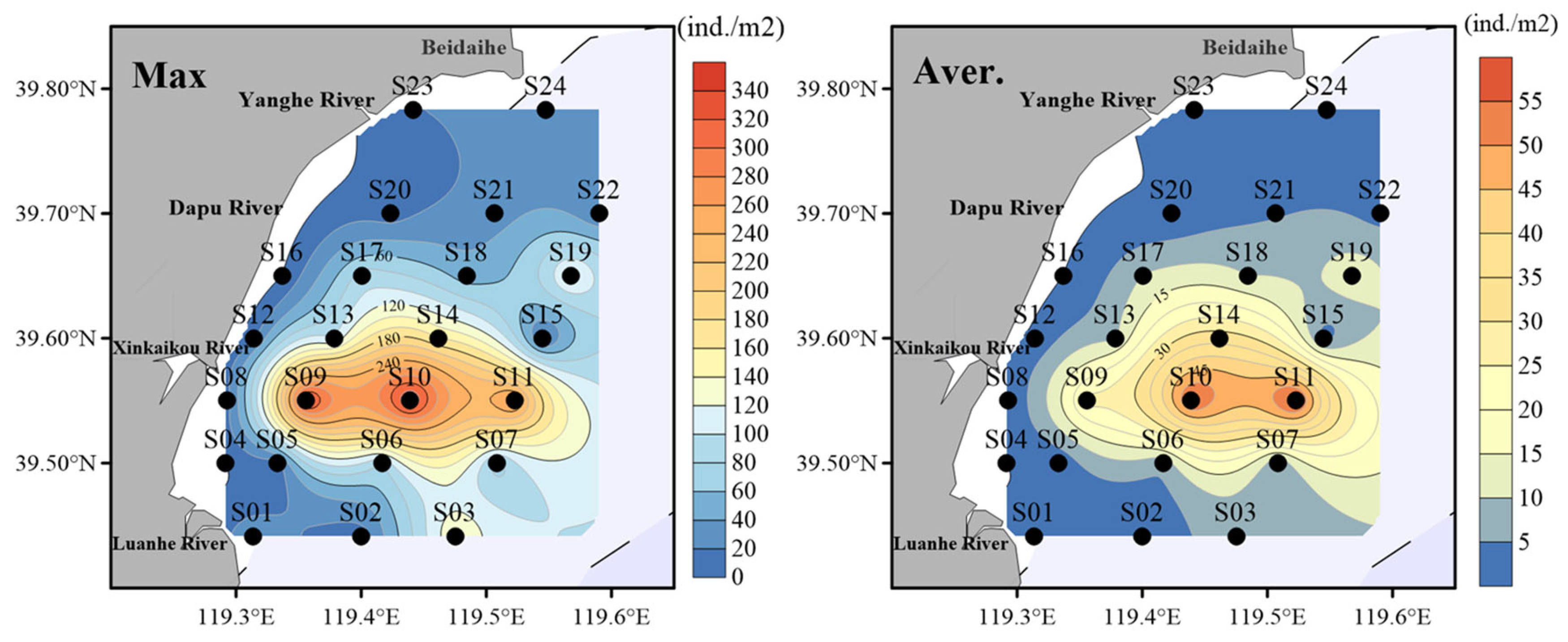
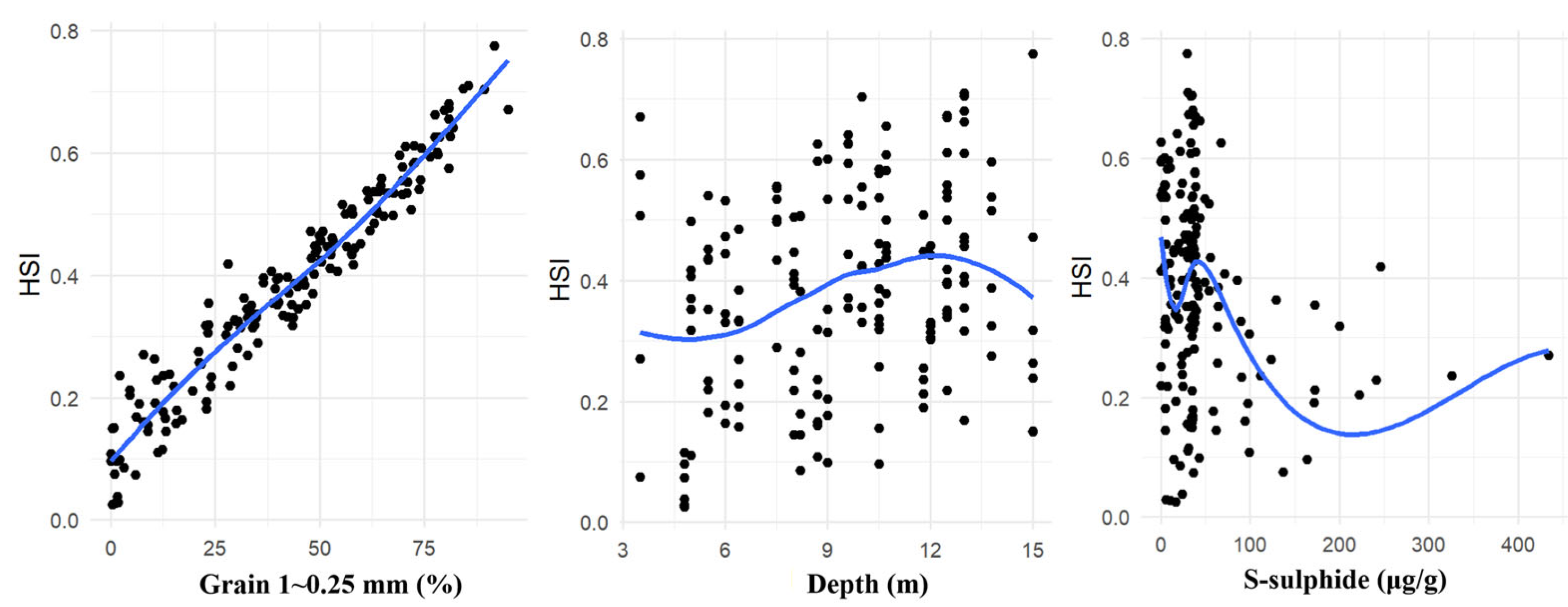
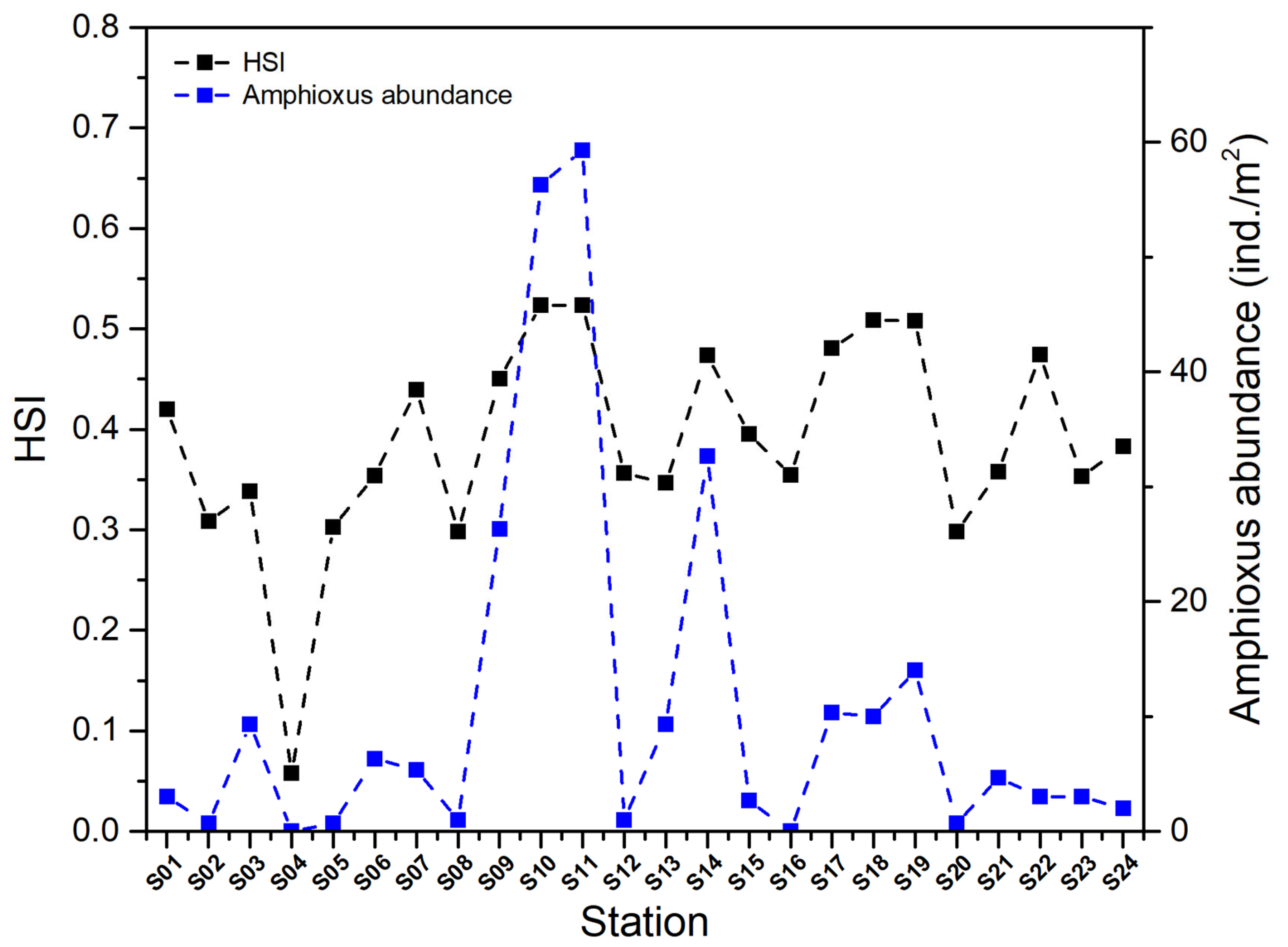
| Factor | Correlation Coefficient | p | Factor | Correlation Coefficient | p |
|---|---|---|---|---|---|
| Depth | 0.155 | 0.003 | S-TOC | −0.183 | 0.001 |
| Grain 1~0.5 mm | 0.337 | 0.000 | S-sulphide | −0.255 | 0.000 |
| Grain 0.5~0.25 mm | 0.443 | 0.000 | S-oil | −0.276 | 0.000 |
| Grain 0.125~0.063 mm | −0.442 | 0.000 | COD | −0.200 | 0.000 |
| Grain < 0.001 mm | −0.216 | 0.006 | NO2 | −0.208 | 0.000 |
| Sand | 0.215 | 0.000 | NO3 | −0.150 | 0.004 |
| Silty sand | −0.216 | 0.000 | DIN | −0.160 | 0.002 |
| Clay | −0.211 | 0.000 | Chl-a | −0.148 | 0.007 |
| Grain 1~0.25 mm | 0.260 | 0.000 | Transparency | 0.235 | 0.005 |
| Factors | S-Sulphide | Water Depth | Grain 1~0.25 mm |
|---|---|---|---|
| S-sulphide | 1 | 0.6 | 0.2 |
| Water depth | 1.67 | 1 | 0.33 |
| Grain 1~0.25 mm | 5 | 3 | 1 |
| Station | HSI | Station | HSI |
|---|---|---|---|
| S01 | 0.42 | S13 | 0.35 |
| S02 | 0.31 | S14 | 0.47 |
| S03 | 0.34 | S15 | 0.40 |
| S04 | 0.06 | S16 | 0.35 |
| S05 | 0.30 | S17 | 0.48 |
| S06 | 0.35 | S18 | 0.51 |
| S07 | 0.44 | S19 | 0.51 |
| S08 | 0.30 | S20 | 0.30 |
| S09 | 0.45 | S21 | 0.36 |
| S10 | 0.52 | S22 | 0.47 |
| S11 | 0.52 | S23 | 0.35 |
| S12 | 0.36 | S24 | 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, Q.; Wang, Q.; Zhao, Q.; Shi, W.; Zhang, Y.; Yao, Y.; Zhang, J. Assessment of Habitat Suitability for Amphioxus in the Changli Marine Reserve and Adjacent Coastal Waters, Hebei Province. Animals 2025, 15, 3203. https://doi.org/10.3390/ani15213203
Zhang Y, Wang Q, Wang Q, Zhao Q, Shi W, Zhang Y, Yao Y, Zhang J. Assessment of Habitat Suitability for Amphioxus in the Changli Marine Reserve and Adjacent Coastal Waters, Hebei Province. Animals. 2025; 15(21):3203. https://doi.org/10.3390/ani15213203
Chicago/Turabian StyleZhang, Yongfeng, Qiuzhen Wang, Quanying Wang, Qianqian Zhao, Weijie Shi, Yong Zhang, Yuan Yao, and Jianle Zhang. 2025. "Assessment of Habitat Suitability for Amphioxus in the Changli Marine Reserve and Adjacent Coastal Waters, Hebei Province" Animals 15, no. 21: 3203. https://doi.org/10.3390/ani15213203
APA StyleZhang, Y., Wang, Q., Wang, Q., Zhao, Q., Shi, W., Zhang, Y., Yao, Y., & Zhang, J. (2025). Assessment of Habitat Suitability for Amphioxus in the Changli Marine Reserve and Adjacent Coastal Waters, Hebei Province. Animals, 15(21), 3203. https://doi.org/10.3390/ani15213203





