Effect of Distillery Spent Wash Utilization on Maize Silage Fermentation and In Vitro Methane Production
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Physical and Chemical Analyses
2.3. Bacterial and Archaeal Community Analysis
2.4. In Vitro Ruminal Fermentation
2.5. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of Silage Samples
3.2. Silage Fermentation Profile
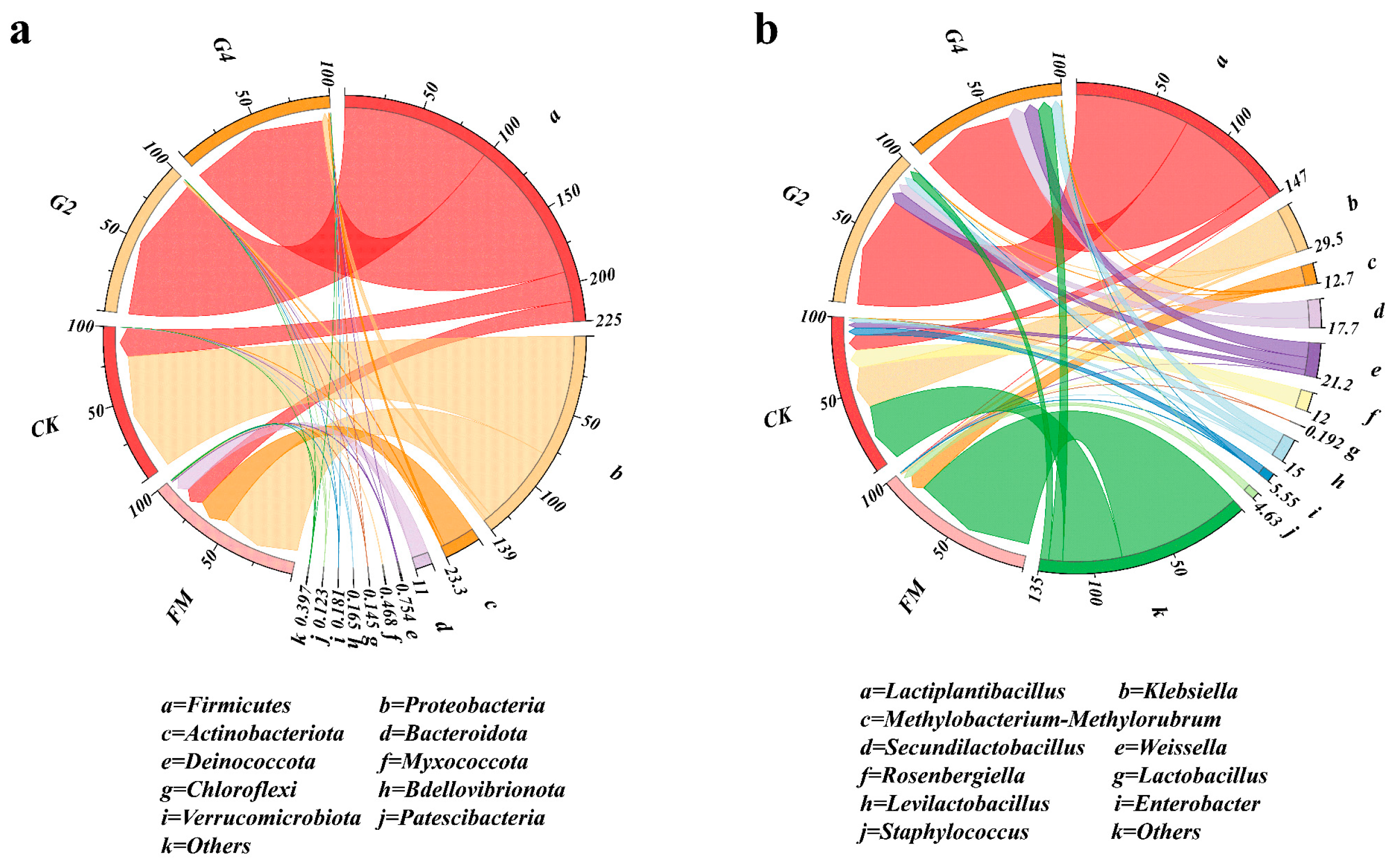
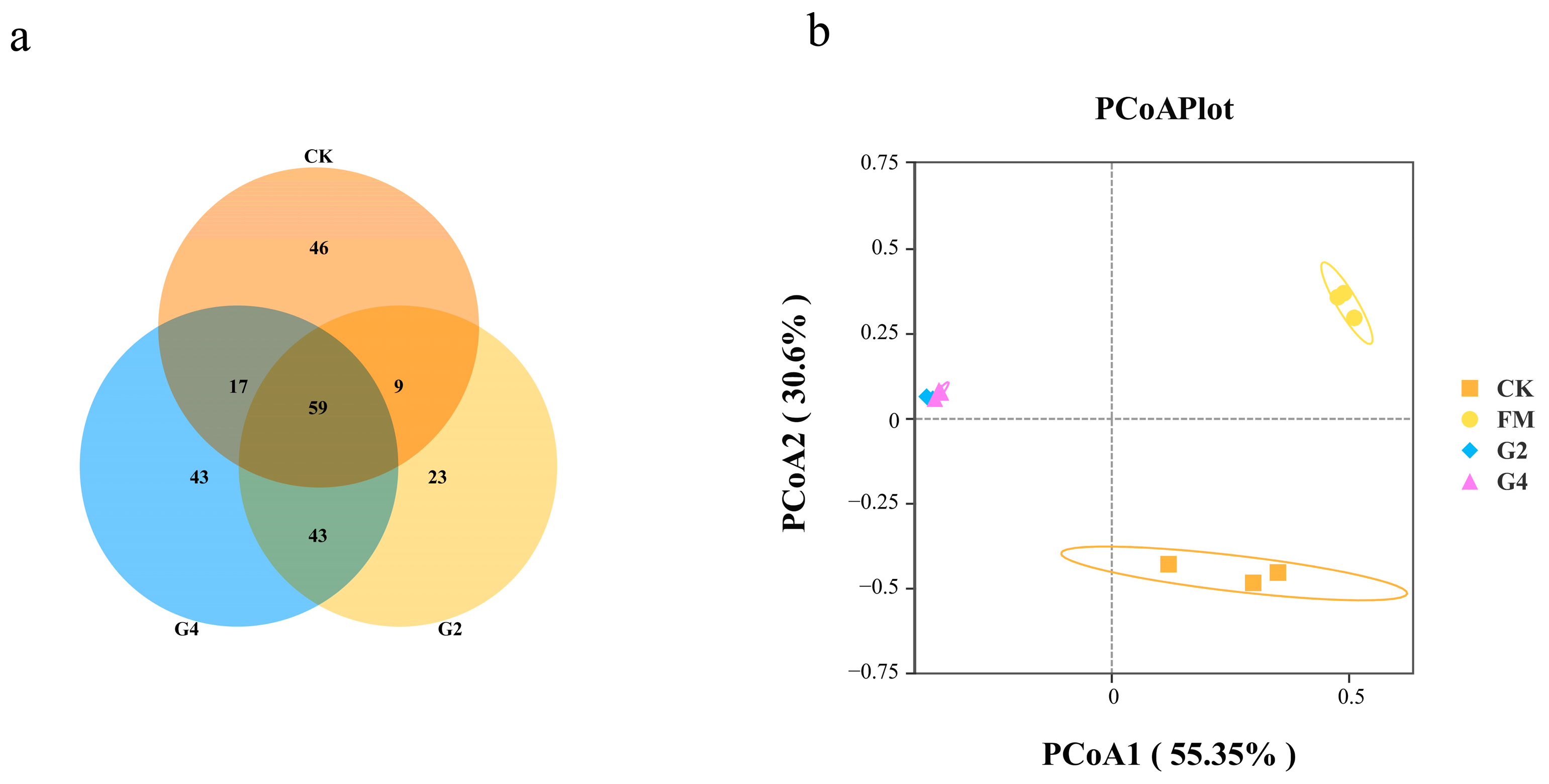
3.3. Analysis of Bacterial Communities in Whole-Plant Maize Silage Samples
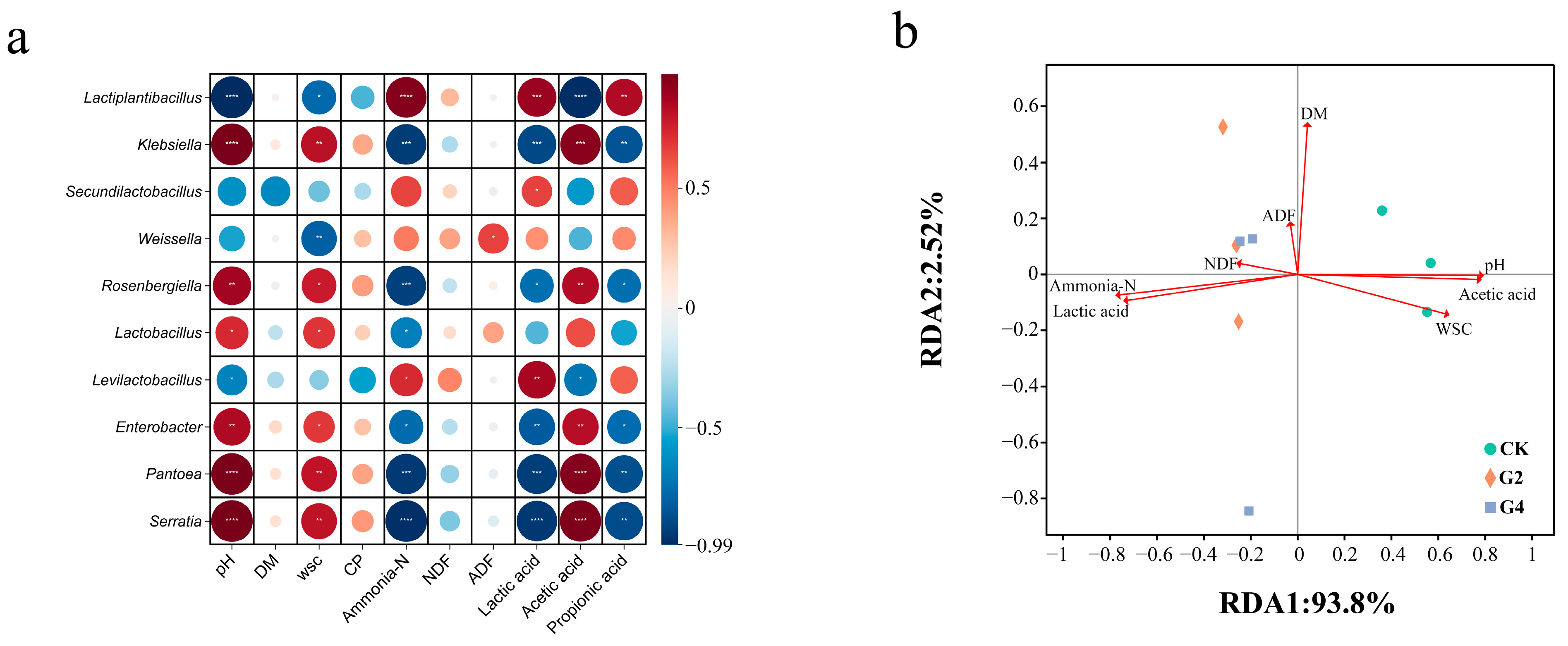
3.4. Correlation Analysis Between Bacterial Community and Environmental Conditions of Samples After 60 Days of Fermentation
3.5. Parameters of In Vitro Fermentation and Methane Emissions
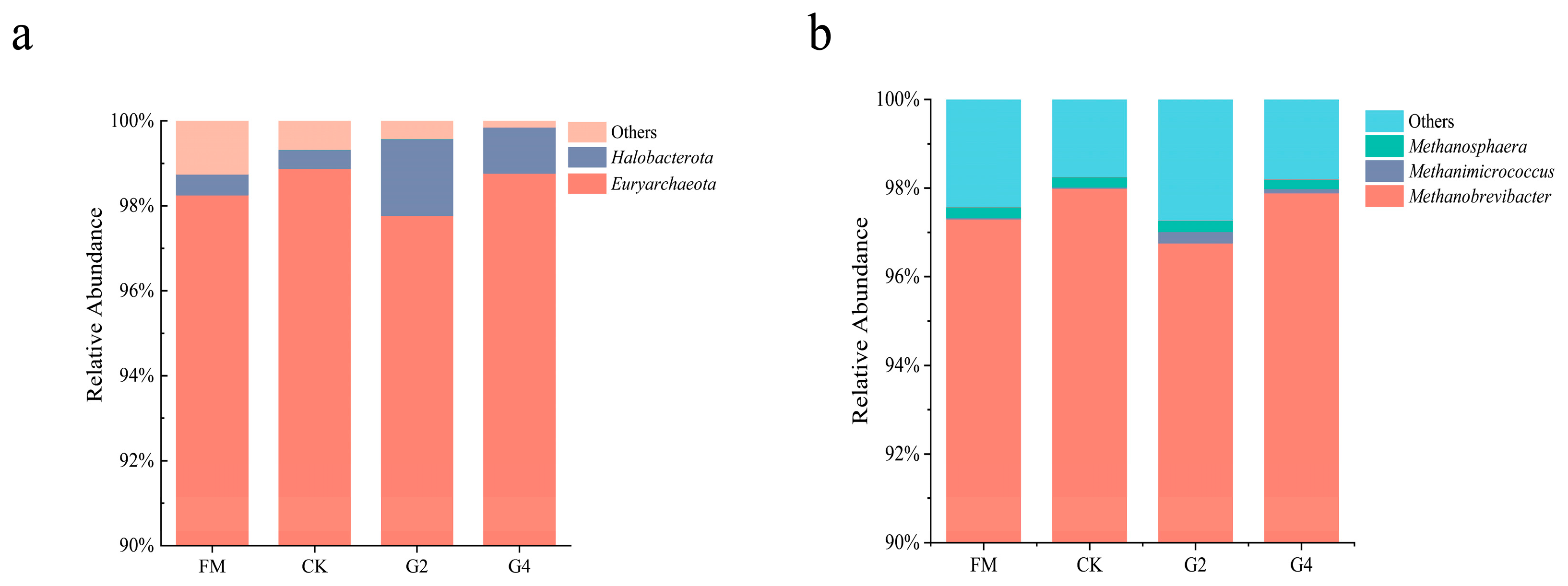
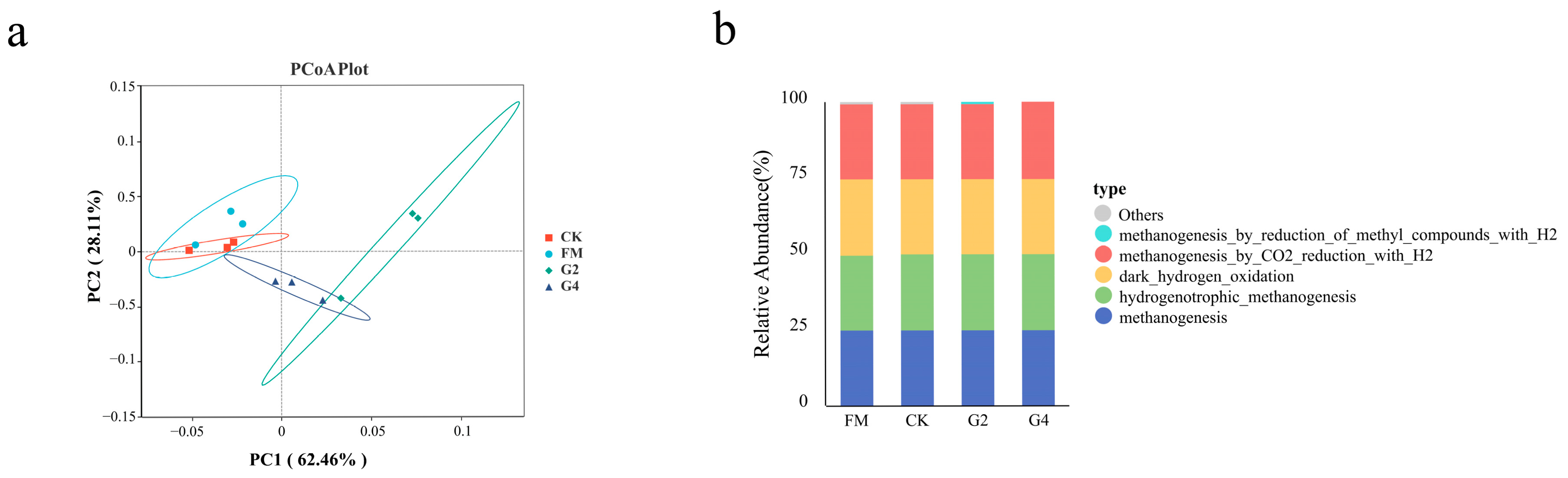
3.6. Analysis of Archaea Community and Functional Relative Abundance of Silage
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gerber, P.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change Through Livestock: A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013. [Google Scholar]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P. Climate Change 2014: Synthesis Report. In Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Qi, Z.; Feng, R. Global natural and anthropogenic methane emissions with approaches, potentials, economic costs, and social benefits of reductions: Review and outlook. J. Environ. Manag. 2025, 373, 123568. [Google Scholar] [CrossRef]
- Wang, M.; Yang, T.; Wang, R.; Fang, X.; Zheng, J.; Zhao, J.; Zhao, S.; Sun, Z.; Zhao, Y. Ruminant methane mitigation: Microbiological mechanisms and integrated strategies for sustainable livestock production in the context of climate change. Renew. Sustain. Energy Rev. 2025, 217, 115741. [Google Scholar] [CrossRef]
- Jeyanathan, J.; Martin, C.; Morgavi, D.P. Screening of bacterial direct-fed microbials for their antimethanogenic potential in vitro and assessment of their effect on ruminal fermentation and microbial profiles in sheep. J. Anim. Sci. 2016, 94, 739–750. [Google Scholar] [CrossRef]
- Wanapat, M.; Kang, S.; Khejornsart, P.; Wanapat, S. Effects of plant herb combination supplementation on rumen fermentation and nutrient digestibility in beef cattle. Asian-Australas. J. Anim. Sci. 2013, 26, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, M.; Diao, Q.; Ma, T.; Tu, Y. Seaweed as a feed additive to mitigate enteric methane emissions in ruminants: Opportunities and challenges. J. Integr. Agric. 2025, 24, 1327–1341. [Google Scholar] [CrossRef]
- Lianhua, L.; Feng, Z.; Yongming, S.; Zhenhong, Y.; Xiaoying, K.; Xianyou, Z.; Hongzhi, N. Low-cost additive improved silage quality and anaerobic digestion performance of napiergrass. Bioresour. Technol. 2014, 173, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H.; Wang, D.; Zhang, Y. From waste to value: Multi-omics reveal pomegranate peel addition improves corn silage antioxidant activity and reduces methane and nitrogen losses. Bioresour. Technol. 2025, 429, 132544. [Google Scholar] [CrossRef]
- Lu, G.; Li, L.; Huang, X.; Hou, P.; Tang, X.; Liao, C.; Cheng, C.; Zhang, M.; Chen, C.; Li, P. Yellow serofluid-inclusion enhanced anaerobic preservation of whole plant maize for subsequently reducing potential methane emission during in vitro ruminal fermentation. BMC Microbiol. 2025, 25, 399. [Google Scholar] [CrossRef]
- Mikucka, W.; Zielińska, M. Distillery Stillage: Characteristics, Treatment, and Valorization. Appl. Biochem. Biotechnol. 2020, 192, 770–793. [Google Scholar] [CrossRef]
- Shinde, P.A.; Ukarde, T.M.; Pandey, P.H.; Pawar, H.S. Distillery spent wash: An emerging chemical pool for next generation sustainable distilleries. J. Water Process Eng. 2020, 36, 101353. [Google Scholar] [CrossRef]
- Pant, D.; Adholeya, A. Biological approaches for treatment of distillery wastewater: A review. Bioresour. Technol. 2007, 98, 2321–2334. [Google Scholar] [CrossRef] [PubMed]
- Kannan, A.; Upreti, R.K. Influence of distillery effluent on germination and growth of mung bean (Vigna radiata) seeds. J. Hazard. Mater. 2008, 153, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Galbally, P.; Fagan, C.; Ryan, D.; Finnan, J.; Grant, J.; McDonnell, K. Biosolids and distillery effluent amendment to Irish Miscanthus ×giganteus plantations: Impacts on groundwater and soil. J. Environ. Qual. 2012, 41, 114–123. [Google Scholar] [CrossRef]
- Fito, J.; Tefera, N.; Van Hulle, S.W. Sugarcane biorefineries wastewater: Bioremediation technologies for environmental sustainability. Chem. Biol. Technol. Agric. 2019, 6, 6. [Google Scholar] [CrossRef]
- Huang, X.; Lu, G.; Li, L.; Liao, C.; Tang, X.; Chen, C.; Zhang, M.; Li, P.; Chen, C. Effect of distillery wastewater on chemical composition and microbial community of Sorghum propinquum silage during micro-permeation of air. Front. Ind. Microbiol. 2024, 2, 1409699. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated Simultaneous Determination of Ammonia and Total Amino Acids in Ruminal Fluid and in vitro Media1. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Sáez-Plaza, P.; Navas, M.J.; Wybraniec, S.; Michałowski, T.; Asuero, A.G. An Overview of the Kjeldahl Method of Nitrogen Determination. Part II. Sample Preparation, Working Scale, Instrumental Finish, and Quality Control. Crit. Rev. Anal. Chem. 2013, 43, 224–272. [Google Scholar] [CrossRef]
- Arthur, T.T. An automated procedure for the determination of soluble carbohydrates in herbage. J. Sci. Food Agric. 1977, 28, 639–642. [Google Scholar] [CrossRef]
- Menke, K.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The estimation of the digestibility and metabolizable energy content of ruminant feedingstuffs from the gas production when they are incubated with rumen liquor in vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- Cai, Y.; Hua, B.; Gao, L.; Hu, Y.; Yuan, X.; Cui, Z.; Zhu, W.; Wang, X. Effects of adding trace elements on rice straw anaerobic mono-digestion: Focus on changes in microbial communities using high-throughput sequencing. Bioresour. Technol. 2017, 239, 454–463. [Google Scholar] [CrossRef]
- Castro-Montoya, J.; De Campeneere, S.; Van Ranst, G.; Fievez, V. Interactions between methane mitigation additives and basal substrates on in vitro methane and VFA production. Anim. Feed Sci. Technol. 2012, 176, 47–60. [Google Scholar] [CrossRef]
- Li, P.; Zhao, W.; Yan, L.; Chen, L.; Chen, Y.; Gou, W.; You, M.; Cheng, Q.; Chen, C. Inclusion of abandoned rhubarb stalk enhanced anaerobic fermentation of alfalfa on the Qinghai Tibetan Plateau. Bioresour. Technol. 2022, 347, 126347. [Google Scholar] [CrossRef]
- Bernardes, T.F.; Gervásio, J.R.S.; De Morais, G.; Casagrande, D.R. Technical note: A comparison of methods to determine pH in silages. J. Dairy Sci. 2019, 102, 9039–9042. [Google Scholar] [CrossRef]
- Converti, A.; Zilli, M.; Del Borghi, M.; Ferraiolo, G. The fluidized bed reactor in the anaerobic treatment of wine wastewater. Bioprocess Eng. 1990, 5, 49–55. [Google Scholar] [CrossRef]
- He, L.; Zhou, W.; Xing, Y.; Pian, R.; Chen, X.; Zhang, Q. Improving the quality of rice straw silage with Moringa oleifera leaves and propionic acid: Fermentation, nutrition, aerobic stability and microbial communities. Bioresour. Technol. 2020, 299, 122579. [Google Scholar] [CrossRef] [PubMed]
- Mohana, S.; Acharya, B.K.; Madamwar, D. Distillery spent wash: Treatment technologies and potential applications. J. Hazard. Mater. 2009, 163, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Yu, C.; Zheng, M.; Ren, J.; Li, C.; Xu, C. Effects of ensiling on Irpex lacteus fermentation in wheat straw: Chemical composition, in vitro rumen digestibility, and fungal community. Anim. Feed Sci. Technol. 2022, 292, 115433. [Google Scholar] [CrossRef]
- Ren, H.; Feng, Y.; Pei, J.; Li, J.; Wang, Z.; Fu, S.; Zheng, Y.; Li, Z.; Peng, Z. Effects of Lactobacillus plantarum additive and temperature on the ensiling quality and microbial community dynamics of cauliflower leaf silages. Bioresour. Technol. 2020, 307, 123238. [Google Scholar] [CrossRef]
- Romero, J.J.; Zhao, Y.; Balseca-Paredes, M.A.; Tiezzi, F.; Gutierrez-Rodriguez, E.; Castillo, M.S. Laboratory silo type and inoculation effects on nutritional composition, fermentation, and bacterial and fungal communities of oat silage. J. Dairy Sci. 2017, 100, 1812–1828. [Google Scholar] [CrossRef]
- Xian, Z.; Wu, J.; Deng, M.; Wang, M.; Tian, H.; Liu, D.; Li, Y.; Liu, G.; Sun, B.; Guo, Y. Effects of Cellulase and Lactiplantibacillus plantarum on the Fermentation Parameters, Nutrients, and Bacterial Community in Cassia alata Silage. Front. Microbiol. 2022, 13, 926065. [Google Scholar] [CrossRef] [PubMed]
- Parvin, S.; Wang, C.; Li, Y.; Nishino, N. Effects of inoculation with lactic acid bacteria on the bacterial communities of Italian ryegrass, whole crop maize, guinea grass and rhodes grass silages. Anim. Feed Sci. Technol. 2010, 160, 160–166. [Google Scholar] [CrossRef]
- Dunière, L.; Sindou, J.; Chaucheyras-Durand, F.; Chevallier, I.; Thévenot-Sergentet, D. Silage processing and strategies to prevent persistence of undesirable microorganisms. Anim. Feed Sci. Technol. 2013, 182, 1–15. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X.; Dong, T.; Bao, Y.; Xu, B.; Shen, Y.; Yuan, X. Sustainable use of agricultural residues to improve corn silage quality: Co-ensiling with oregano oil residues. J. Environ. Manag. 2024, 371, 123172. [Google Scholar] [CrossRef]
- Tüzel, E.; Tegün, E.; Aydin, R. Effects of sodium benzoate on the quality and aerobic stability of corn silage contaminated with Listeria monocytogenes. Anim. Feed Sci. Technol. 2025, 321, 116238. [Google Scholar] [CrossRef]
- Faniyi, T.O.; Adegbeye, M.J.; Elghandour, M.; Pilego, A.B.; Salem, A.Z.M.; Olaniyi, T.A.; Adediran, O.; Adewumi, M.K. Role of diverse fermentative factors towards microbial community shift in ruminants. J. Appl. Microbiol. 2019, 127, 2–11. [Google Scholar] [CrossRef]
- Zhang, G.; Du, Z.; Liu, S.; Zhang, J.; Lv, L.; Sun, L.; Liang, J. Anaerobic fermentation of spent mushroom substrate with rumen microbiota for volatile fatty acid production: Performance, community, and metabolic pathway. J. Water Process Eng. 2024, 67, 106133. [Google Scholar] [CrossRef]
- Meng, Y.; Mumme, J.; Xu, H.; Wang, K. A biologically inspired variable-pH strategy for enhancing short-chain fatty acids (SCFAs) accumulation in maize straw fermentation. Bioresour. Technol. 2016, 201, 329–336. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Christophe, G.; Fontanille, P.; Larroche, C. Biological upgrading of volatile fatty acids, key intermediates for the valorization of biowaste through dark anaerobic fermentation. Bioresour. Technol. 2013, 145, 166–174. [Google Scholar] [CrossRef]
- Sirohi, S.K.; Pandey, N.; Singh, B.; Puniya, A.K. Rumen methanogens: A review. Indian J. Microbiol. 2010, 50, 253–262. [Google Scholar] [CrossRef]
- Sun, H.; Liao, C.; Lu, G.; Zheng, Y.; Cheng, Q.; Xie, Y.; Wang, C.; Chen, C.; Li, P. Role of Lactiplantibacillus paraplantarum during anaerobic storage of ear-removed corn on biogas production. Bioresour. Technol. 2022, 364, 128061. [Google Scholar] [CrossRef]
- Xu, S.; Han, R.; Zhang, Y.; He, C.; Liu, H. Differentiated stimulating effects of activated carbon on methanogenic degradation of acetate, propionate and butyrate. Waste Manag. 2018, 76, 394–403. [Google Scholar] [CrossRef]
- Calabro, S.; Cutrignelli, M.I.; Bovera, F.; Piccolo, G.; Infascelli, F. In vitro fermentation kinetics of carbohydrate fractions of fresh forage, silage and hay of Avena sativa. J. Sci. Food Agric. 2005, 85, 1838–1844. [Google Scholar] [CrossRef]
- Stieb, M.; Schink, B. Anaerobic degradation of isovalerate by a defined methanogenic coculture. Arch. Microbiol. 1986, 144, 291–295. [Google Scholar] [CrossRef]
- Blümmel, M.; Makkar, H.; Becker, K. In vitro gas production: A technique revisited. J. Anim. Physiol. Anim. Nutr. 1997, 77, 24–34. [Google Scholar] [CrossRef]
- Olijhoek, D.W.; Lamminen, M.; Hellwing, A.L.F.; Larsen, M.; Weisbjerg, M.R.; Bach Knudsen, K.E.; Lund, P. Effect of substituting maize silage with fresh or ensiled sugar beets on nutrient digestibility, rumen fermentation and microbial synthesis, and enteric methane emission in dairy cows. Anim. Feed Sci. Technol. 2023, 303, 115715. [Google Scholar] [CrossRef]
- Di Marco, O.; Aello, M.; Arias, S. Digestibility and ruminal digestion kinetics of corn silage. Arq. Bras. Med. Vet. Zootec. 2005, 57, 223–228. [Google Scholar] [CrossRef]
- Yan, S.; Wang, M.; Zhang, S.; Tong, Z.; Li, S.; Yong, X.; Zhang, X.; Zhou, J. Fe-doped hydrochar facilitating simultaneous methane production and pharmaceutical and personal care products (PPCPs) degradation in co-anaerobic digestion of municipal sludge and food waste. Chem. Eng. J. 2023, 474, 146001. [Google Scholar] [CrossRef]

| Items | CK | G2 | G4 | SEM | p-Value |
|---|---|---|---|---|---|
| DM, % FM | 32.57 | 32.78 | 32.19 | 0.264 | 0.764 |
| CP, % DM | 10.11 | 9.73 | 9.92 | 0.122 | 0.503 |
| WSC, % DM | 5.72 a | 4.41 b | 4.32 b | 0.721 | 0.001 |
| NDF, % DM | 61.95 | 62.60 | 62.22 | 0.251 | 0.751 |
| ADF, % DM | 36.00 | 35.72 | 36.20 | 0.184 | 0.707 |
| Ash, % | 2.15 | 2.15 | 2.07 | 0.027 | 0.461 |
| HC, % | 25.95 | 26.87 | 26.02 | 0.062 | 0.383 |
| Items | CK | G2 | G4 | SEM | p-Value |
|---|---|---|---|---|---|
| pH | 4.80 a | 4.03 c | 4.15 b | 0.357 | <0.001 |
| Lactic acid, % DM | 2.35 b | 3.40 a | 3.43 a | 0.603 | 0.012 |
| Acetic acid, % DM | 1.25 a | 0.53 b | 0.70 b | 0.338 | <0.001 |
| Lactic acid/Acetic acid | 1.93 c | 6.45 a | 4.93 b | 0.686 | <0.001 |
| Propionic acid, % DM | 0.63 b | 0.70 a | 0.68 a | 0.039 | 0.021 |
| Butyric acid, % DM | 0.36 | 0.16 | 0.20 | 0.103 | 0.053 |
| Ammonia-N, % DM | 0.34 b | 0.80 a | 0.84 a | 0.251 | <0.001 |
| Items | FM | CK | G2 | G4 | SEM | p-Value |
|---|---|---|---|---|---|---|
| Goods coverage | 0.861 b | 0.972 a | 0.985 a | 0.978 a | 0.016 | <0.001 |
| Observed Otus | 527.33 a | 145.33 b | 60.00 c | 87.67 c | 198.320 | <0.001 |
| Dominance | 0.01 c | 0.06 c | 0.41 a | 0.32 b | 0.177 | <0.001 |
| Chao1 | 844.43 a | 204.93 b | 93.74 c | 135.34 bc | 93.158 | <0.001 |
| Pielou_e | 0.87 a | 0.73 b | 0.40 c | 0.44 c | 0.059 | <0.001 |
| Shannon | 7.88 a | 5.20 b | 2.38 c | 2.84 c | 0.662 | <0.001 |
| Simpson | 0.99 a | 0.94 a | 0.59 c | 0.68 b | 0.051 | <0.001 |
| Items | CK | G2 | G4 | FM | SEM | p-Value |
|---|---|---|---|---|---|---|
| pH | 6.85 c | 7.11 a | 6.96 b | 6.92 b | 0.031 | <0.001 |
| NH3-N (mmol/L) | 8.33 a | 9.19 a | 8.70 a | 7.38 b | 1.436 | 0.008 |
| TVFAs (mmol/L) | 77.55 b | 77.18 b | 84.25 a | 76.44 b | 1.088 | 0.008 |
| Acetate (mmol/L) | 54.57 ab | 52.89 b | 57.12 a | 48.11 c | 1.069 | <0.001 |
| Propionate (mmol/L) | 12.54 c | 13.82 bc | 15.19 ab | 16.07 a | 0.445 | 0.002 |
| Butyrate (mmol/L) | 6.39 b | 6.49 b | 7.44 a | 8.00 a | 0.232 | 0.007 |
| Isobutyrate (mmol/L) | 1.15 ab | 1.11 ab | 1.25 a | 1.07 b | 0.028 | 0.129 |
| Valerate (mmol/L) | 1.01 c | 1.04 bc | 1.16 ab | 1.17 a | 0.026 | 0.037 |
| Isovalerate (mmol/L) | 1.89 ab | 1.83 b | 2.10 a | 2.01 ab | 0.041 | 0.080 |
| Item | FM | CK | G2 | G4 | SEM | p-Value |
|---|---|---|---|---|---|---|
| Goods coverage | 1.00 | 1.00 | 1.00 | 1.00 | - | - |
| Observed Otus | 121.67 a | 108.00 ab | 88.67 ab | 69.67 b | 28.970 | 0.117 |
| Dominance | 0.82 a | 0.82 a | 0.67 c | 0.75 b | 0.065 | <0.001 |
| Chao1 | 127.00 a | 118.46 ab | 90.12 ab | 69.85 b | 9.452 | 0.097 |
| Pielou_e | 0.13 c | 0.13 c | 0.22 a | 0.19 b | 0.012 | <0.001 |
| Shannon | 0.89 c | 0.90 c | 1.42 a | 1.15 b | 0.069 | <0.001 |
| Simpson | 0.18 c | 0.18 c | 0.33 a | 0.25 b | 0.019 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Lu, G.; Zhao, H.; Li, L.; Liao, C.; Wang, P.; Zhang, Y.; Zhang, M.; Li, P.; Gou, W. Effect of Distillery Spent Wash Utilization on Maize Silage Fermentation and In Vitro Methane Production. Animals 2025, 15, 3146. https://doi.org/10.3390/ani15213146
Tang Y, Lu G, Zhao H, Li L, Liao C, Wang P, Zhang Y, Zhang M, Li P, Gou W. Effect of Distillery Spent Wash Utilization on Maize Silage Fermentation and In Vitro Methane Production. Animals. 2025; 15(21):3146. https://doi.org/10.3390/ani15213146
Chicago/Turabian StyleTang, Yu, Guangrou Lu, Hongxiang Zhao, Lin Li, Chaosheng Liao, Pan Wang, Yubo Zhang, Meiyan Zhang, Ping Li, and Wenlong Gou. 2025. "Effect of Distillery Spent Wash Utilization on Maize Silage Fermentation and In Vitro Methane Production" Animals 15, no. 21: 3146. https://doi.org/10.3390/ani15213146
APA StyleTang, Y., Lu, G., Zhao, H., Li, L., Liao, C., Wang, P., Zhang, Y., Zhang, M., Li, P., & Gou, W. (2025). Effect of Distillery Spent Wash Utilization on Maize Silage Fermentation and In Vitro Methane Production. Animals, 15(21), 3146. https://doi.org/10.3390/ani15213146





