RNA Sequencing and Metabolomic Analyses Reveal Differences in Muscle Characteristics and Metabolic Profiles Between Purebred and Crossbred Huainan Pigs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sample Collection
2.3. Carcass and Muscle Characteristics Measurements
2.4. IMF and Moisture
2.5. RNA Extraction, Library Construction, and Sequencing
2.6. Quantitative Real-Time PCR (qRT-PCR) Validation
2.7. Analysis of DEGs and Pathways
2.8. Untargeted Metabolomics Profiling
2.9. Mass Spectrometry Data Analysis
2.10. Statistical Analysis
3. Results
3.1. Evaluation of Carcass and Muscle Characteristics
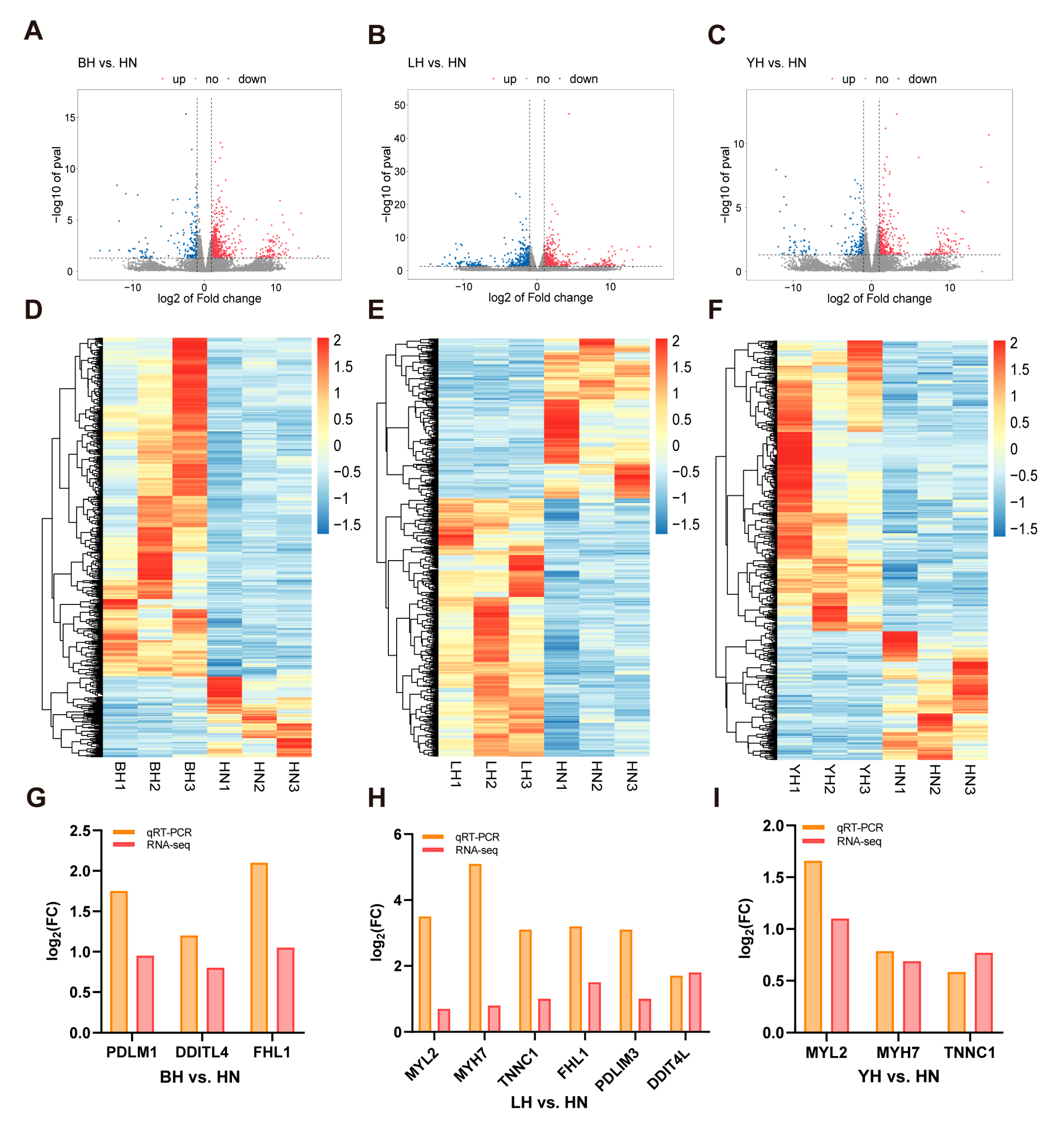
3.2. Analysis of DEGs in Each Hybrid Group Compared to the Purebred Group
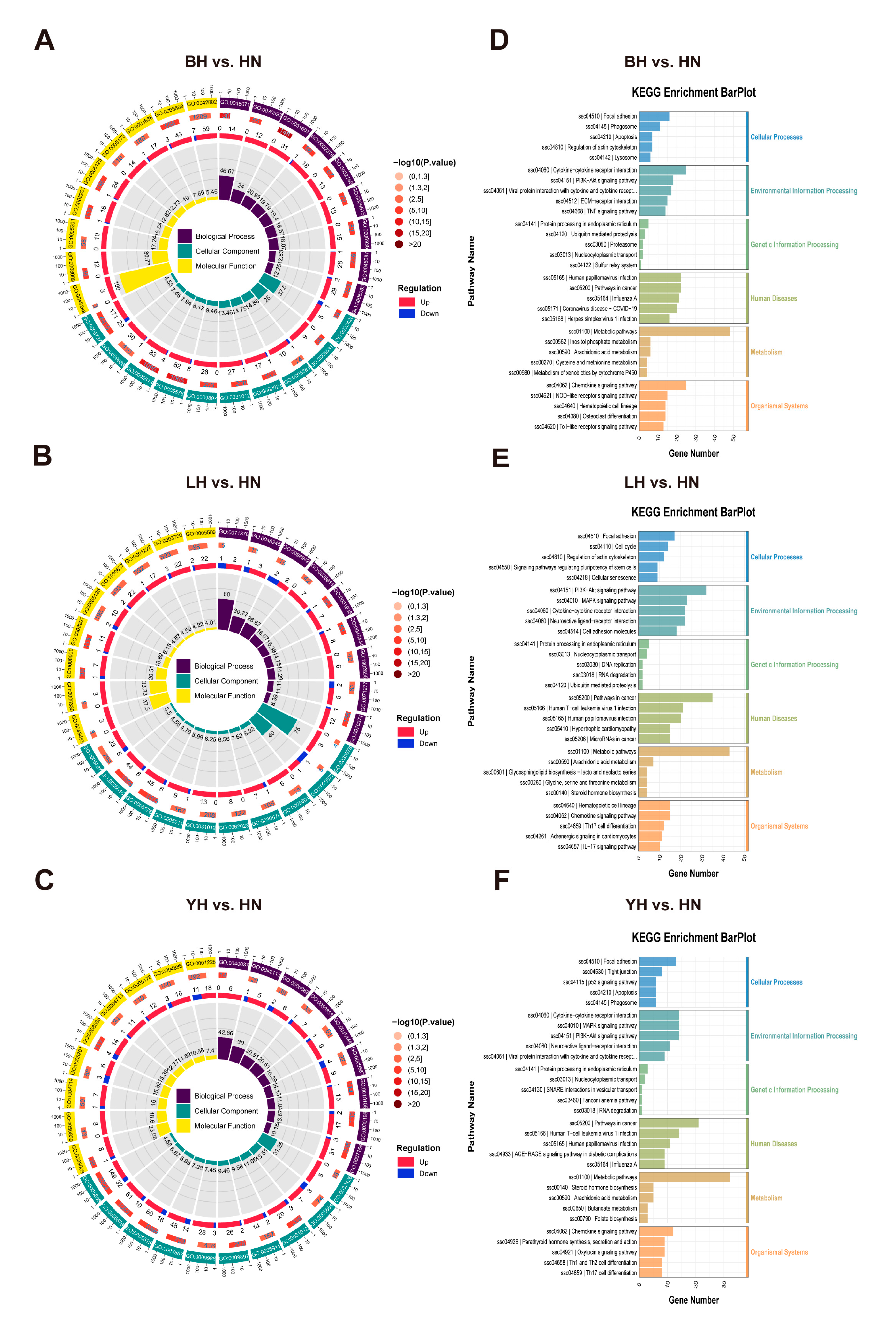
3.3. GO and KEGG Enrichment Analysis
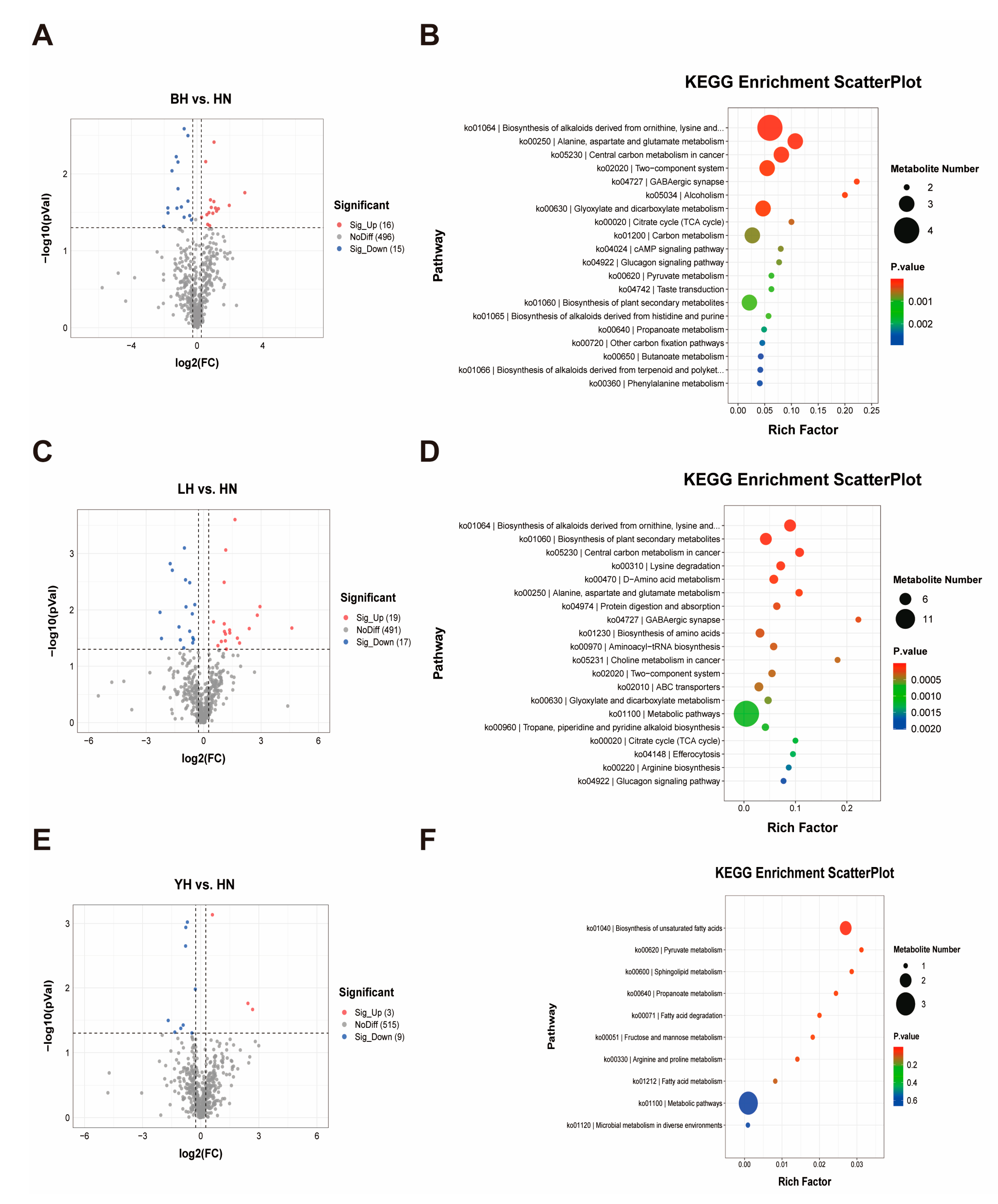
3.4. DAMs and Metabolome Data Analysis
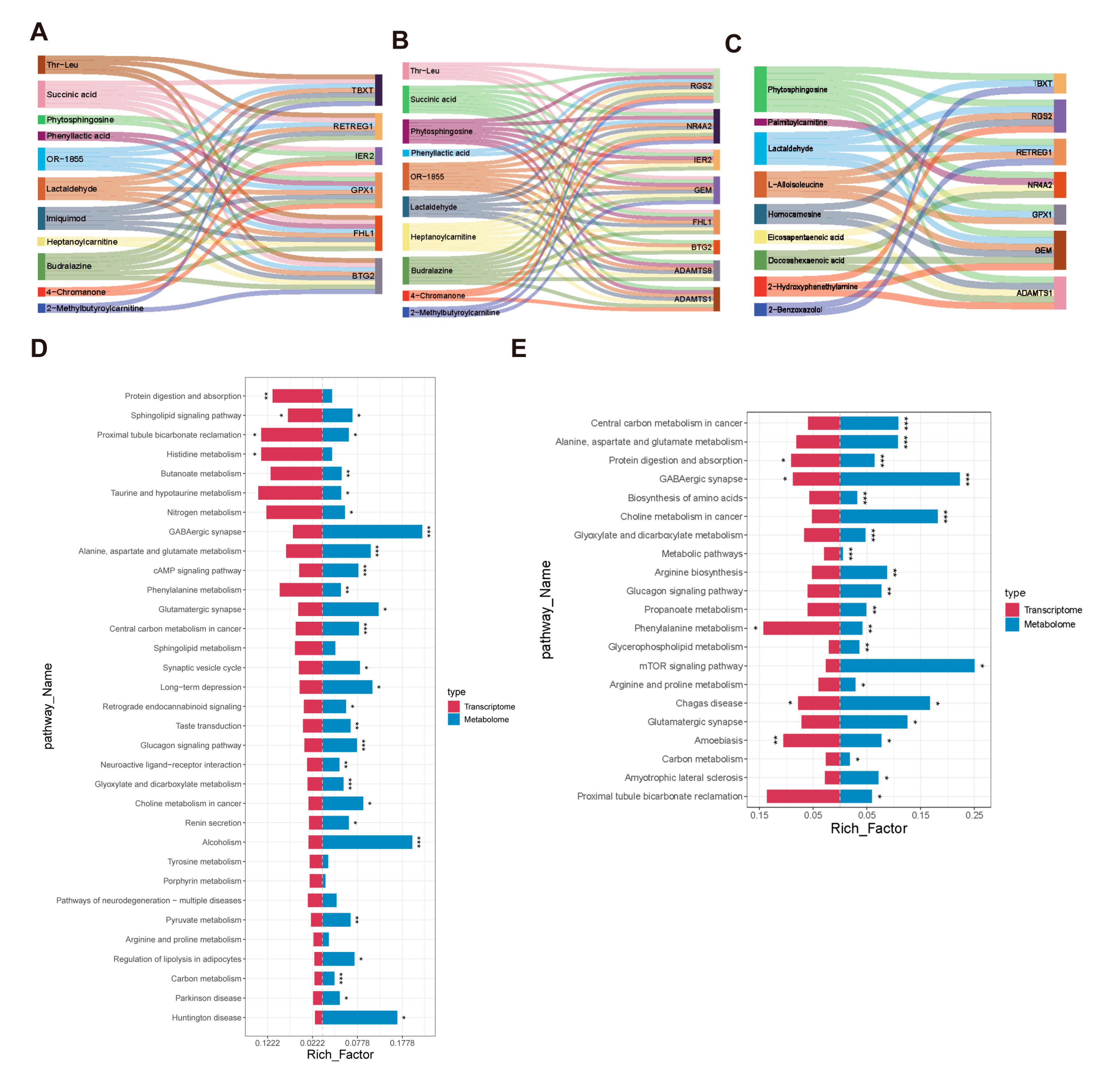
3.5. Combined Analysis of Transcriptome and Metabolomics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, G.; Shen, Y.; Li, C.; Zhu, Q.; ZhanBota, A. The Regulatory Effect of Herd Structure on Pig Production under the Environmental Regulation. PLoS ONE 2022, 17, e0266687. [Google Scholar] [CrossRef]
- Wang, J.; Hua, L.; Chen, J.; Zhang, J.; Bai, X.; Gao, B.; Li, C.; Shi, Z.; Sheng, W.; Gao, Y.; et al. Identification and Characterization of Long Non-Coding RNAs in Subcutaneous Adipose Tissue from Castrated and Intact Full-Sib Pair Huainan Male Pigs. BMC Genom. 2017, 18, 542. [Google Scholar] [CrossRef]
- Fernandez, X.; Monin, G.; Talmant, A.; Mourot, J.; Lebret, B. Influence of Intramuscular Fat Content on the Quality of Pig Meat—2. Consumer Acceptability of m. Longissimus Lumborum. Meat Sci. 1999, 53, 67–72. [Google Scholar] [CrossRef]
- Park, B.-Y.; Cho, S.-H.; Kim, J.-H.; Seong, P.-N.; Kang, G.-H.; Jeong, D.-W.; Kim, C.-W.; Park, H.-C.; Jeong, J.-H.; Choi, J.-S.; et al. Comparison of Pork Quality by Different Berkshire Line. Food Sci. Anim. Resour. 2010, 30, 867–871. [Google Scholar] [CrossRef][Green Version]
- Suzuki, K.; Shibata, T.; Kadowaki, H.; Abe, H.; Toyoshima, T. Meat Quality Comparison of Berkshire, Duroc and Crossbred Pigs Sired by Berkshire and Duroc. Meat Sci. 2003, 64, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Li, S.; Deng, L.; Guan, Y.; Chen, D.; Yuan, X.; Xia, T.; He, X.; Shan, Y.; Li, C. Transcriptome Analysis Reveals Long Intergenic Noncoding RNAs Contributed to Growth and Meat Quality Differences between Yorkshire and Wannanhua Pig. Genes 2017, 8, 203. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Teng, J.; Wang, X.; Xu, B.; Niu, Y.; Ma, L.; Yan, X. Multi-Omics Analysis Reveals Gut Microbiota-Induced Intramuscular Fat Deposition via Regulating Expression of Lipogenesis-Associated Genes. Anim. Nutr. 2022, 9, 84–99. [Google Scholar] [CrossRef]
- Zhang, L.; An, X.; Xu, Z.; Niu, C.; Geng, Z.; Zhang, J.; Shi, H.; Chen, Z.; Zhang, R.; Yue, Y. Transcriptome-Metabolome Analysis Reveals That Crossbreeding Improves Meat Quality in Hu Sheep and Their F1-Generation Sheep. Foods 2025, 14, 1384. [Google Scholar] [CrossRef]
- Yu, T.; Tian, X.; Li, D.; He, Y.; Yang, P.; Cheng, Y.; Zhao, X.; Sun, J.; Yang, G. Transcriptome, Proteome and Metabolome Analysis Provide Insights on Fat Deposition and Meat Quality in Pig. Food Res. Int. 2023, 166, 112550. [Google Scholar] [CrossRef]
- Dan, H.; Liu, C.; Zhang, H.; Gan, M.; Wang, Y.; Chen, L.; Zhao, Y.; Liu, B.; Zhu, K.; Niu, L.; et al. Integrated Transcriptomic and Metabolomic Analyses Reveal Heterosis for Meat Quality of Neijiang Pigs. Front. Vet. Sci. 2024, 11, 1493284. [Google Scholar] [CrossRef]
- Salomão, R.; Neto, I.V.d.S.; Ramos, G.V.; Tibana, R.A.; Durigan, J.Q.; Pereira, G.B.; Franco, O.L.; Royer, C.; Neves, F.d.A.R.; Carvalho, A.C.A.d.; et al. Paternal Resistance Exercise Modulates Skeletal Muscle Remodeling Pathways in Fathers and Male Offspring Submitted to a High-Fat Diet. Front. Physiol. 2021, 12, 706128. [Google Scholar] [CrossRef]
- Henckel, P.; Oksbjerg, N.; Erlandsen, E.; Barton-Gade, P.; Bejerholm, C. Histo- and Biochemical Characteristics of the Longissimus Dorsi Muscle in Pigs and Their Relationships to Performance and Meat Quality. Meat Sci. 1997, 47, 311–321. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Xing, B.; Chen, J.; Lu, Q.; Zhang, J.; Ren, Q.; Ma, Q.; Guo, H.; Cao, H. Castration Induced circRNA Expressional Changes in Subcutaneous Adipose Tissue of Male Pigs. Anim. Sci. J. 2021, 92, e13648. [Google Scholar] [CrossRef]
- Li, Y.; Jia, M.; Chen, J.; Liu, F.; Ren, Q.; Yan, X.; Xing, B.; Pan, C.; Wang, J. A Comparative Metabolomics Study of the Potential Marker Compounds in Feces from Different Hybrid Offspring of Huainan Pigs. Animals 2024, 14, 3282. [Google Scholar] [CrossRef]
- NY/T 65-2004 English Version, NY/T 65-2004 Feeding Standard of Swine (English Version)—Code of China. Available online: https://codeofchina.com/standard/NYT65-2004.html (accessed on 23 October 2025).
- Wang, D.; Chen, G.; Chai, M.; Shi, C.; Geng, Y.; Che, Y.; Li, Y.; Liu, S.; Gao, Y.; Hou, H. Effects of Dietary Protein Levels on Production Performance, Meat Quality and Flavor of Fattening Pigs. Front. Nutr. 2022, 9, 910519. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, G.; Motoyama, M.; Nakajima, I.; Sasaki, K. Relationship between Water-Holding Capacity and Intramuscular Fat Content in Japanese Commercial Pork Loin. Asian-Australas. J. Anim. Sci. 2018, 31, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Bu, D.; Luo, H.; Huo, P.; Wang, Z.; Zhang, S.; He, Z.; Wu, Y.; Zhao, L.; Liu, J.; Guo, J.; et al. KOBAS-i: Intelligent Prioritization and Exploratory Visualization of Biological Functions for Gene Enrichment Analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef]
- Goethals, S.; Rombouts, C.; Hemeryck, L.Y.; Van Meulebroek, L.; Van Hecke, T.; Vossen, E.; Van Camp, J.; De Smet, S.; Vanhaecke, L. Untargeted Metabolomics to Reveal Red versus White Meat-Associated Gut Metabolites in a Prudent and Western Dietary Context. Mol. Nutr. Food Res. 2020, 64, 2000070. [Google Scholar] [CrossRef]
- Thomann, J.; Ley, L.; Klaiber, A.; Liechti, M.E.; Duthaler, U. Development and Validation of an LC-MS/MS Method for the Quantification of Mescaline and Major Metabolites in Human Plasma. J. Pharm. Biomed. Anal. 2022, 220, 114980. [Google Scholar] [CrossRef]
- Khanal, S.; Bai, Y.; Ngo, W.; Nichols, K.K.; Wilson, L.; Barnes, S.; Nichols, J.J. Human Meibum and Tear Film Derived Cholesteryl and Wax Esters in Meibomian Gland Dysfunction and Tear Film Structure. Ocul. Surf. 2022, 23, 12–23. [Google Scholar] [CrossRef]
- Rodrigues, S.; Teixeira, A. Effect of Breed and Sex on Pork Meat Sensory Evaluation. Food Nutr. Sci. 2014, 5, 599–605. [Google Scholar] [CrossRef][Green Version]
- Yang, S.-L.; Wang, Z.-G.; Liu, B.; Zhang, G.-X.; Zhao, S.-H.; Yu, M.; Fan, B.; Li, M.-H.; Xiong, T.-A.; Li, K. Genetic Variation and Relationships of Eighteen Chinese Indigenous Pig Breeds. Genet. Sel. Evol. GSE 2003, 35, 657–671. [Google Scholar] [CrossRef]
- Jun, W.; Zhe, Z.; Jiaqi, L.; Xiaodian, C.; Qing, L.; Zhanming, Z.; Haoqiang, Y.; Chen, W.; Zhiting, X.; Xibo, W.; et al. Weighted Single-step GWAS of Eye Muscle Area, Predicted Lean Meat Percentage and Average Backfat Thickness in A Yorkshire Pig Population. Acta Vet. Zootech. Sin. 2023, 54, 1403–1414. [Google Scholar]
- Song, B.; Zheng, C.; Zheng, J.; Zhang, S.; Zhong, Y.; Guo, Q.; Li, F.; Long, C.; Xu, K.; Duan, Y.; et al. Comparisons of Carcass Traits, Meat Quality, and Serum Metabolome between Shaziling and Yorkshire Pigs. Anim. Nutr. 2022, 8, 125–134. [Google Scholar] [CrossRef]
- Li, Y.; He, Y.; Ran, J.; Huang, Y.; Li, X.; Jiang, H.; Li, X.; Pan, Y.; Zhao, S.; Song, C.; et al. Comparison of Meat Quality and Glycolysis Potential of Two Hybrid Pigs in Three-Way Hybrid Model. Front. Vet. Sci. 2023, 10, 1136485. [Google Scholar] [CrossRef]
- Wang, Z.; Zeng, X.; Zhang, C.; Wang, Q.; Zhang, W.; Xie, J.; Chen, J.; Hu, Q.; Wang, Q.; Yang, H.; et al. Higher Niacin Intakes Improve the Lean Meat Rate of Ningxiang Pigs by Regulating Lipid Metabolism and Gut Microbiota. Front. Nutr. 2022, 9, 959039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Song, Q.; Wu, F.; Zhang, J.; Xu, M.; Li, H.; Han, Z.; Gao, H.; Xu, N. Evaluation of the Four Breeds in Synthetic Line of Jiaxing Black Pigs and Berkshire for Meat Quality Traits, Carcass Characteristics, and Flavor Substances. Anim. Sci. J. 2019, 90, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Font-i-Furnols, M.; Čandek-Potokar, M.; Daumas, G.; Gispert, M.; Judas, M.; Seynaeve, M. Comparison of National ZP Equations for Lean Meat Percentage Assessment in SEUROP Pig Classification. Meat Sci. 2016, 113, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Font-i-Furnols, M.; Tous, N.; Esteve-Garcia, E.; Gispert, M. Do All the Consumers Accept Marbling in the Same Way? The Relationship between Eating and Visual Acceptability of Pork with Different Intramuscular Fat Content. Meat Sci. 2012, 91, 448–453. [Google Scholar] [CrossRef]
- Fernandez, X.; Monin, G.; Talmant, A.; Mourot, J.; Lebret, B. Influence of Intramuscular Fat Content on the Quality of Pig Meat—1. Composition of the Lipid Fraction and Sensory Characteristics of m. Longissimus Lumborum. Meat Sci. 1999, 53, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Huang, Q.; Wang, Y.; Shan, T. Lipo-Nutritional Quality of Pork: The Lipid Composition, Regulation, and Molecular Mechanisms of Fatty Acid Deposition. Anim. Nutr. 2023, 13, 373. [Google Scholar] [CrossRef]
- Szulc, K.; Wojtysiak, D.; Migdał, Ł.; Migdał, W. The Muscle Fibre Characteristics and the Meat Quality of m. Longissimus Thoracis from Polish Native Złotnicka Spotted Pigs and the Crossbreed Fatteners from the Crossing of Duroc and Polish Large White Boars. Appl. Sci. 2022, 12, 3051. [Google Scholar] [CrossRef]
- Ayuso, M.; Fernández, A.; Núñez, Y.; Benítez, R.; Isabel, B.; Barragán, C.; Fernández, A.I.; Rey, A.I.; Medrano, J.F.; Cánovas, Á.; et al. Comparative Analysis of Muscle Transcriptome between Pig Genotypes Identifies Genes and Regulatory Mechanisms Associated to Growth, Fatness and Metabolism. PLoS ONE 2015, 10, e0145162. [Google Scholar] [CrossRef]
- Platko, K.; Lebeau, P.F.; Byun, J.H.; Poon, S.V.; Day, E.A.; MacDonald, M.E.; Holzapfel, N.; Mejia-Benitez, A.; Maclean, K.N.; Krepinsky, J.C.; et al. GDF10 Blocks Hepatic PPARγ Activation to Protect against Diet-Induced Liver Injury. Mol. Metab. 2019, 27, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Svensson, K.J.; Long, J.Z.; Jedrychowski, M.P.; Cohen, P.; Lo, J.C.; Serag, S.; Kir, S.; Shinoda, K.; Tartaglia, J.A.; Rao, R.R.; et al. A Secreted Slit2 Fragment Regulates Adipose Tissue Thermogenesis and Metabolic Function. Cell Metab. 2016, 23, 454–466. [Google Scholar] [CrossRef]
- Qin, D.; Yang, Y.; Pu, Z.; Liu, D.; Yu, C.; Gao, P.; Chen, J.; Zong, C.; Zhang, Y.; Li, X.; et al. NR4A1 Retards Adipocyte Differentiation or Maturation via Enhancing GATA2 and P53 Expression. J. Cell Mol. Med. 2018, 22, 4709–4720. [Google Scholar] [CrossRef]
- John, S.; Bhowmick, K.; Park, A.; Huang, H.; Yang, X.; Mishra, L. Recent Advances in Targeting Obesity, with a Focus on TGF-β Signaling and Vagus Nerve Innervation. Bioelectron. Med. 2025, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Xu, J.; Zhou, W.; Chen, S.; Shi, H.; Han, M.; Yang, Z.; Duan, Y.; Pang, W.; Yin, Y.; et al. Metabolome and RNA-Seq Reveal Discrepant Metabolism and Secretory Metabolism Profile in Skeletal Muscle between Obese and Lean Pigs at Different Ages. Sci. China Life Sci. 2025, 68, 1102–1117. [Google Scholar] [CrossRef]
- Fu, L.; Xu, Y.; Hou, Y.; Qi, X.; Zhou, L.; Liu, H.; Luan, Y.; Jing, L.; Miao, Y.; Zhao, S.; et al. Proteomic Analysis Indicates That Mitochondrial Energy Metabolism in Skeletal Muscle Tissue Is Negatively Correlated with Feed Efficiency in Pigs. Sci. Rep. 2017, 7, 45291. [Google Scholar] [CrossRef]
- Rimmer, L.A.; Geisbrecht, E.R.; Chao, M.D.; O’Quinn, T.G.; Woodworth, J.C.; Zumbaugh, M.D. Skeletal Muscle Metabolism Is Dynamic during Porcine Postnatal Growth. Metabolites 2024, 14, 357. [Google Scholar] [CrossRef]
- Stanko, R.T.; Ferguson, T.L.; Newman, C.W.; Newman, R.K. Reduction of Carcass Fat in Swine with Dietary Addition of Dihydroxyacetone and Pyruvate1. J. Anim. Sci. 1989, 67, 1272–1278. [Google Scholar] [CrossRef]
- Liu, J.; Sebastià, C.; Jové-Juncà, T.; Quintanilla, R.; González-Rodríguez, O.; Passols, M.; Castelló, A.; Sánchez, A.; Ballester, M.; Folch, J.M. Identification of Genomic Regions Associated with Fatty Acid Metabolism across Blood, Liver, Backfat and Muscle in Pigs. Genet. Sel. Evol. 2024, 56, 66. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Q.; Pan, C.-Y.; Lan, X.-Y. The Free Fatty Acid Receptor 2 (FFA2): Mechanisms of Action, Biased Signaling, and Clinical Prospects. Pharmacol. Ther. 2025, 272, 108878. [Google Scholar] [CrossRef] [PubMed]
- Malgwi, I.H.; Halas, V.; Grünvald, P.; Schiavon, S.; Jócsák, I. Genes Related to Fat Metabolism in Pigs and Intramuscular Fat Content of Pork: A Focus on Nutrigenetics and Nutrigenomics. Animals 2022, 12, 150. [Google Scholar] [CrossRef] [PubMed]
- Kihara, A. Synthesis and Degradation Pathways, Functions, and Pathology of Ceramides and Epidermal Acylceramides. Prog. Lipid Res. 2016, 63, 50–69. [Google Scholar] [CrossRef]
- Kitamura, T.; Seki, N.; Kihara, A. Phytosphingosine Degradation Pathway Includes Fatty Acid α-Oxidation Reactions in the Endoplasmic Reticulum. Proc. Natl. Acad. Sci. USA 2017, 114, E2616–E2623. [Google Scholar] [CrossRef]
- Nagahara, Y.; Shinomiya, T.; Kuroda, S.; Kaneko, N.; Nishio, R.; Ikekita, M. Phytosphingosine Induced Mitochondria-Involved Apoptosis. Cancer Sci. 2005, 96, 83–92. [Google Scholar] [CrossRef]
- Nikolova-Karakashian, M.N.; Reid, M.B. Sphingolipid Metabolism, Oxidant Signaling, and Contractile Function of Skeletal Muscle. Antioxid. Redox Signal. 2011, 15, 2501–2517. [Google Scholar] [CrossRef] [PubMed]

| Ingredients | Contents | Nutrient Components | Contents |
|---|---|---|---|
| Corn | 58.00 | CP | 13.13 |
| Soybean meal | 14.00 | DE/(MJ/kg) | 11.33 |
| Wheat bran | 9.00 | ||
| Grass meal | 15.00 | ||
| Premix | 4.00 | ||
| Total | 100.00 |
| Traits | HN (n = 3) | BH (n = 3) | LH (n = 3) | YH (n = 3) | p-Value |
|---|---|---|---|---|---|
| Eye muscle area (cm2) | 31.933 b ± 1.608 | 39.333 a ± 1.167 | 37.833 a ± 1.167 | 41.267 a ± 2.022 | 0.013 |
| Lean meat percentage (%) | 45.903 B ± 1.397 | 50.537 AB ± 1.047 | 56.871 A ± 1.407 | 57.522 A ± 2.613 | 0.007 |
| IMF content (%) | 7.653 a ± 0.251 | 4.800 ab ± 0.916 | 5.033 ab ± 0.698 | 3.167 b ± 1.068 | 0.025 |
| Muscle fiber area (μm2) | 4486.000 A ± 95.970 | 3788.250 BC ± 92.813 | 3523.835 C ± 106.220 | 4011.435 B ± 74.525 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Li, Y.; Zhang, M.; Chen, J.; Lu, Q.; Zhang, H.; Yan, X.; Pan, C.; Zhang, X.; Xing, B. RNA Sequencing and Metabolomic Analyses Reveal Differences in Muscle Characteristics and Metabolic Profiles Between Purebred and Crossbred Huainan Pigs. Animals 2025, 15, 3144. https://doi.org/10.3390/ani15213144
Wang J, Li Y, Zhang M, Chen J, Lu Q, Zhang H, Yan X, Pan C, Zhang X, Xing B. RNA Sequencing and Metabolomic Analyses Reveal Differences in Muscle Characteristics and Metabolic Profiles Between Purebred and Crossbred Huainan Pigs. Animals. 2025; 15(21):3144. https://doi.org/10.3390/ani15213144
Chicago/Turabian StyleWang, Jing, Yufu Li, Mengyang Zhang, Junfeng Chen, Qingxia Lu, Hanbing Zhang, Xiangzhou Yan, Chuanying Pan, Xuelian Zhang, and Baosong Xing. 2025. "RNA Sequencing and Metabolomic Analyses Reveal Differences in Muscle Characteristics and Metabolic Profiles Between Purebred and Crossbred Huainan Pigs" Animals 15, no. 21: 3144. https://doi.org/10.3390/ani15213144
APA StyleWang, J., Li, Y., Zhang, M., Chen, J., Lu, Q., Zhang, H., Yan, X., Pan, C., Zhang, X., & Xing, B. (2025). RNA Sequencing and Metabolomic Analyses Reveal Differences in Muscle Characteristics and Metabolic Profiles Between Purebred and Crossbred Huainan Pigs. Animals, 15(21), 3144. https://doi.org/10.3390/ani15213144








