Study of the Nasal Cavity of the Cadaveric Yellow-Legged Gull (Larus michahellis atlantis) Through Anatomical Cross-Sections and Computed Tomography
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimens
2.2. CT Technique
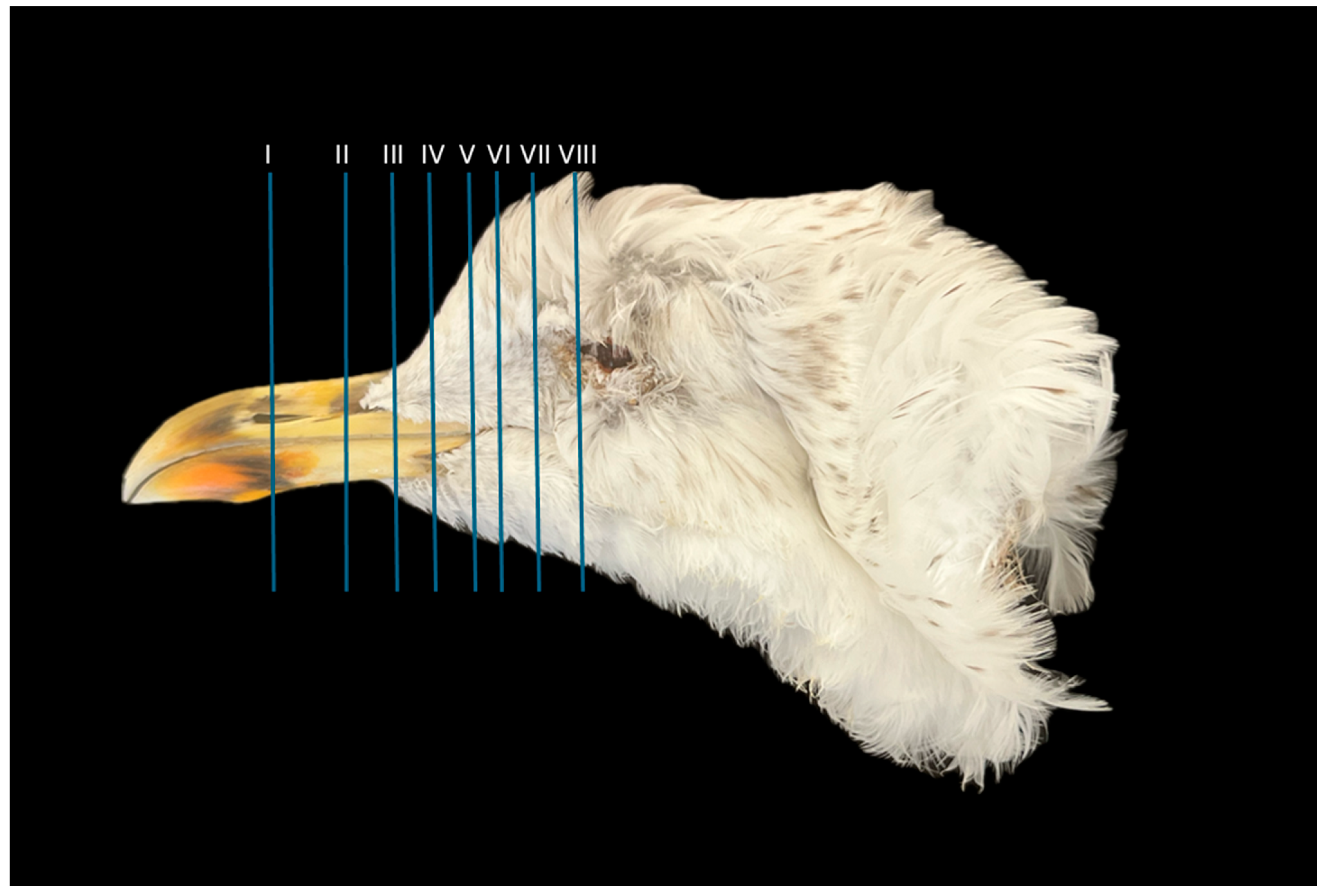
2.3. Anatomical Sections
2.4. Anatomical Evaluation
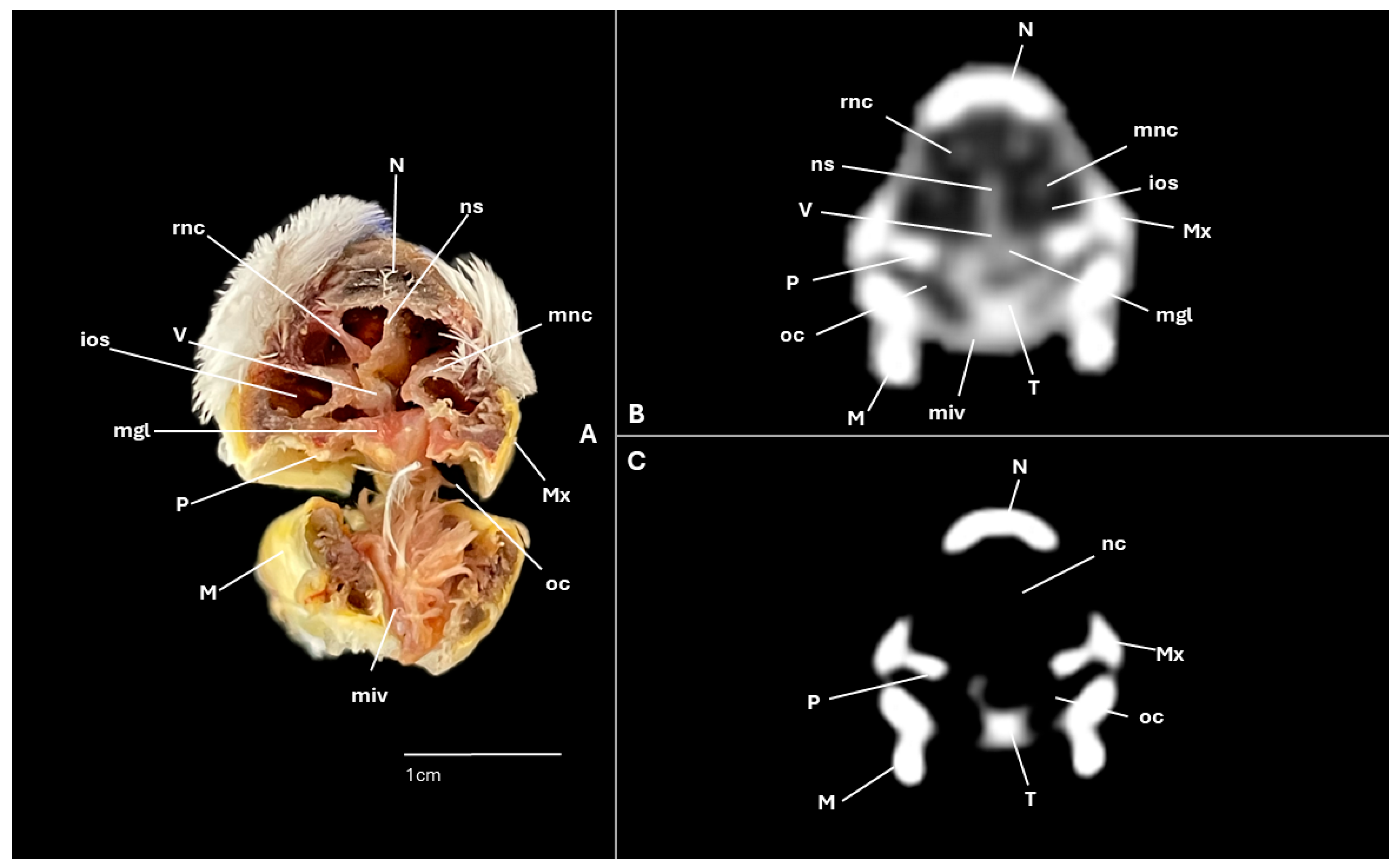
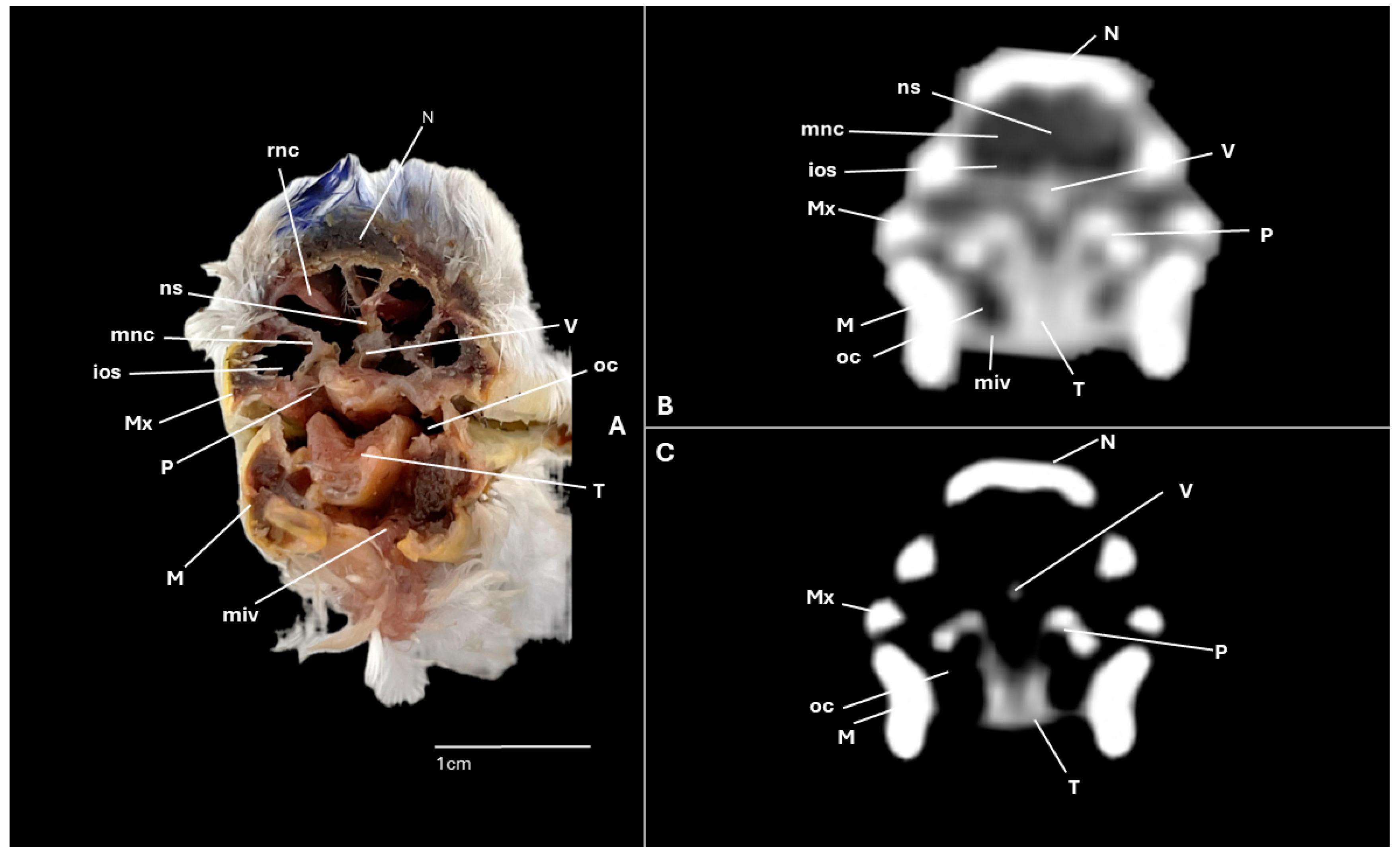
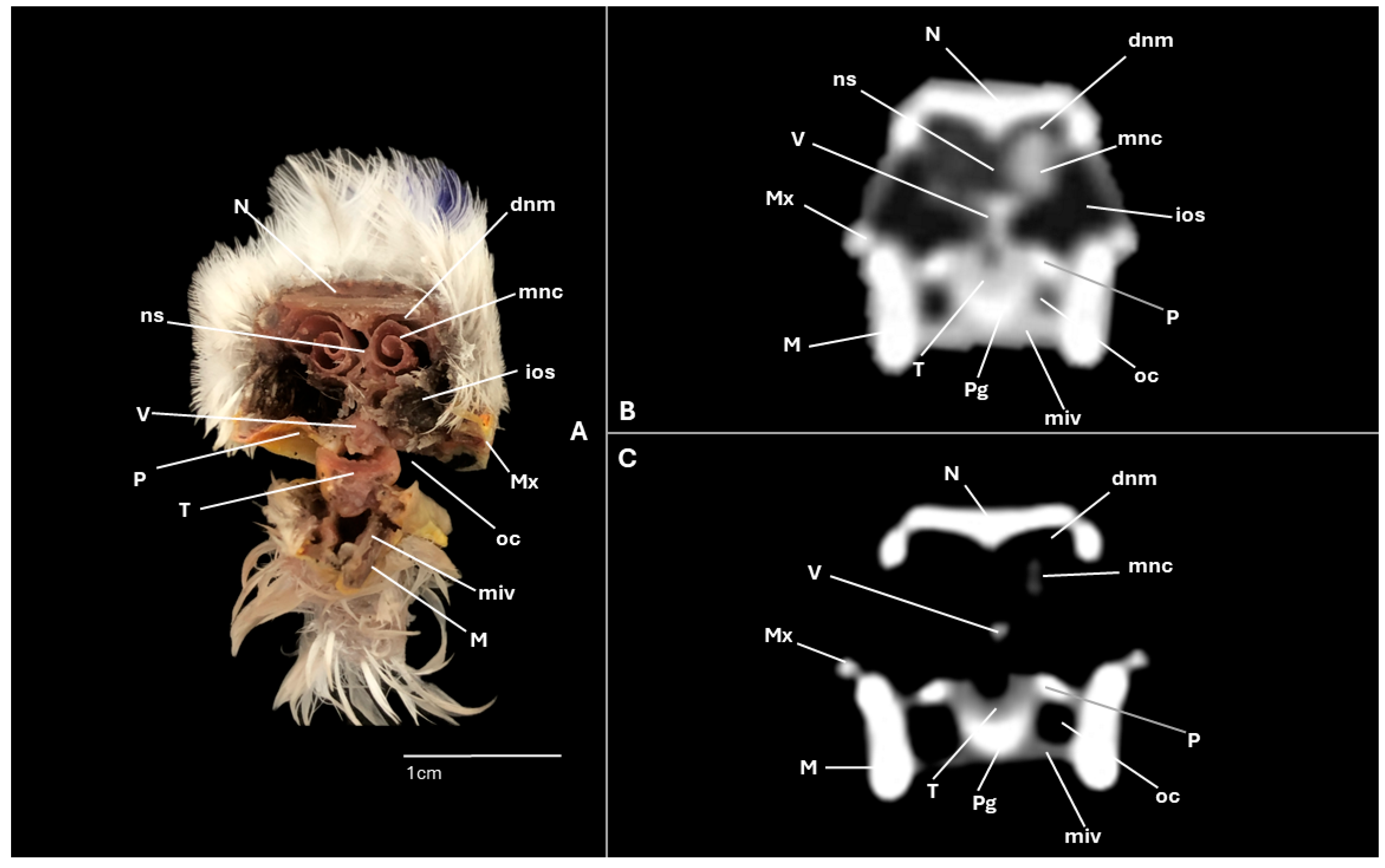
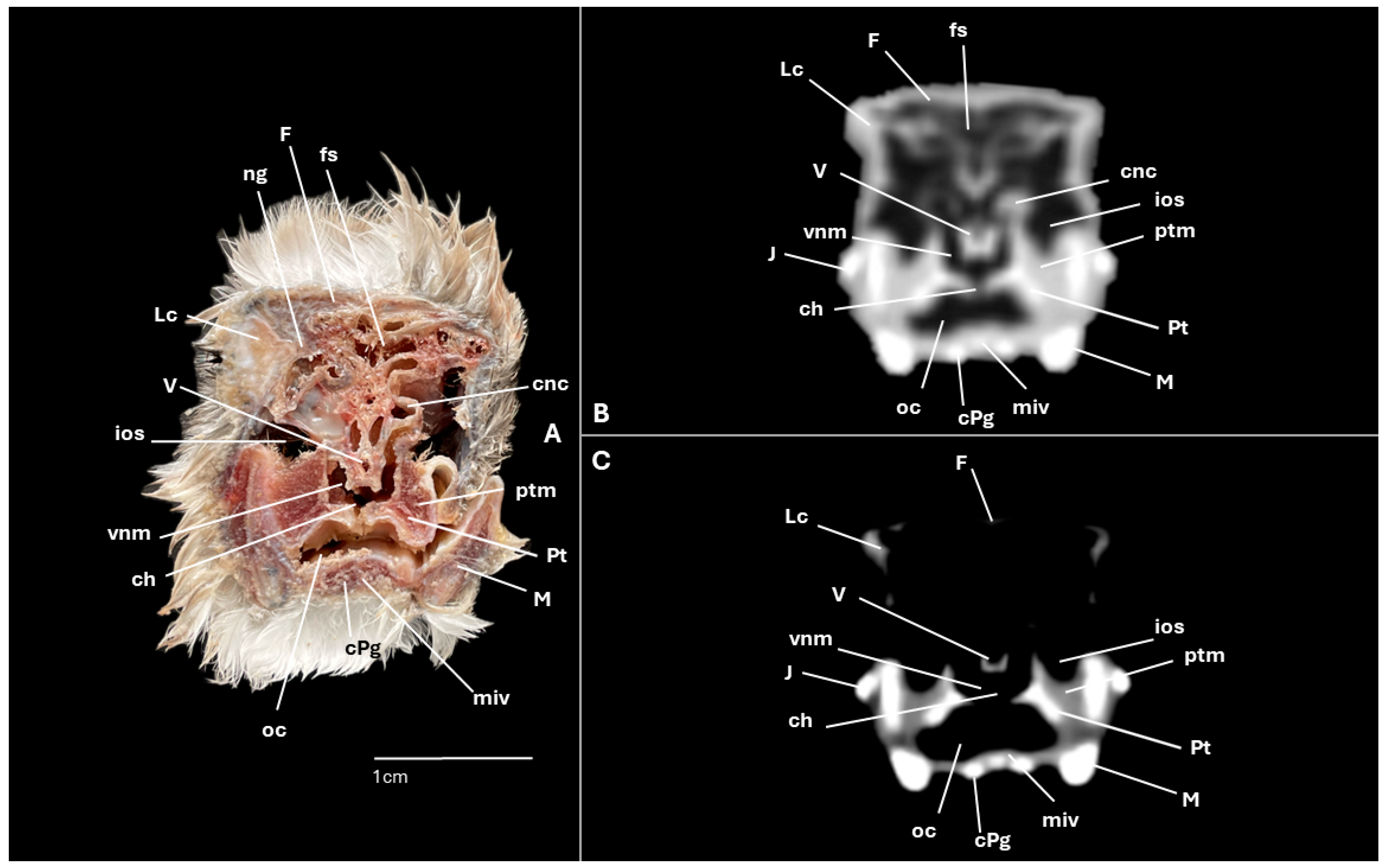
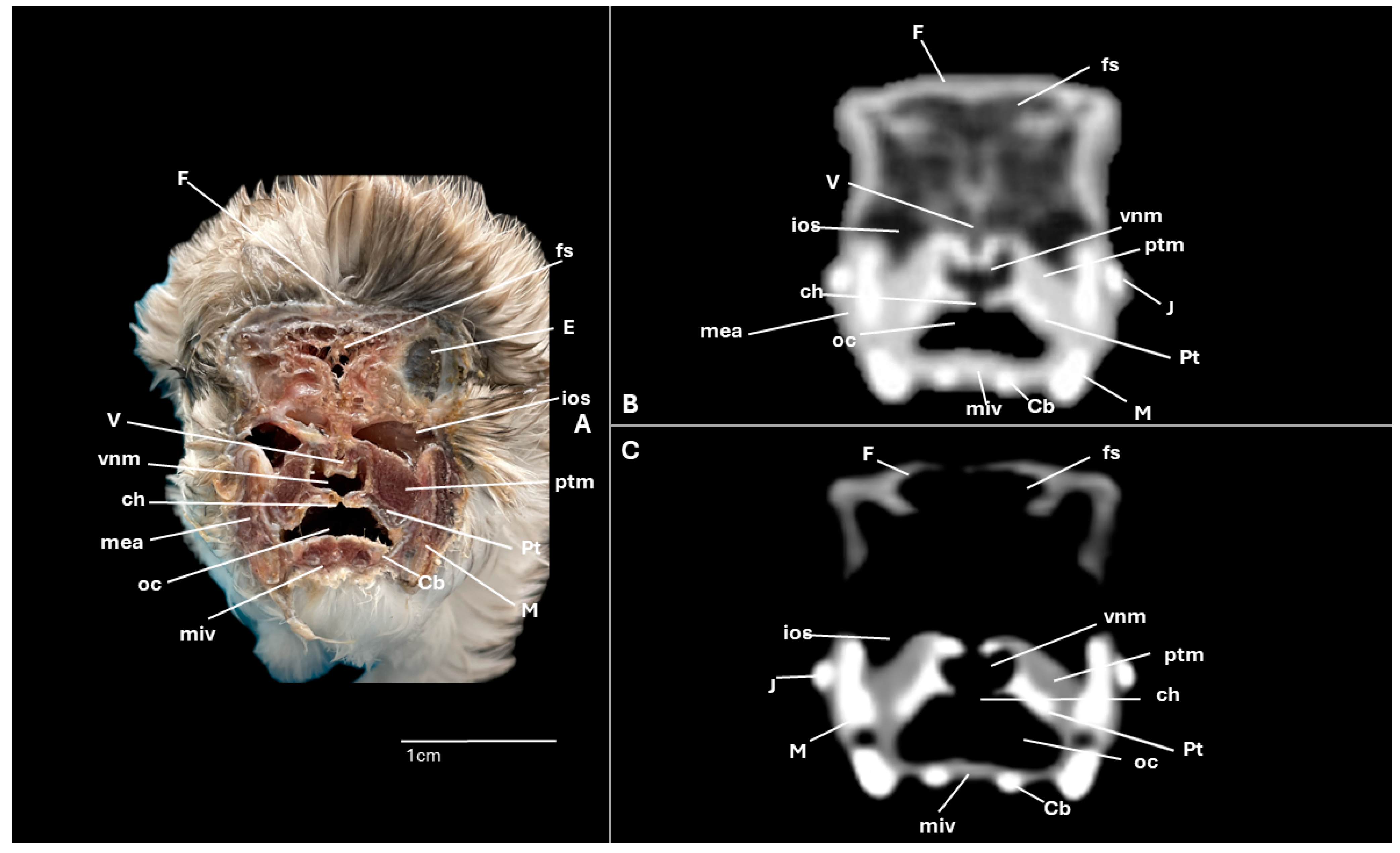
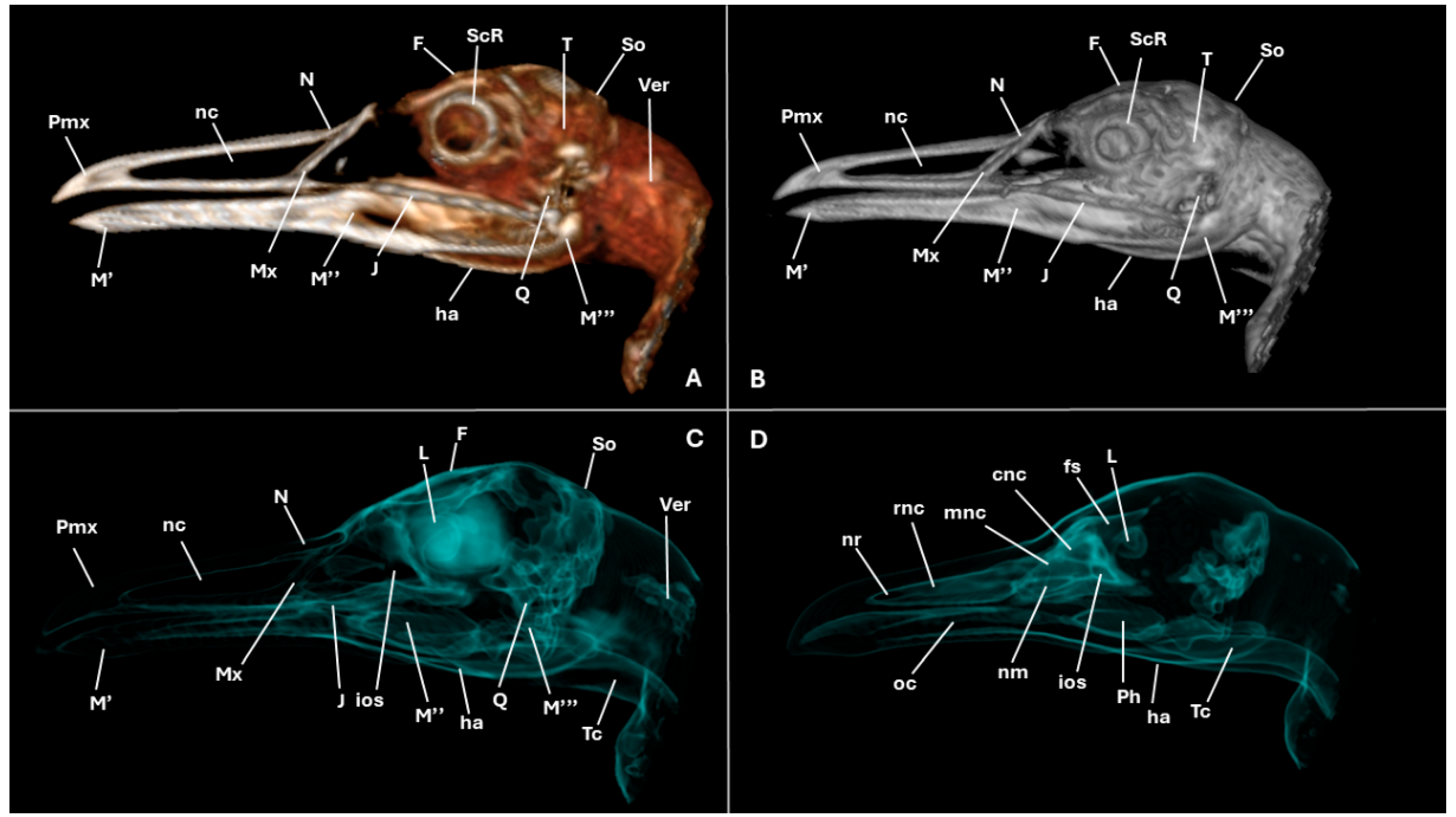
3. Results
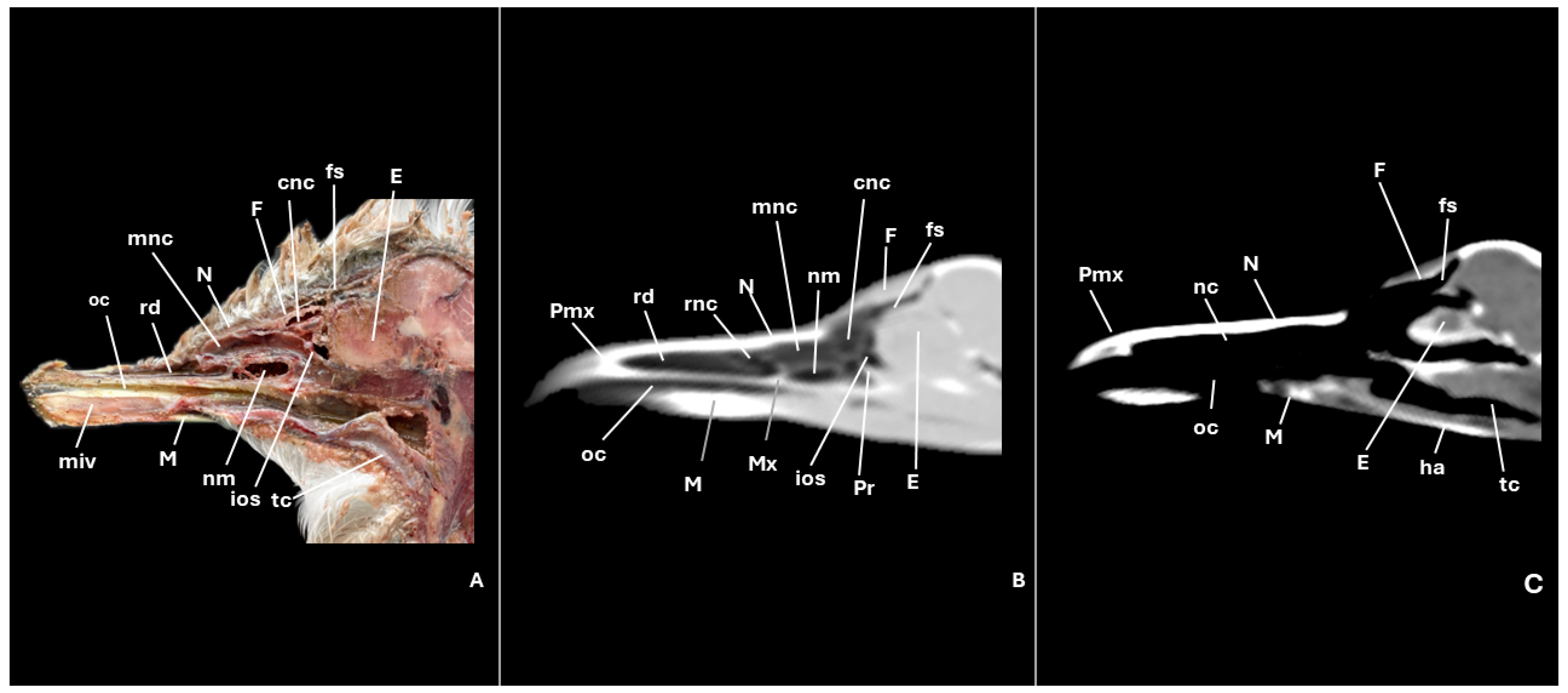
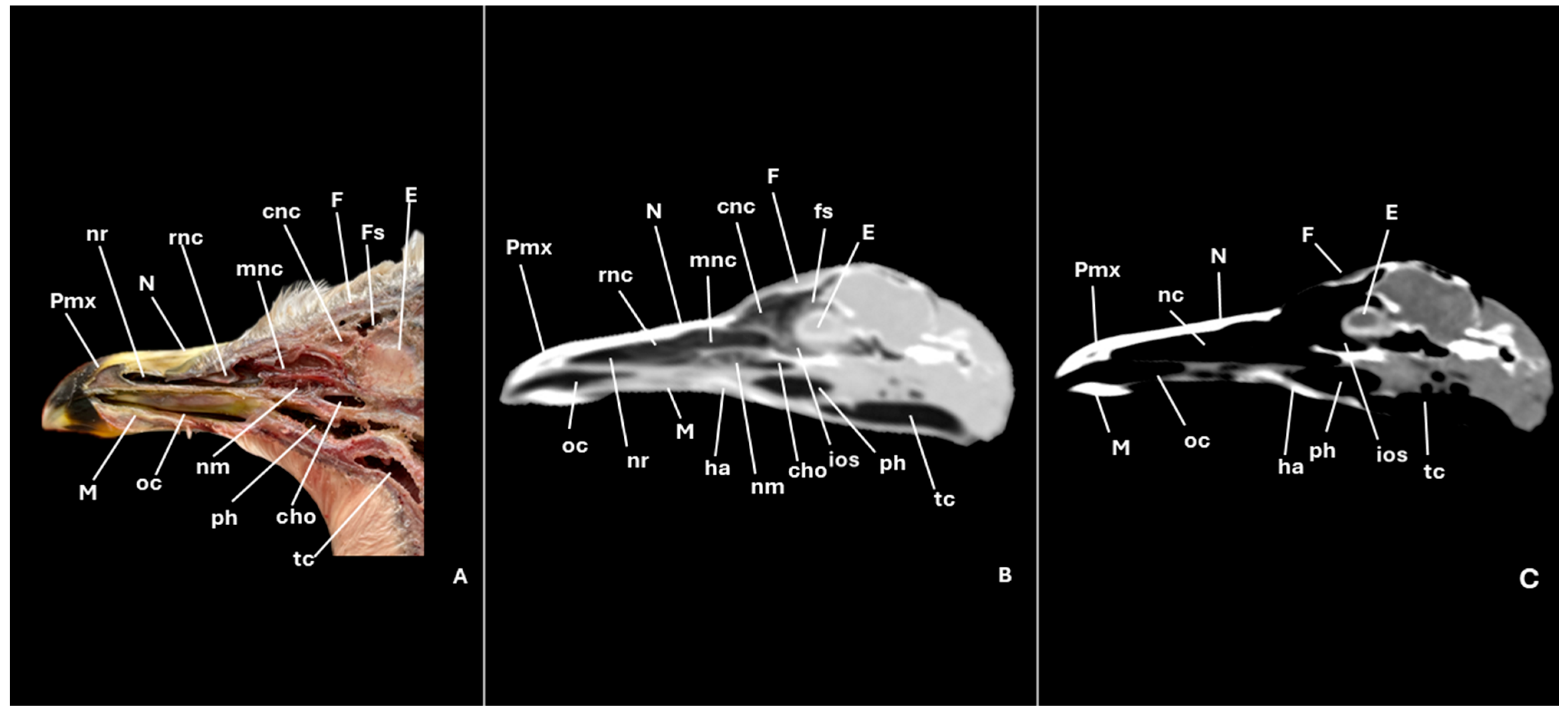
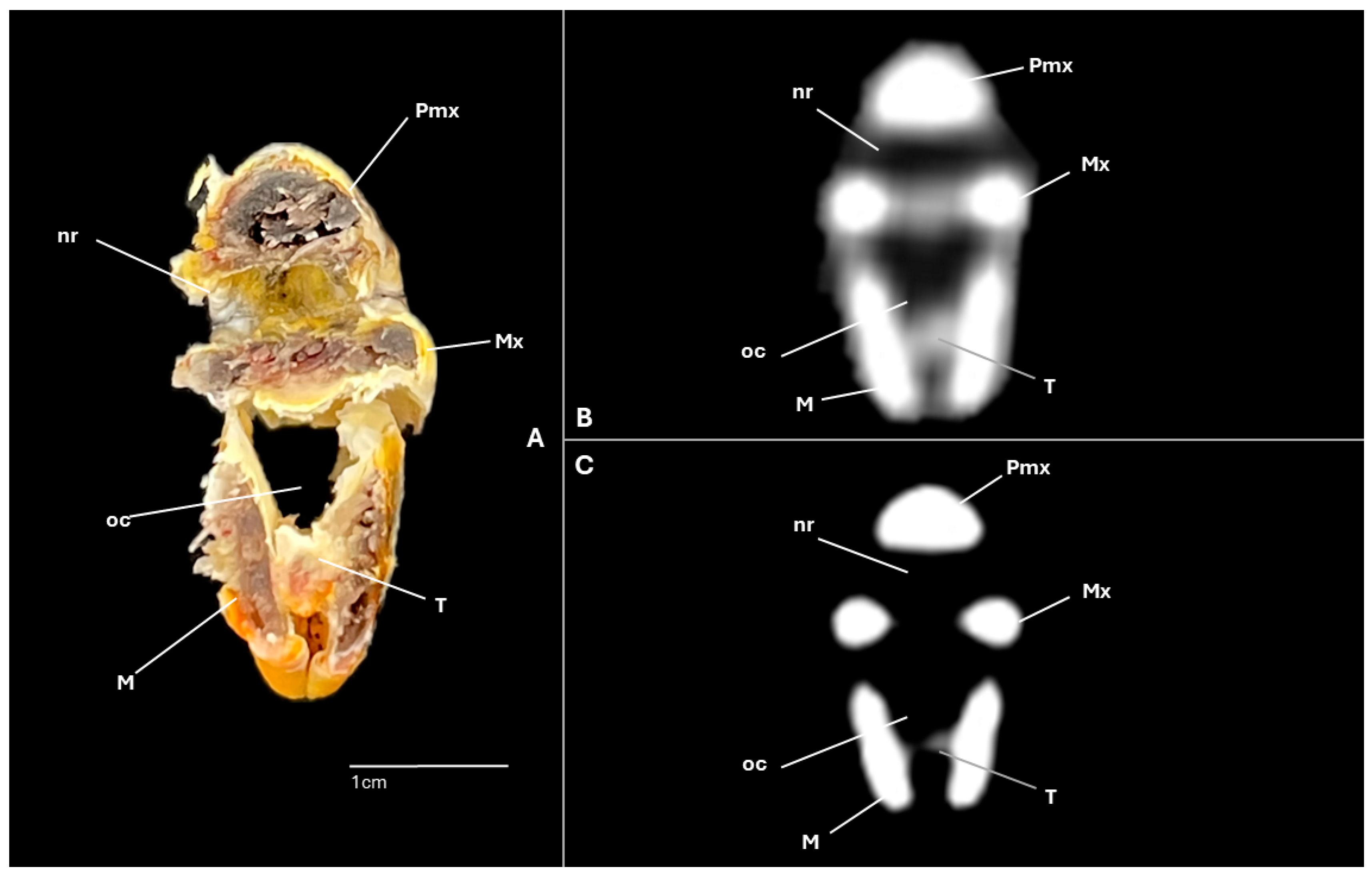
3.1. Anatomical Cross-Sections
3.2. Computed Tomography (CT)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coccon, F.; Vanni, L.; Dabalà, C.; Giunchi, D. The Abundance of Yellow-Legged Gulls Larus Michahellis Breeding in the Historic Centre of Venice, Italy and the Initial Effects of the New Waste Collection Policy on the Population. Urban Ecosyst. 2022, 25, 643–656. [Google Scholar] [CrossRef]
- SEO/BirdLife. Gaviota Patiamarilla. Available online: https://seo.org/ave/gaviota-patiamarilla/ (accessed on 3 July 2025).
- BiObserva. Gaviota Patiamarilla (Larus michahellis). Available online: https://www.biobserva.com/main/especies/LARMIC (accessed on 3 July 2025).
- ten Cate, C. Niko Tinbergen and the red patch on the herring gull’s beak. Anim. Behav. 2009, 77, 785–794. [Google Scholar] [CrossRef]
- Audubon Society. Ivory Gull. Available online: https://www.audubon.org/birds-of-america/ivory-gull (accessed on 3 July 2025).
- Ritchison, G. Bird Nostrils. Available online: https://avesbiology.com/birdnostrils.html (accessed on 3 July 2025).
- Cornell Lab of Ornithology. American Herring Gull. Available online: https://www.allaboutbirds.org/guide/American_Herring_Gull/overview (accessed on 3 July 2025).
- Ceccherelli, R.; Ebani, V.V.; Pesaro, S.; Rossi, G.; Perrucci, S. Reighardia Sternae Infection and Associated Lesions in a Yellow-Legged Gull (Larus Michahellis) in Italy. Vet. Sci. 2025, 12, 411. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wille, M.; Benkaroun, J.; Munro, H.; Bond, A.L.; Fifield, D.A.; Robertson, G.J.; Ojkic, D.; Whitney, H.; Lang, A.S. Perpetuation and Reassortment of Gull Influenza A Viruses in Atlantic North America. Virology 2014, 456–457, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Orosz, S.E.; Lichtenberger, M. Avian Respiratory Distress: Etiology, Diagnosis, and Treatment. Vet. Clin. N. Am. Exot. Anim. Pract. 2011, 14, 241–255. [Google Scholar] [CrossRef]
- Heneberg, P.; Sitko, J.; Yakovleva, G.; Lebedeva, D. Severe Decline in Abundance of Cyathostoma Lari, a Parasite of the Nasal and Orbital Sinuses of Gulls, at Their Central European Nesting Grounds. J. Helminthol. 2024, 98, e1. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, H.; Lei, F.; Zhu, Q.; Qin, K.; Zhang, X.-W.; Zhang, X.-L.; Zhao, D.; Wang, G.; Feng, Y.; et al. Highly Pathogenic H5N1 Influenza Virus Infection in Migratory Birds. Science 2005, 309, 1206. [Google Scholar] [CrossRef]
- Hammouda, A.; Pearce-Duvet, J.; Chokri, M.A.; Arnal, A.; Gauthier-Clerc, M.; Boulinier, T.; Selmi, S. Prevalence of Influenza A Antibodies in Yellow-Legged Gull (Larus michahellis) Eggs and Adults in Southern Tunisia. Vector Borne Zoonotic Dis. 2011, 11, 1583–1590. [Google Scholar] [CrossRef]
- Koenhemsi, L. Liver ultrasonography of healthy yellow-legged gull. Int. J. Agric. 2019, 4, 159. [Google Scholar]
- Noemi, V.; Segovia, Y.; García, M. Cone Distribution and Visual Resolution of the Yellow-Legged Gull (Larus michahellis) in relation to habitat and behaviour. Anat. Histol. Embryol. 2022, 51, 197–214. [Google Scholar]
- Codner, E.C.; Lurus, A.G.; Miller, J.B.; Gavin, P.R.; Gallina, A.; Barbee, D.D. Comparison of Computed Tomography with Radiography as a Noninvasive Diagnostic Technique for Chronic Nasal Disease in Dogs. J. Am. Vet. Med. Assoc. 1993, 202, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, T.; Sullivan, M.; Hartung, K. Radiographic Detection of Defects of the Nasal Boundaries. Vet. Radiol. Ultrasound 2000, 41, 226–230. [Google Scholar] [CrossRef]
- Gibbs, C.; Lane, J.G.; Denny, H.R. Radiologic features of intra-nasal lesions in the dog: A review of 100 cases. J. Small Anim. Pract. 1979, 205, 15–535. [Google Scholar]
- Wilson, M.; Mauragis, C.V.D.; Berry, C.R. Small Animal Skull & Nasofacial Radiography, Including the Nasal Cavity & Frontal Sinuses. Vet. Radiol. Ultrasound 2025, 66, 205–212. [Google Scholar]
- Morales-Espino, A.; Fumero-Hernández, M.; Suárez-Cabrera, F.; Encinoso, M.; Conde-Felipe, M.M.; Jaber, J.R. Computed Tomography Anatomy of the Juvenile Cory’s Shearwater (Calonectris Borealis) Normal Nasal Cavity. Animals 2024, 14, 3015. [Google Scholar] [CrossRef]
- Burk, R.L. Computed Tomographic Anatomy of the Canine Nasal Passages. Vet. Radiol. Ultrasound 1992, 33, 170–176. [Google Scholar] [CrossRef]
- Arencibia, A.; Vázquez, J.M.; Jaber, R.; Gil, F.; Ramírez, J.A.; Rivero, M.; González, N.; Wisner, E.R. Magnetic resonance imaging and cross-sectional anatomy of the normal equine sinuses and nasal passages. Vet. Radiol. Ultrasound 2000, 41, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Rycke, L.M.D.; Saunders, J.H.; Gielen, I.M.; van Bree, H.J.; Simoens, P.J. Magnetic resonance imaging, computed tomography, and cross-sectional views of the anatomy of normal nasal cavities and paranasal sinuses in mesocephalic dogs. Am. J. Vet. Res. 2003, 64, 1093–1098. [Google Scholar] [CrossRef]
- Fleming, G.J.; Lester, N.V.; Stevenson, R.; Silver, X.S. High field strength (4.7T) magnetic resonance imaging of hydrocephalus in an African Grey parrot (Psittacus erithacus). Vet. Radiol. Ultrasound 2003, 44, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Díaz Martínez, E.; Arencibia Espinosa, A.; Soler Laguía, M.; Kilroy, D.; Martínez Gomariz, F.; Casas García, D.L.; Sánchez Collado, C.; Gil Cano, F.; Jaber, J.R.; Ramírez Zarzosa, G. An Anatomical Study Using Computed Tomography, Magnetic Resonance Imaging, and Rhinoscopy of the Nasal Cavity of Domestic Cat (Felis silvestris catus L.) and Big Cats: Lion (Panthera leo leo L.), Leopard (Panthera pardus kotiya L.), and Cheetah (Acinonyx jubatus jubatus S.). Animals 2024, 14, 1172. [Google Scholar] [CrossRef]
- Aref, M.; Raouf, M.A.E.; Youssef, W.O.M.; Abdelbaset-Ismail, A.; Salem, G.A.; Nassan, M.A.; Rutland, C.S.; Mahdy, E.A.A. Structural Investigations of the Normal Ostrich Head Using Anatomical Sections, Computed Tomography, and Magnetic Resonance Imaging. Open Vet. J. 2024, 14, 3487–3497. [Google Scholar] [CrossRef] [PubMed]
- Faillace, A.C.L.; Vieira, K.R.A.; Santana, M.I.S. Computed Tomographic and Gross Anatomy of the Head of the Blue-Fronted Amazon Parrot (Amazona aestiva). Anat. Histol. Embryol. 2021, 50, 192–205. [Google Scholar] [CrossRef]
- Veladiano, I.A.; Banzato, T.; Bellini, L.; Montani, A.; Catania, S.; Zotti, A. Computed Tomographic Anatomy of the Heads of Blue-and-Gold Macaws (Ara ararauna), African Grey Parrots (Psittacus erithacus), and Monk Parakeets (Myiopsitta monachus). Am. J. Vet. Res. 2016, 77, 1346–1356. [Google Scholar] [CrossRef]
- Hadden, P.W.; Ober, W.C.; Gerneke, D.A.; Thomas, D.; Scadeng, M.; McGhee, C.N.J.; Zhang, J. Micro-CT Guided Illustration of the Head Anatomy of Penguins (Aves: Sphenisciformes: Spheniscidae). J. Morphol. 2022, 283, 827–851. [Google Scholar] [CrossRef]
- Baumel, J.J.; King, A.S.; Breazile, J.E.; Evans, H.E.; Vanden Berge, J.C. Handbook of Avian Anatomy, 2nd ed.; World Association of Veterinary Anatomists: Ithaca, NY, USA, 1993; pp. 45–132. [Google Scholar]
- Ali, S. Gross Anatomical Studies on The Nasal Cavity of The Ostrich. Benha Vet. Med. J. 2015, 29, 326–332. [Google Scholar] [CrossRef]
- Mushi, E.Z.; Isa, J.F.W.; Chabo, R.G.; Segaise, T.T. Growth rate of ostrich (Struthio camelus) chicks under intensive management in Botswana. Trop. Anim. Health Prod. 1998, 30, 197–203. [Google Scholar] [CrossRef]
- Onuk, B.; Kabak, M.; Sahin, B.; Gezer İnce, N. Volumetric and Volume Fractional Comparison of the Nasal Structures of the Stork (Ciconia ciconia) and Seagull (Larus fuscus) Using Computed Tomography Images. Pak. J. Zool. 2018, 50, 963–967. [Google Scholar] [CrossRef]
- Peng, K.; Feng, Y.; Zhang, G.; Liu, H.; Song, H. Anatomical Study of the Brain of the African Ostrich. Turk. J. Vet. Anim. Sci. 2010, 34, 235–241. [Google Scholar] [CrossRef]
- Hanafy, B. Structural Adaption of the Nasal Conchae of Eurasian Common Moorhen (Gallinula chloropus chloropus, Linnaeus, 1758): Histomorphological Study. Microsc. Res. Tech. 2021, 84, 2195–2202. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, F.; Tellhelm, B. Beak, Oropharyngeal and Nasal Cavities of Broad Breasted White Turkey (Meleagris gallopavo): Gross Anatomical and Morphometrical Study. J. Adv. Vet. Res. 2022, 12, 99–106. [Google Scholar]
- Hassan, S. Gross anatomical features of the nasal cavity of the hooded crow (Corvus cornix). Suez Canal Univ. Med. J. 2012, 17, 119–127. [Google Scholar]
- Madkour, F.A. Anatomical descriptions of the nasal cavity of aquatic and non-aquatic birds. Int. J. Vet. Sci. 2019, 2, 101–110. [Google Scholar] [CrossRef]
- Schmidt, R.; Struthers, J.; Phalen, D. Pathology of Pet and Aviary Birds; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Casteleyn, C.; Cornillie, P.; Van Cruchten, S.; Van den Broeck, W.; Van Ginneken, C.; Simoens, P. Anatomy of the upper respiratory tract in domestic birds, with emphasis on vocalization. Anat. Histol. Embryol. 2018, 47, 100–109. [Google Scholar] [CrossRef]
- Maina, J.N. Perspectives on the Structure and Function of the Avian Respiratory System: Functional Efficiency Built on Structural Complexity. Front. Anim. Sci. 2022, 3, 851574. [Google Scholar] [CrossRef]
- Witmer, L.M.; Ridgely, R.C. The Paranasal Air Sinuses of Predatory and Armored Dinosaurs (Archosauria: Theropoda and Ankylosauria) and Their Contribution to Cephalic Structure. Anat. Rec. 2008, 291, 1362–1388. [Google Scholar] [CrossRef]
- Mohamed, S. Some Morphological Studies of the Nasal Cavity of Ostrich (Struthio camelus). Ph.D. Thesis, Zagazig University, Zagazig, Egypt, 2008. [Google Scholar]
- Moselhy, A.A.A.; Mohamed, S.K.A.; El-Ghazali, H.M. Anatomical Features of Bones and Bony Cavities of the Ostrich (Struthio camelus). Int. J. Anat. Res. 2018, 6, 5390–5398. [Google Scholar] [CrossRef]
- Bourke, J.M.; Witmer, L.M. Nasal Conchae Function as Aerodynamic Baffles: Experimental Computational Fluid Dynamic Analysis in a Turkey Nose (Aves: Galliformes). Respir. Physiol. Neurobiol. 2016, 234, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Morales-Espino, A.; Déniz, S.; Paz-Oliva, P.; Roldán-Medina, N.; Encinoso, M.; Suárez-Cabrera, F.; Jaber, J.R. Cory’s Shearwater (Calonectris borealis): Exploring Normal Head Anatomy through Cross-Sectional Anatomy, Computed Tomography and Magnetic Resonance Imaging. Animals 2024, 14, 1962. [Google Scholar] [CrossRef] [PubMed]
- Banzato, T.; Hellebuyck, T.; Van Caelenberg, A.; Saunders, J.H.; Zotti, A. A review of diagnostic imaging of snakes and lizards. Vet. Rec. 2013, 173, 43–49. [Google Scholar] [CrossRef] [PubMed]


| Animal | Weight (g) | Head Length (cm) | Beak Length (cm) |
|---|---|---|---|
| 1 | 957.4 | 14.5 | 8.0 |
| 2 | 663.3 | 11.5 | 7.5 |
| 3 | 776.9 | 12.4 | 7.5 |
| 4 | 785.1 | 12.4 | 7.8 |
| 5 | 631.9 | 13.2 | 7.8 |
| 6 | 673.5 | 11.3 | 7.4 |
| 7 | 807.2 | 12.5 | 8.0 |
| 8 | 665.5 | 12.5 | 7.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaber, J.R.; Morales, M.; Ros, A.; Paz-Oliva, P.; Roldán-Medina, N.; Morales-Espino, A.; Arencibia, A.; Déniz, S. Study of the Nasal Cavity of the Cadaveric Yellow-Legged Gull (Larus michahellis atlantis) Through Anatomical Cross-Sections and Computed Tomography. Animals 2025, 15, 3114. https://doi.org/10.3390/ani15213114
Jaber JR, Morales M, Ros A, Paz-Oliva P, Roldán-Medina N, Morales-Espino A, Arencibia A, Déniz S. Study of the Nasal Cavity of the Cadaveric Yellow-Legged Gull (Larus michahellis atlantis) Through Anatomical Cross-Sections and Computed Tomography. Animals. 2025; 15(21):3114. https://doi.org/10.3390/ani15213114
Chicago/Turabian StyleJaber, Jose Raduan, Manuel Morales, Alvaro Ros, Pablo Paz-Oliva, Natalia Roldán-Medina, Alejandro Morales-Espino, Alberto Arencibia, and Soraya Déniz. 2025. "Study of the Nasal Cavity of the Cadaveric Yellow-Legged Gull (Larus michahellis atlantis) Through Anatomical Cross-Sections and Computed Tomography" Animals 15, no. 21: 3114. https://doi.org/10.3390/ani15213114
APA StyleJaber, J. R., Morales, M., Ros, A., Paz-Oliva, P., Roldán-Medina, N., Morales-Espino, A., Arencibia, A., & Déniz, S. (2025). Study of the Nasal Cavity of the Cadaveric Yellow-Legged Gull (Larus michahellis atlantis) Through Anatomical Cross-Sections and Computed Tomography. Animals, 15(21), 3114. https://doi.org/10.3390/ani15213114







