1. Introduction
Nutrients are essential for the life and development of an animal. Generally, the chicken embryo obtains nutrients from the egg yolk, which mainly contains lipids [
1]. Lipids are energy sources and are very important for chick hatching because the hatching period is a very energy-demanding stage [
2,
3]. The later stage of chicken embryo development is the fastest growing and most crucial period for functional maturity, with energy requirements increasing by three to five times compared to the initial and middle stages. After hatching, chicks are generally deprived of feed for a period of 24 to 84 h [
4,
5,
6]. Numerous studies have reported that delayed access to feed reduces post-hatch performance in broilers [
7,
8,
9,
10,
11]. Indeed, in ovo feeding is a technique that allows nutrients to be supplied to the chicken embryo during incubation. This helps the chick to overcome nutritional deficiencies after hatching. The study has found that in ovo feeding is an alternative method to increase the hatching rate and muscle growth weight of chicks [
12]. Thus, Bhanja and Mandal [
13] demonstrated in their study that in ovo injection influences embryonic development and the growth of chicks after hatching. The work of Kadam et al. [
14] showed that in ovo injection of amino acids, carbohydrates, and vitamins improved hatchability and chick growth after hatching. Similarly, Ohta et al. [
15] and Zielinska et al. [
16] also showed that in ovo injection of amino acids supports embryonic development. According to Kpodo and Proszkowiec-Weglarz [
17], in ovo feeding during early embryonic development directly influences organ formation, whereas during late development in ovo injection increases the energy reserves that will support the hatching process.
Vegetable oils have already been used to feed chicken embryos. Sulaiman and Tayeb [
18] evaluated the effect of in ovo injection of 0.1 mL of some vegetable oils, such as rosemary oil, black cumin oil, olive oil, and almond oil, on the hatching rate and subsequent physiological responses of post-hatching broilers. Following the results of their work, hatching and post-hatching performance were improved in the test batches compared to the control lots. Currently, there has been a growing need to explore alternative energy sources, with insects gaining attention due to their favorable nutritional profile. Black soldier fly (BSF) maggots, which contain 40% to 45% protein and 17% to 49% lipids, are known as a promising feed ingredient in animal nutrition. However, to our knowledge, very few studies have been conducted on in ovo injections of maggot oil from BSF. Could in ovo injection of BSF larvae oil also improve hatchability and post-hatch performance of chicks? Further research is needed to find out. It is in this context that this work was undertaken to know the effect of in ovo oil injection of BSF maggots on the hatching rate and some physiological parameters after the hatching of broiler chicks.
2. Materials and Methods
The study was carried out in two experimental stages at the experimental farm of the College of Animal Science and Technology of Shandong Agricultural University, China.
2.1. Experiment 1 (Incubation)
For this study, a total of 2300 eggs from Arbor Acres broiler breeders of 50 weeks of age were obtained from a commercial farm (Weifang Hengrui Animal Co., Ltd., Weifang, China).
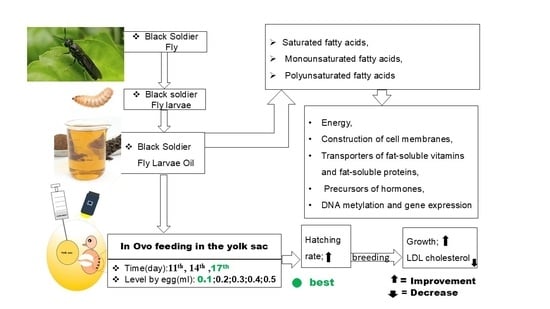
The birds were confined to ten hens per a cock in cages. Water and feed were provided ad libitum. All necessary vaccines and medications were provided according to the guidelines of the Veterinary Service. Before incubation, the eggs were numbered and weighed; the mean egg weight was 65.03 g. Incubation was performed in an incubator where the temperature and humidity were set to 37.8 °C and 60%, respectively, during the first 18 days of incubation. From the 19th to the 21st day, the eggs were subjected to the same temperature (37.8 °C), but the humidity was set to 70% in the hatchers. Indeed, on the 10th day of incubation, the infertile eggs were excluded, and 2268 fertile eggs were selected and then divided into three homogeneous groups, A, B, and C. Each group of 756 eggs was subdivided into seven lots of 108 eggs, labeled as CON−, CON+, L0.1, L0.2, L0.3, L0.4, and L0.5, for an in ovo injection of maggot oil from black soldier flies, i.e., six replicates of 18 eggs each. All the eggs were placed in the same incubator, and the eggs from each treatment of the different groups were distributed evenly across all the incubation trays. The in ovo injection of maggot oil from the black soldier flies was made through the yolk sac on the 11th, 14th, and 17th day of incubation for groups A, B, and C, respectively.
On the day of the injection, oils from BSF larvae were placed under the standard incubation temperature (37.8 °C) for 15 min before the injection. For this operation, the eggs were scanned with a light source, and the yolk sac was identified. The eggs were pierced using a 21-gauge needle drill 5 mm deep. The oil injection was performed with a semi-automatic syringe according to the method of Zhu et al. [
19]. As soon as it was injected, the injection site of each egg was disinfected with 75% alcohol and then closed with paraffin. For each group, CON− had not been pierced or injected but was removed from the incubator and then replaced, along with the other batches. CON+ was punctured but had not received oil injection. On the other hand, each of the eggs in batches L0.1, L0.2, L0.3, L0.4, and L0.5 received 0.1 mL, 0.2 mL, 0.3 mL, 0.4 mL, and 0.5 mL of BSF maggot oil, respectively.
2.2. Oil from Black Soldier Fly Maggot
The maggot oil from the BSF was obtained from Sino Crown Biological Engineering Co., Ltd. (Qingdao, China) and stored at 4 °C for use. The fatty acid composition of BSF maggot oil is shown in
Table 1.
2.3. Data Collection
From day 19 until hatching, external pipping data were recorded for each batch by observing the eggs every six hours. Early and late embryonic mortalities were determined after breaking the unhatched eggs and observing the developmental status of the embryos. The cumulative external pipping, hatch rates, and embryonic mortality rates of the chicks in each batch were calculated using Formulas (1), (2), and (3), respectively.
2.4. Experiment 2 (Breeding)
After hatching, mixed-sex chicks (male and female) from each of the seven lots of each group were obtained according to the incubation setup. A total of 336 chicks per group, or 48 chicks by lot, were randomly selected due to six replications of eight, and placed in different cages measuring 95 cm × 75 cm × 60 cm. At the start, each lot had three cages housing sixteen chicks. During the growing phase of the chicks, the number of cages for each lot increased from three to six, housing eight chicks. The sanitation, temperature, lighting, and humidity were managed according to the requirements of broiler chickens. All were fed the same diet and had access to water ad libitum. The starter and grower feeds were purchased from Golden Rooster Hatching Co., Ltd. and Antimp Solar Feeds Co., Ltd. (Tai’an, China), respectively. The experimentation was carried out over six weeks.
2.5. Data Collection
The chicks were weighed at the beginning of the experiment and then weekly. The quantity of feed consumed was recorded by daily weighing of the feed distributed and the feed remaining unconsumed. The feed conversion ratio by weight was determined for each lot.
At the third and sixth weeks of age, six chickens were randomly selected from each lot due to one chicken per replication, and blood samples were taken by wing vein from chickens of each lot into dry tubes (without anticoagulants) and centrifuged at 3500 rpm for 10 min. The serum was obtained, and the biochemical parameters such as total protein (TP), glucose (GLU), albumin (ALB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), triglyceride (TG), total cholesterol (TCHO), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) were measured using the automatic biochemical analyzer (Hitachi High-Technologies Corp., Tokyo, Japan) with commercial reagent kits (Maccura Biotechnology Co., Ltd., Chengdu, China).
2.6. Statistical Analysis
The data were analyzed using GraphPad Prism version 8.02 (263) statistical software. One-way ANOVA was chosen for the data analysis of the effect of in ovo injection of BSF larvae oil on the chicks. Tukey’s test was chosen for comparisons between the different lots. The results were presented as means with standard error (Mean ± SEM). p < 0.05 was considered the threshold for significance.
4. Discussion
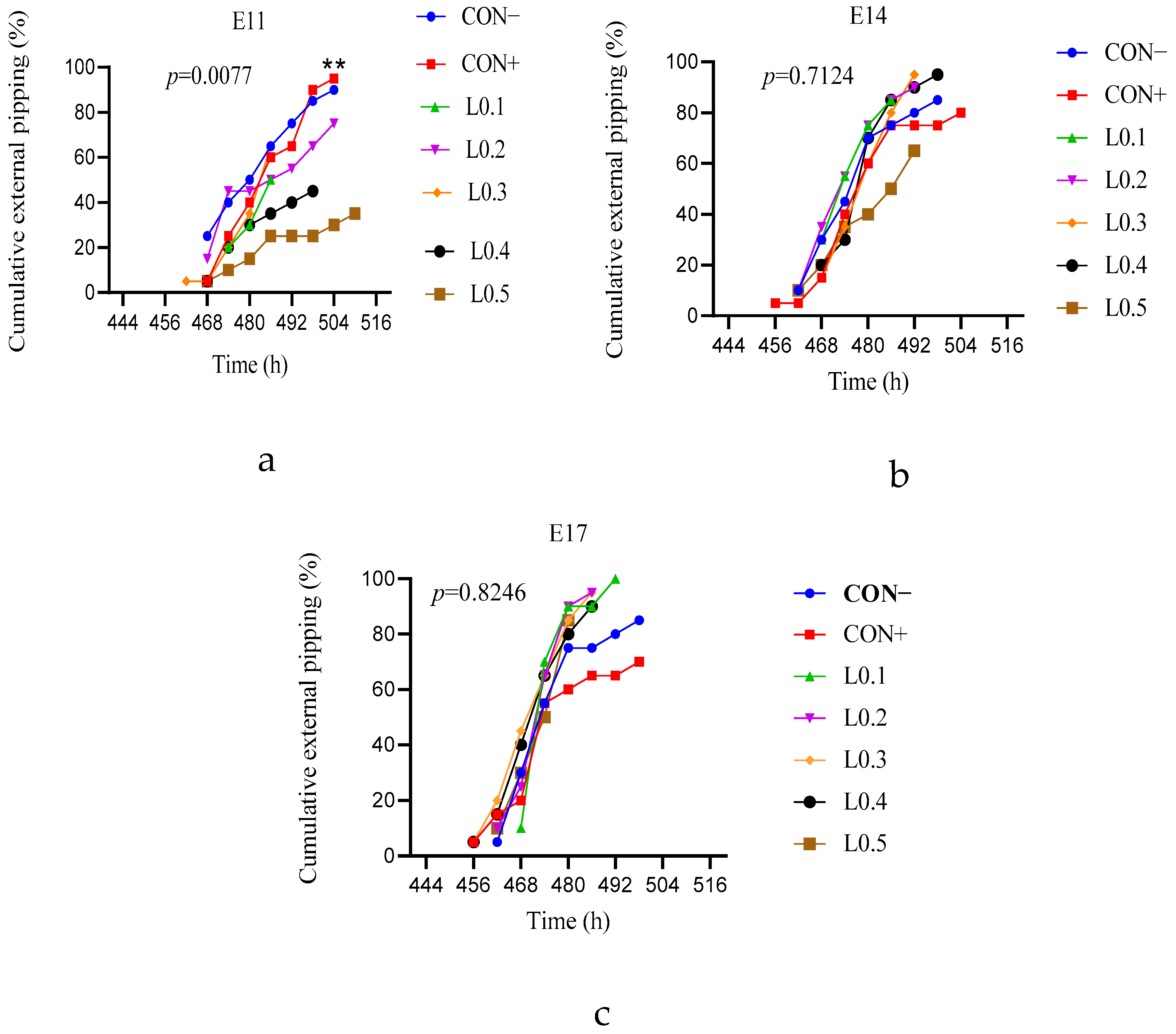
Hatching is a phenomenon in which the chicken embryo goes through several processes, namely assuming the right position, internal pipping, and external pipping, to be able to come out of the eggshell and lead a free life. All these physical steps require enough energy. The cumulative external pipping scores of the control lots from the 11th day of incubation in ovo injection were better than the test lots. Aberbour et al. [
20] had suggested that an in ovo injection of three microliters of rosemary essential oil into quail eggs on the 9th day of incubation was toxic to the embryo and led to a decrease in hatchability. Compared with the different doses of BSF larvae oil used in the present study, this suggests that the various doses have negatively affected the embryos of injections on the 11th day of incubation. But as age increases, the embryo can tolerate certain doses. This is why the external pipping of lots L0.1, L0.2, and L0.3 from the in ovo injection on the 14th day of incubation was better compared to the control batch. Similarly, except for lot L0.5, all external pipping of all test batches from the in ovo injection on the 17th day of incubation was also better. According to Kpodo and Proszkowiec-Weglarz [
17], in ovo feeding during late embryonic development increases the energy reserves and supports the hatching process. With the dose being appropriate at this stage, BSF larvae oil provided the necessary energy, which allowed chicks from the test lots to break the eggshell. The external pipping results corroborate the hatchability results.
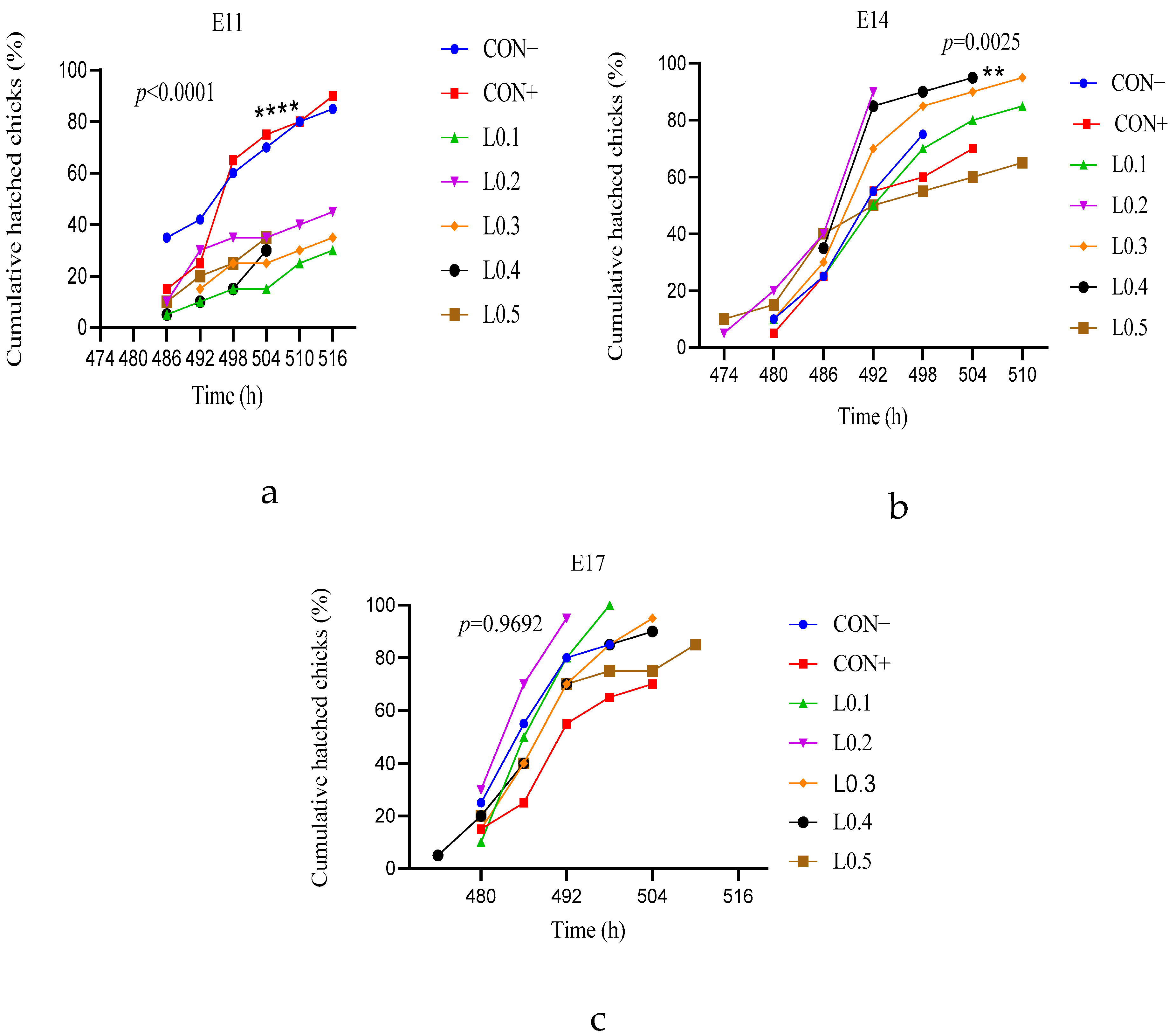
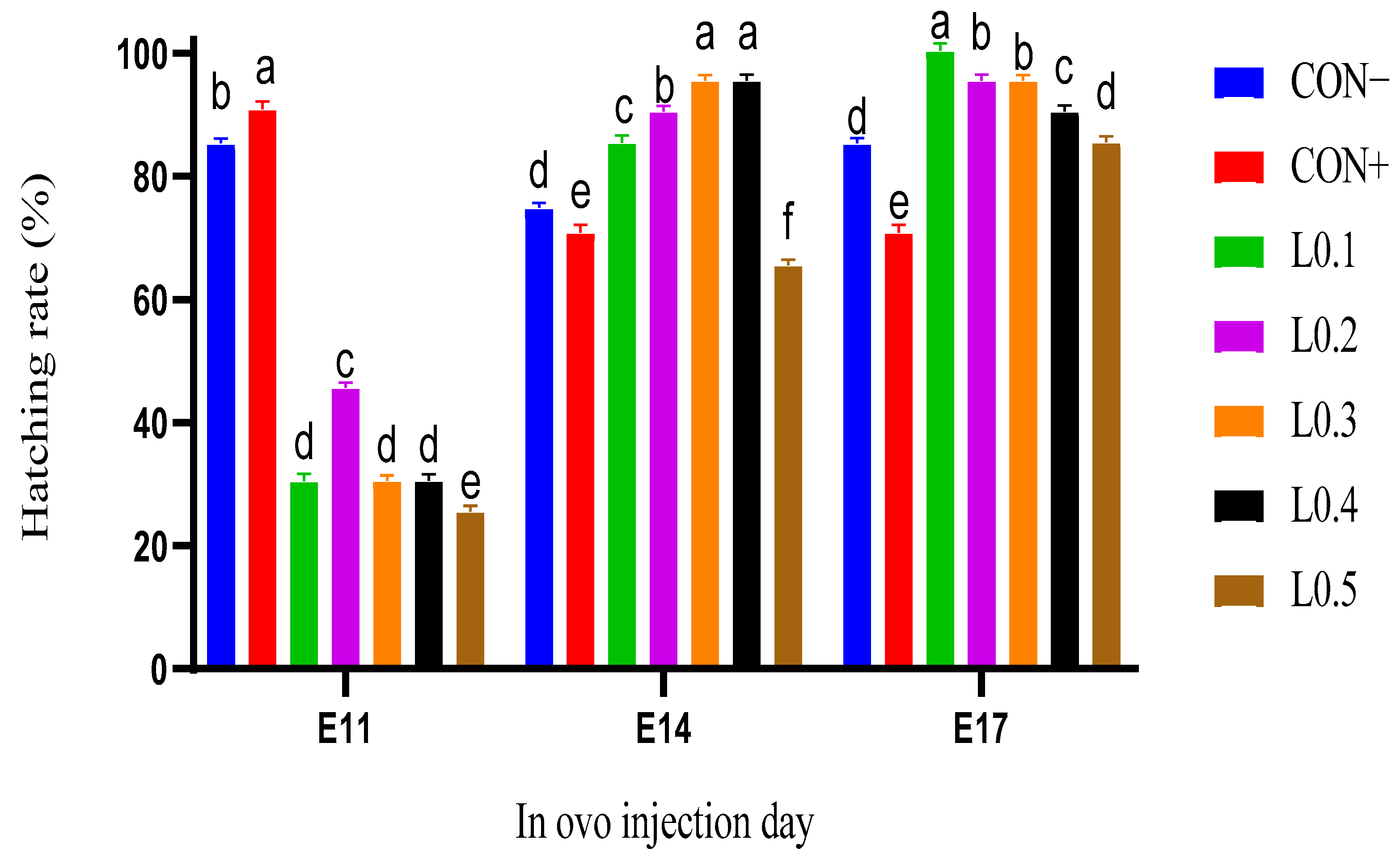
The group injected on the 11th day of incubation gave a better hatching rate in the control lots compared to all the test lots. On the other hand, the in ovo injected groups of the 14th and 17th days of incubation have the best hatching rate for the lots injected with the doses of 0.1 mL, 0.2 mL, 0.3 mL, or 0.4 mL. The cumulative rate of chicks in the L0.2 and L0.1 lots which were injected on the 14th and 17th day of incubation was higher than that of the other lots, and the time between the emergence of the first and last chick was also shorter compared to the different lots, indicating a reduction in the hatching window, which is important because this allows for obtaining high-quality chicks and the best post-hatching performance. However, the highest hatching rates were recorded for the lots injected on the 17th day of incubation, where a 0.1 mL dose was considered a success index, yielding a hatching rate of 100%. Indeed, lipid nutrients are an energy source. By injecting oil from BSF maggots in ovo, additional energy is produced that can be used in the chicken embryo for the best hatching. This was the case for the in ovo injected groups on days 14 and 17 of incubation. In ovo feeding lots, the hatching rate of those injected on the 17th day was better than those of the 14th day, which would mean that the amount of energy produced by the oil of BSF maggots was sufficient to support the 17th day embryo until hatching, more so than that of the 14th day embryo. Similarly, Sulaiman and Tayeb [
18] injected 0.1 mL of vegetable oils in ovo on the 18th day of incubation, and also observed an improvement in the hatching rate. However, the difference in hatching rates between treatments shows that the injection dose has an effect on the chicken embryo, and this was negative for high doses, particularly the volume of 0.5 mL, showing a low rate for each group. Regardless of the type of substance used, an appropriate dose is required for in ovo injection. Other studies have already suggested this with substances other than maggot oil. When Zhai et al. [
21] injected 0.1, 0.4, 0.7, or 1.0 mL of diluent carbohydrate into eggs at 18.5 days of incubation, the hatching rate decreased according to the dose, and they suggested that to achieve a 90% hatch rate, the injection volume should not exceed 0.4 mL for fructose or sucrose and should not exceed 0.7 mL for glucose, maltose, or dextrin.
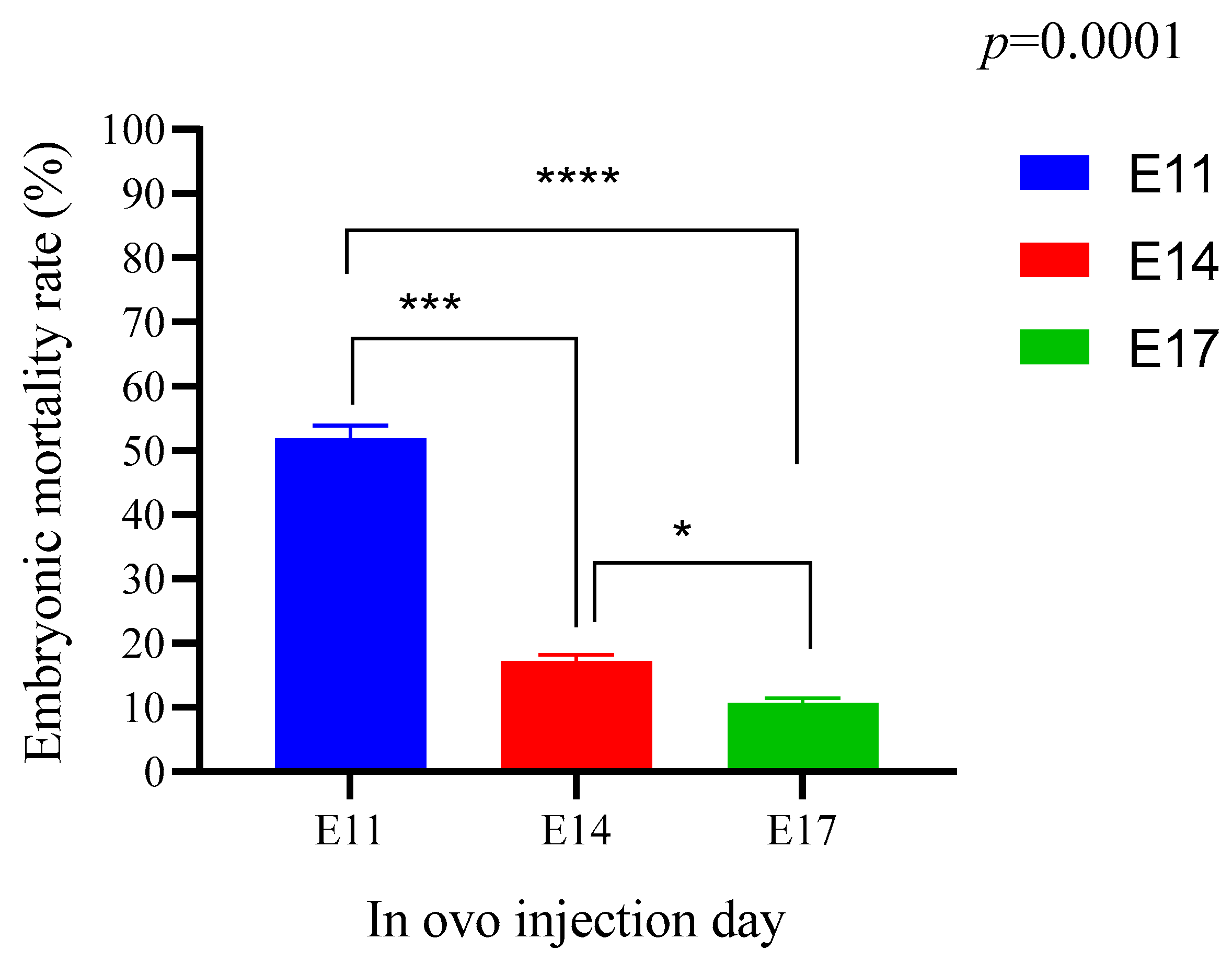
The results of hatching rates are related to the results of embryonic mortality rates shown in
Figure 4. The mortality rate was low for all lots injected on the 17th day of incubation. During the hatching period, the embryo needs energy to break the eggshell. A deficit of energy makes this operation difficult, even leading to late mortality. This explains the increase in the late mortality rate of the control lots compared to the test lots injected on the 17th day of incubation (
Figure 5c).
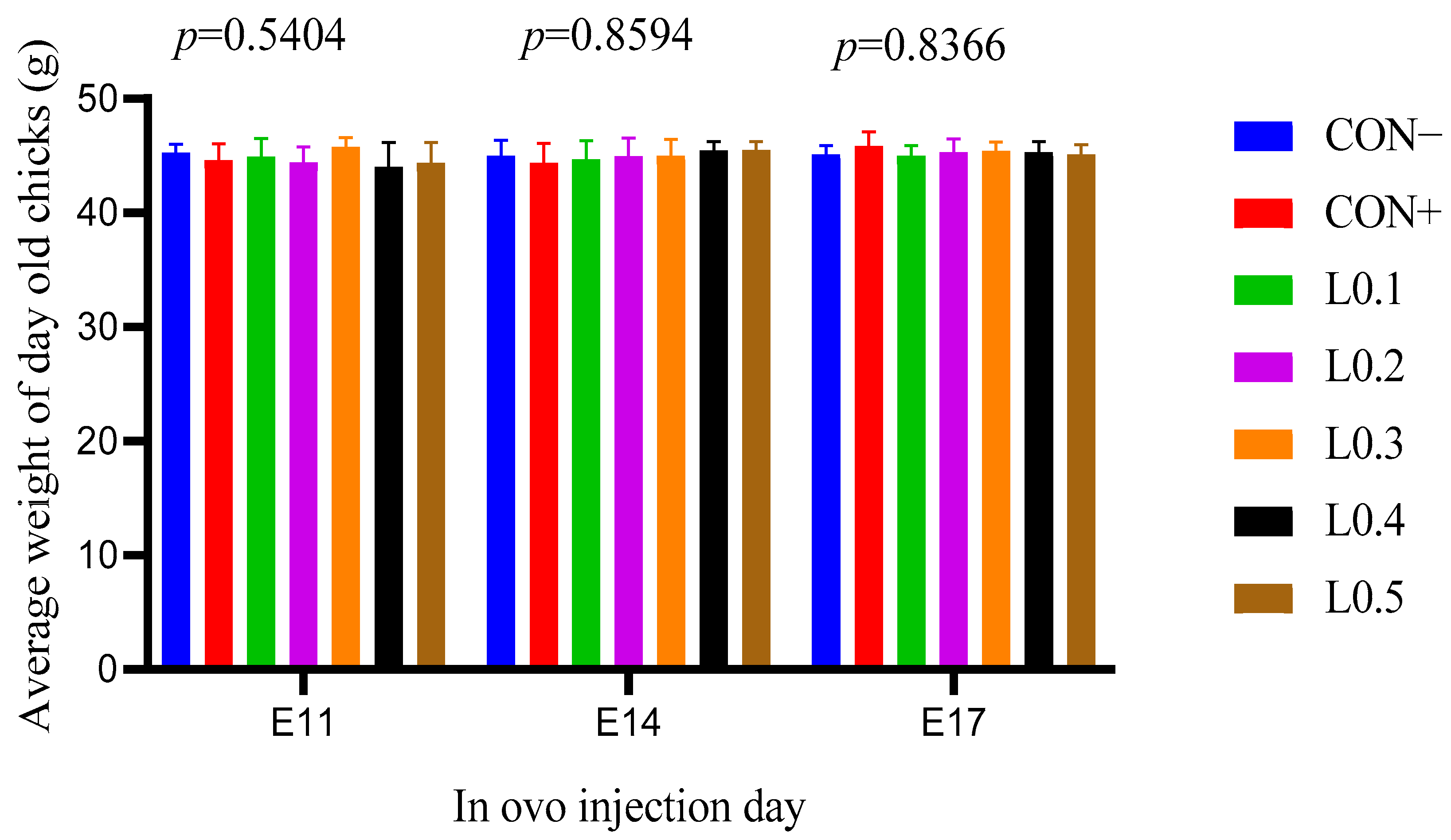
At hatching, no significant difference was observed for chick weights in all lots. Thus, the maggot oil from BSF did not play a significant role in the process of muscle development. Similar results were reported by many works. Ma et al. [
22] did not find a significant difference in day-old chick weight when they injected flaxseed oil and soybean oil in ovo. Also, Samson et al. [
23] did not find a significant difference in the weights of one-day-old chicks when they performed the in ovo injection of 0.2 mL of essential oil into the amniotic fluid of broiler chicken embryos on the 18th day of incubation. Some substances other than oil used for in ovo injection did not result in a difference in the weight of chicks at hatching. This is the case for an in ovo injection of L-glutamine into the amniotic sac of embryos performed on the 18th day of incubation [
24]; in ovo injection of gamma-aminobutyric acid performed in the amniotic fluid of the breeding chickens’ eggs at 17.5 days of incubation [
25]; and in ovo injection of creatine monohydrate carried out in the air chamber of broiler chicken eggs at 17.5 days of incubation [
26]. However, when Majida et al. [
27] performed an in ovo injection of omega-3 oil at different doses, such as 0.1 mL, 0.2 mL, and 0.3 mL per egg, into the amniotic fluid of broiler chicken eggs at 18 days of incubation, the weights of the day-old chicks in the injected groups were higher compared to the control. The difference in this result compared to the result of this current work may be due partly to the difference in injection time (18th/17th day) or to the difference in the fatty acids.
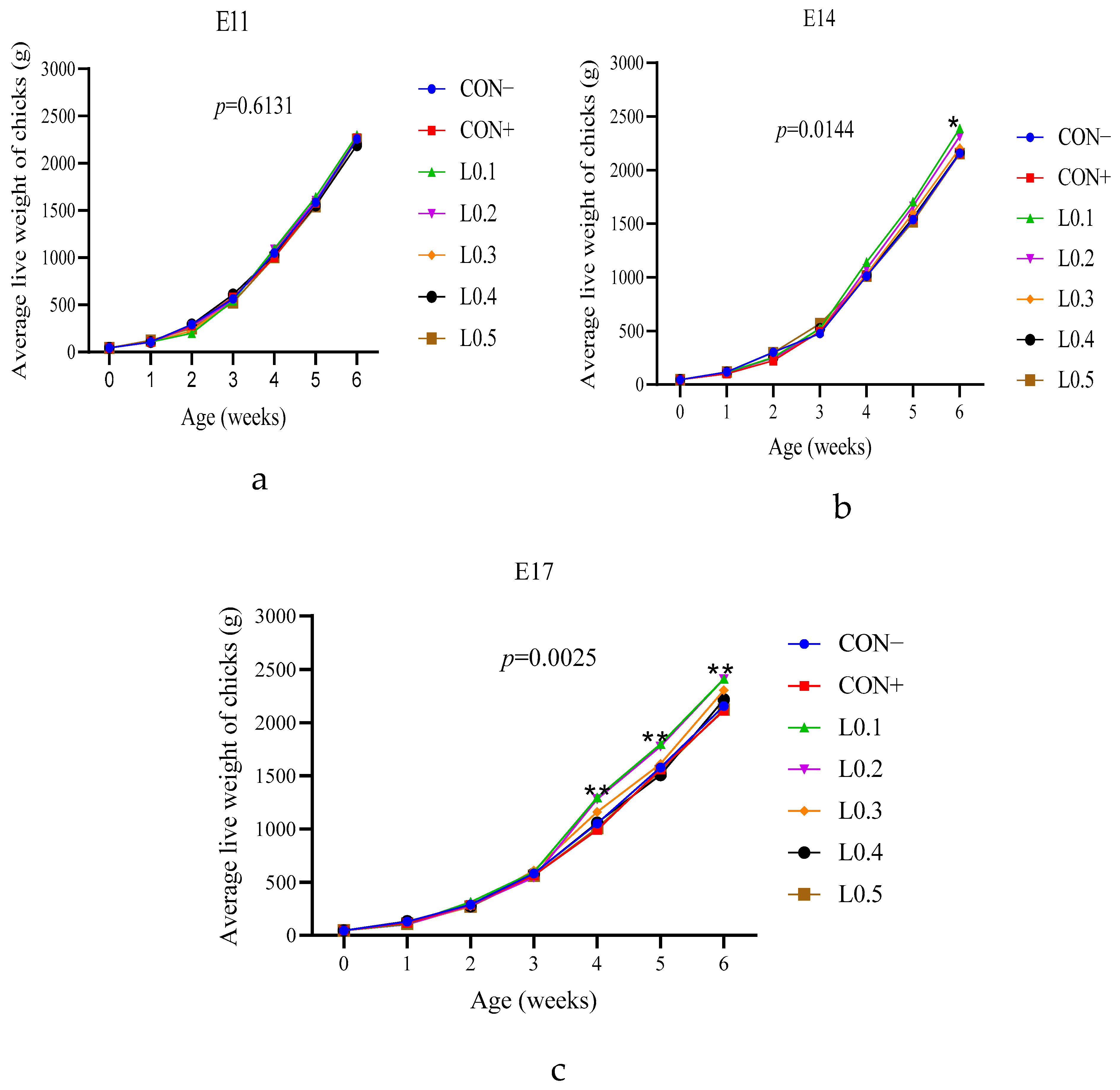
The chick weights of all the 11th day lots of injection were similar. On the other hand, the injected lots L0.1 and L0.2 had better weights compared to the other lots on the 14th and 17th day of injection. This showed the positive long-term impact of in ovo injection of BSF maggot oil in chick production. Sulaiman and Tayeb [
18] also reported similar results when they injected natural oils. In addition, due to the antimicrobial role of lauric acid in BSF maggot oil, it can also act on the intestinal ecosystem by reducing the number of pathogenic microorganisms in the gastrointestinal tract of chickens. The stability of the intestinal microflora promotes the ideal pH for the activation of digestive enzymes [
28].
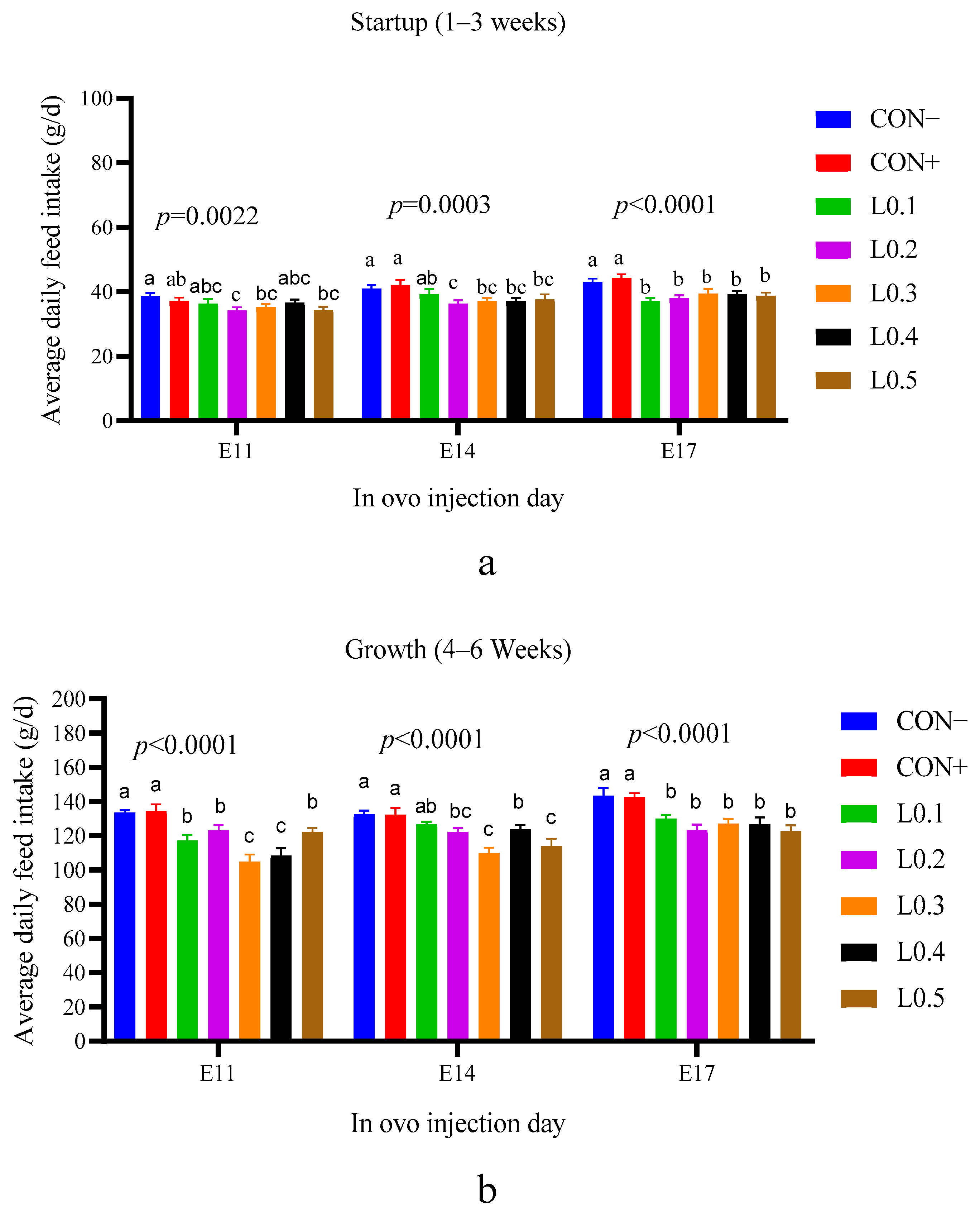
Feed consumption influences the growth and weight of an animal. During the start-up phase, on the 11th day of incubation, feed intake of all lots was practically similar. The difference was remarkable between the control lots and the test lots of in ovo injection on the 14th and 17th days of incubation. On the 11th day of incubation, the yolk sac of the hen’s egg is still filled with substance, so injecting another substance is only a substitution and not an addition. But as time passes, a certain amount of yolk sac nutrient will be used for physiological needs, and the remaining space can be used for in ovo nutrient addition. After hatching, the chick has nutrients in the yolk sac, which are adapted to the digestion process and can be used for the first 5 days of its life [
29]. In addition, the first days of a chick’s life are also a time of dietary transition, and chicks take time to adapt to solid feed. For this reason, on the 11th day of incubation, the feed intakes of all the chicks of the in ovo injected lots were similar. On the other hand, the decrease in feed intake of chicks in the test lots compared to the control lots on the 14th and 17th day of incubation is explained by the fact that, at this time, in ovo injection allows nutrients to be added quantitatively or qualitatively that can accompany the chick for a long time after hatching, therefore, the chicks’ feed intake decreased. However, even if the difference in feed intake was observed between the injected lots of the chicks of the 17 day incubation, it should be noted that no significant difference in average body weight was noticed between all the chicks of all lots of all injection times. During the hatching of domestic pigeons, Zhu et al. [
30] evaluated the effect of in ovo feeding L-lysine and also found no significant difference between the lots.
For all in ovo injection timings, the injected groups consumed less feed at the growth stage compared to the control groups. The decrease in feed intake in the treated groups suggests that the oil from BSF larvae may have developed the intestinal cells. In ovo injection allows for directly providing chicks with essential nutrients to optimize the growth and maturation of the digestive tract. Chen et al. [
31] had already found that chicks receiving in ovo methionine injection into the yolk sac showed better intestinal development and growth performance. Indeed, intestinal cells regulate nutrient absorption through the intestinal wall. Furthermore, the enteroendocrine cells of the intestine produce hormones such as serotonin, somatostatin, and cholecystokinin, which regulate appetite and metabolism, thus influencing feed consumption. Thus, the work of El-Sayed et al. [
32] has shown that the use of certain essential oils in the chick ration leads to the development of intestinal cells.
The feed conversion ratio was also lower for all test lots compared to control lots, particularly for the chicks in the growth phase. This allows us to affirm that the in ovo injection of maggot oil from BSF has led to the development of intestinal cells, resulting in the valorization of nutrients and a decrease in the feed consumption. These results are consistent with the work of Kim et al. [
33], who also showed that the use of oil from BSF larvae as an alternative fat source in broiler feed improves the feed conversion ratio. Ma et al. [
22] also found that feeding soybean oil and flaxseed oil in ovo improved feed conversion in broilers. In addition, when El-Sayed et al. [
32] evaluated the stimulating effect of Bacillus subtilis essential oil in broilers, they also found that the oil improved feed conversion.
Blood serum parameters are indicators that make it possible to assess the state of health of animals. The activities of AST and ALT are often biomarkers of liver health [
34]. In the growth phase, whether it is the in ovo injection on the 11th, 14th, or 17th day of incubation, no significant differences were observed between the test and control lots for AST and ALT. Thus, the in ovo injection of maggot oil from BSF had no negative impact on the liver, which is a main organ for the digestion of lipids. Although the AST concentration showed a significant difference during the start-up phase, it should be noted that AST can be secreted by several organs, such as muscles, kidneys, and the brain, not just the liver. Therefore, liver dysfunction cannot be determined only based on the results of AST. Total protein and albumin also did not show a significant difference in all lots during both the start-up and growth phase. Serum protein and albumin levels are endpoints for assessing animal health because they are related to stress and disease [
35,
36]. This means that the in ovo injection had not stressed the chicks as much. In addition, LDL cholesterol levels were significantly lower in the test lots than in the control lots. The oil extracted from BSF larvae contains compounds that can help lower LDL cholesterol levels. One such compound present in the oil, constituting 19% of its composition in this study, is linoleic acid (also known as octadecadienoic acid). In an experiment conducted by Mona et al. [
37], linoleic acid was injected into the air chamber of broiler chicken eggs on the 18th day of incubation, resulting in a reduction in LDL levels. These works are consistent with those of Kisun et al. [
38], who found that administering 0.5 g of linoleic acid daily to rabbits led to a decrease in blood LDL concentrations. LDL cholesterol is known as bad cholesterol, whose excess in an organism leads to cardiovascular disease. Thus, the in ovo injection of maggot oil from BSF likely reduces the risk of chickens’ cardiovascular disease by lowering the LDL cholesterol level. Apart from the positive effect of maggot oil on the health of the chicks through the biochemical blood parameters, it can also improve the quality of the chicken meat since the LDL cholesterol has been lowered [
39].